Abstract
Objective
To estimate the cumulative incidence of severe complications associated with genital chlamydia infection in the general female population.
Methods
The Uppsala Women's Cohort Study was a retrospective population based cohort study in Sweden, linking laboratory, hospital, and population registers. We estimated the cumulative incidence of hospital diagnosed pelvic inflammatory disease, ectopic pregnancy, and infertility, and used multivariable regression models to estimate hazard ratios according to screening status.
Results
We analysed complete data from 43 715 women in Uppsala aged 15–24 years between January 1985 and December 1989. Follow up until the end of 1999 included 709 000 woman years and 3025 events. The cumulative incidence of pelvic inflammatory disease by age 35 years was 3.9% (95% CI 3.7% to 4.0%) overall: 5.6% (4.7% to 6.7%) in women who ever tested positive for chlamydia, 4.0% (3.7% to 4.4%) in those with negative tests, and 2.9% (2.7% to 3.2%) in those who were never screened. The corresponding figures were: for ectopic pregnancy, 2.3% (2.2% to 2.5%) overall, 2.7% (2.1% to 3.5%), 2.0% (1.8% to 2.3%), and 1.9% (1.7% to 2.1%); and for infertility, 4.1% (3.9% to 4.3%) overall, 6.7% (5.7% to 7.9%), 4.7% (4.4% to 5.1%), and 3.1% (2.8% to 3.3%). Low educational attainment was strongly associated with the development of all outcomes.
Conclusions
The incidence of severe chlamydia associated complications estimated from ours, and other population based studies, was lower than expected. Studies that incorporate data about pelvic inflammatory disease diagnosed in primary care and behavioural risk factors would further improve our understanding of the natural history of chlamydia. Our results provide reassurance for patients, but mean that the benefits of chlamydia screening programmes might have been overestimated.
Keywords: chlamydia infections, pelvic inflammatory disease, ectopic pregnancy, infertility, cohort studies
Infection with Chlamydia trachomatis is the most common preventable cause of pelvic inflammatory disease in young women, and may lead to ectopic pregnancy and tubal infertility.1 Reliable information about the complications of lower genital tract chlamydia infection is required so that patients can be better informed of the risks of infection, and the likely impact of preventive interventions can be determined. The fertility outcomes of women admitted to hospital with pelvic inflammatory disease have been studied in detail in Lund, Sweden,2,3,4 although information about previous chlamydia infections is lacking. In addition, authoritative sources state that up to 40% of untreated endocervical chlamydia will progress to pelvic inflammatory disease.5,6 There are several reasons, however, for questioning the generalisability of this information.7 Firstly, data from studies of clinical populations might overestimate progression rates if they include more severe cases.7,8 Secondly, the symptoms and signs of pelvic inflammatory disease are non‐specific, and diagnosis without the aid of laparoscopy is difficult.9 Thirdly, there are many causes of pelvic inflammatory disease and an aetiological role for C trachomatis cannot be unequivocally established or excluded, particularly after the acute infection has resolved. Finally, recorded numbers of chlamydia associated complications in population based studies are lower than would be expected from clinical research (table 1).8,10,11,12,13,14,15
Table 1 Population based estimates of chlamydia associated complications.
| Outcome and study setting | Ref | Period | Population | Study design | Cases | Age (years) | Incidence per 1000 woman years | ||
|---|---|---|---|---|---|---|---|---|---|
| Pelvic inflammatory disease | |||||||||
| All women | Overall | Screened | Unscreened | ||||||
| Amsterdam, Netherlands | 8 | 1990 | Population based | Disease register | Hospital† | 15–44 | 4.85 | .. | .. |
| South Carolina, USA | 10 | 1996–8 | Military recruits | Cohort study | Clinical‡ | 80%<25 at entry | .. | 4.6 | 5.1 |
| Aarhus, Denmark | 11 | 1997–8 | Population based | Randomised trial | Hospital§ | 16–17 at entry | 6.5 | 2.4 | 11.5 |
| All cases§ | 31.2 | 19.2 | 53.2 | ||||||
| Seattle, USA | 12 | 1990–2 | High risk women | Randomised trial | All cases¶ | 18–34 at entry | 16.9‡ | 9.6 | 21.6 |
| England and Wales | 13 | 1991–2 | Population based | Cross sectional | Primary care | 16–46 | 16.7 | .. | .. |
| Women with chlamydia | |||||||||
| Massachussetts, USA | 14 | 1988–1 | Population based | Cohort study | All cases¶ | 20–29 at entry | 56.3† | .. | .. |
| Ectopic pregnancy | Overall | Chlamydia + | Chlamydia ‐ | ||||||
| N Jutland, Denmark | 15 | 1984–2003 | Population based | Cohort study | Hospital** | 15–43 at entry | 3.5 | 8.6 | |
†Data from city disease registry. Not clear if primary care cases are included. Authors estimated rate of chlamydial pelvic inflammatory disease as 1.7 cases per 1000 woman years.
‡Inpatient episodes only of pelvic inflammatory disease as a result of any cause.
§Hospital cases from hospital registers. All cases ascertained by patient report. Data collected at 1 year after intervention so assumed that each woman contributed 1 woman year of follow up. 45% lost to follow up. Includes pelvic inflammatory disease as a result of any cause.
¶Cases ascertained from health insurance records.
**From record linkage.
Investigating the natural history of genital chlamydia remains problematic since prospective long term studies of untreated infection would clearly be unethical. The best opportunity to investigate associations between chlamydia and its long term consequences therefore comes from places in which chlamydia status and outcomes of chlamydia associated disease can be ascertained for large numbers of women. Sweden has the world's longest established chlamydia control activities: opportunistic screening has been undertaken nationwide since the late 1980s.16,17,18 In Uppsala County (population 270 000), chlamydia screening is advised for women aged 15–29 years attending family planning clinics or other consultations for contraceptive purposes, pregnant women, and all attenders at five youth clinics,17 but there is no stated screening interval and no monitoring of uptake. The objective of this study was to describe the frequency of hospital diagnosed complications of chlamydia and to estimate their incidence in young women in Uppsala County, Sweden.
Methods
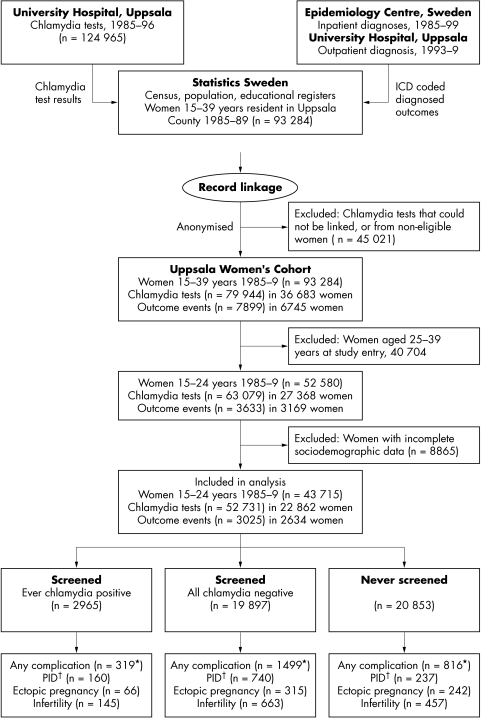
The Uppsala Women's Cohort Study included all 93 284 women resident in Uppsala and aged 15–39 years at any time between 1 January 1985 and 31 December 1989 (fig 1).19 We attempted to follow the chlamydia screening histories and hospital diagnosed complications of these women from 1 January 1985 to 31 December 1999. We used the “person number,” a unique identifier assigned at birth or immigration to link the results of laboratory tests for chlamydia, hospital diagnoses, and sociodemographic data. The study was approved by the University of Uppsala research ethics committee. The source databases are described below.
Figure 1 Record linkage in the Uppsala Women's Cohort Study. *Some women experienced more than one complication so the sum of individual complications is greater than the number with any of the three; †PID, pelvic inflammatory disease.
Demographic and socioeconomic information
Statistics Sweden used population registers to construct a dataset of eligible women and provided data on country of birth, migration, educational attainment, and numbers of births and deaths. We measured the material conditions of each woman based on her family's income recorded in the census closest to her 15th birthday because many women in the cohort were still at school or college during the study period. We did not have data about age at sexual debut, contraceptive practices, sexual behaviour, or healthcare seeking behaviour.
Chlamydia screening
All tests for C trachomatis are carried out at the University Hospital of Uppsala. Culture (sensitivity 75% compared to polymerase chain reaction20) was the diagnostic method for most samples until polymerase chain reaction (Amplicor CT Test, Roche Diagnostics, Basel, Switzerland) sensitivity compared to a combined reference standard 85–90%20) was introduced in 1996. We used a database of all chlamydia tests from 1985 to 1996, after which record linkage was not possible. Tests done outside Uppsala County were not available. Information about symptoms was not available: in this paper we refer to tests done for any purpose as screening tests.
Hospital diagnoses
The Centre for Epidemiology provided information about hospital diagnoses anywhere in Sweden from 1985 to 1999 so follow up for inpatient diagnoses was complete. We also used the Uppsala Hospital Discharge Database, which allowed us to include cases of ectopic pregnancy and pelvic inflammatory disease managed as outpatients from 1993 to 1999. We included all diagnoses of ectopic pregnancy (International Classification of Disease 10th Revision codes corresponding to ICD 10.00), pelvic inflammatory disease (N70, 71, 73, 74), and infertility (N97).
Record linkage
Statistics Sweden supplied a list of person numbers of the whole cohort to the Centre for Epidemiology and Uppsala University Hospital, both of which returned data on hospital diagnoses. Statistics Sweden then linked chlamydia test results, hospital diagnoses, and census data to the population register before deleting the person number and anonymising the dataset. For this study, we restricted the cohort to women resident in Uppsala who were 15–24 years old from 1985 to 1989 because chlamydia testing histories were most complete in this age group.
Statistical analysis
We restricted analysis to women with complete data on socioeconomic variables. We used survival analysis methods with age as the time axis and delayed entry at the date when each woman was first living in Uppsala County and aged 15–24 years. Each woman's screening test history was used to divide her follow up time into one or more of three exposure groups. Until the date of her first screening test, follow up time was classified as “never tested.” If the test was negative, subsequent follow up time was classified as “negative.” All follow up time subsequent to a positive test result was classified as “positive.” Follow up was censored when the woman experienced an outcome, when she left Uppsala County, or on 31 December 1999, whichever came first. We derived Kaplan‐Meier estimates of the cumulative incidence (%, 95% confidence intervals, CI) of each outcome, and used Cox proportional hazards regression models to estimate the association of screening status with the hazard of developing each outcome, controlling for potential confounding by age, education, income, and housing. We used likelihood ratio tests to investigate interactions. Statistical analyses were done using Stata (version 8.2, Stata Corporation, College Station, Austin, Texas).
Results
Among the female resident population of Uppsala County from 1985 to 1989 there were 52 580 women aged 15–24 (fig 1). The subsequent analyses include 43 715 (83.1%) with complete information about socioeconomic characteristics, who contributed 709 000 (median 15 years) woman years of follow up until 31 December 1999. The characteristics of those with and without socioeconomic information were similar, although more younger women had complete data (data not shown).
The cumulative probability of ever being screened for chlamydia was 70.7% (95% CI 70.3% to 71.1%) by age 35 years. Of 22 862 women ever tested 47.8% (n = 10945) were tested once, 22.2% (n = 5081) had two tests, and 30.0% (n = 6877) had three or more tests. There were only 259 (1.1%) women who had 10 or more tests over the entire follow up period. Younger women, aged 15–19, were slightly more likely than 20–24 year olds to have been tested more than once (52.8% v 50.5%, p = 0.002). Among women screened, 11.5% (n = 2626) had one positive chlamydia test, 1.3% (n = 298) had two, and 0.2% (n = 41) had three or four episodes of infection.
During the study period 2634 women experienced any hospital diagnosed complication, including 1138 episodes of pelvic inflammatory disease, 623 ectopic pregnancies and 1265 women diagnosed with infertility (fig 1). Of these women, 816 (31.0%) had never been screened, 1499 (56.9%) had only negative tests, and 319 (12.1%) had had a positive chlamydia test. The proportions of women experiencing any outcome among those who had had none, one, two, or three or more tests were 4.0%, 6.1%, 7.2%, and 11.3%, respectively (p for trend <0.0001). The odds of experiencing any complication were slightly higher in women with more than one episode of infection (odds ratio 1.35, 95% CI 0.97 to 1.9, p = 0.076).
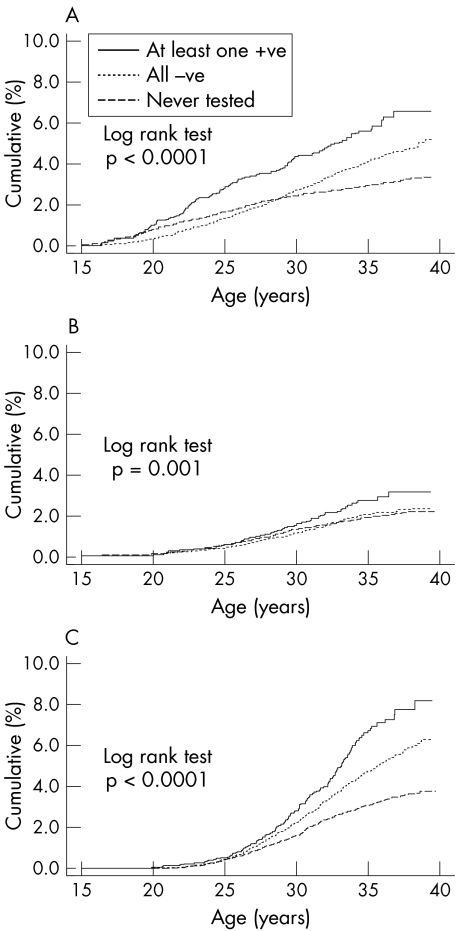
Figure 2 shows the cumulative incidence of hospital diagnosed chlamydia associated conditions by age and time updated screening status. The cumulative incidence of experiencing pelvic inflammatory disease by age 35 years was 3.9% (95% CI 3.7% to 4.0%) overall: 5.6% (4.7% to 6.7%) in women who ever tested positive for chlamydia, 4.0% (3.7% to 4.4%) in those with negative tests, and 2.9% (2.7% to 3.2%) in those who were never screened. The corresponding figures were: for ectopic pregnancy, 2.3% (95% CI 2.2% to 2.5%) overall, 2.7% (2.1% to 3.5%), 2.0% (1.8% to 2.3%), and 1.9% (1.7% to 2.1%); and for infertility, 4.1% (95% CI 3.9% to 4.3%) overall, 6.7% (5.7% to 7.9%), 4.7% (4.4% to 5.1%), and 3.1% (2.8% to 3.3%).
Figure 2 Incidence of hospital diagnosed conditions associated with chlamydia. (A) Pelvic inflammatory disease; (B) ectopic pregnancy; (C) infertility.
Table 2 shows associations between chlamydia screening status and each outcome. In univariable analyses, the crude hazard of developing each event was higher in women with previous positive chlamydia tests than those with previous negative tests and in women with fewer years of schooling. These associations were attenuated after controlling for socioeconomic factors; the adjusted hazard of experiencing each event was about 30% higher in women with previous positive compared to previous negative tests for all three complications. Low educational level remained strongly associated with developing complications before and after controlling for other factors, including chlamydia screening. There was no evidence of interaction between chlamydia screening status and educational level (likelihood ratio tests for pelvic inflammatory disease, p = 0.258; ectopic pregnancy, p = 0.223; infertility, p = 0.397).
Table 2 Associations between chlamydia screening, hospital diagnosed complications, and socioeconomic factors.
| Variable | Pelvic inflammatory disease* | Ectopic pregnancy* | Infertility* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||||||
| Crude | Adjusted† | p value‡ | Crude | Adjusted† | p value‡ | Crude | Adjusted† | p value‡ | |
| Chlamydia test | |||||||||
| Never | 0.69 (0.61 to 0.78) | 0.72 (0.63 to 0.82) | <0.0001 | 0.98 (0.83 to 1.15) | 0.95 (0.80 to 1.13) | 0.160 | 0.60 (0.53 to 0.67) | 0.65 (0.57 to 0.73) | <0.0001 |
| All negative | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Positive | 1.50 (1.23 to 1.82) | 1.27 (1.04 to 1.55) | 1.42 (1.06 to 1.88) | 1.26 (0.94 to 1.67) | 1.38 (1.15 to 1.66) | 1.31 (1.09 to 1.57) | |||
| Educational level | |||||||||
| Graduate | 1 (reference) | 1 (reference) | <0.0001 | 1 (reference) | 1 (reference) | <0.0001 | 1 (reference) | 1 (reference) | <0.0001 |
| High school | 1.52 (1.25 to 1.84) | 1.48 (1.22 to 1.80) | 1.22 (0.97 to 1.54) | 1.28 (1.01 to 1.62) | 1.26 (1.09 to 1.47) | 1.23 (1.06 to 1.43) | |||
| Vocational | 3.20 (2.66 to 3.84) | 2.86 (2.36 to 3.47) | 2.32 (1.86 to 2.89) | 2.16 (1.70 to 2.73) | 1.88 (1.63 to 2.18) | 1.85 (1.58 to 2.16) | |||
| Basic | 5.38 (4.39 to 6.59) | 4.51 (3.62 to 5.61) | 3.43 (2.64 to 4.45) | 3.04 (2.29 to 4.04) | 1.55 (1.25 to 1.92) | 1.51 (1.20 to 1.89) | |||
| Total numbers | 1095 events | 621 events | 1265 events | ||||||
*Models restricted to 43715 women under 25 years at study entry with complete data. All models controlled for age.
†Adjusted models control for chlamydia test status, educational level, housing type, income, number of births, and census year.
‡p Value refers to adjusted model.
Discussion
In this large Swedish population based cohort, 71% of women aged 15–24 at study entry were screened for chlamydia and 13% of those screened had at least one episode of chlamydia. Nearly half of screened women had only one test. Between 2% and 4% overall and between 3% and 7% of women with a previously diagnosed episode of chlamydia experienced a hospital diagnosed episode of ectopic pregnancy, pelvic inflammatory disease or infertility by age 35 years. Low educational status was strongly independently associated with all three chlamydia associated complications.
Methodological issues
The strengths of this study are that it is a large long term population based cohort study that included all young women in a Swedish county from the time that widespread chlamydia testing was introduced. The level of record linkage was excellent, and ascertainment of both exposure and outcomes was high. Our longitudinal analyses took into account both age and the temporal sequence of chlamydia testing and development of outcomes.
The limitations of the study relate to the use of routine datasets and to inherent problems in studying the complications of a largely asymptomatic but curable condition. Firstly, our outcomes were defined by ICD coded hospital discharge records with diagnoses made by clinicians who might have applied different diagnostic criteria. Ectopic pregnancy diagnosis is objective and specific and our estimated incidence should be complete because we included outpatient cases from 1993, when methotrexate treatment became widespread.21 The sensitivity and specificity of hospital diagnosed pelvic inflammatory disease and infertility are, however, imperfect. Complete ascertainment of infertility is not possible because it depends on women presenting for investigation. For pelvic inflammatory disease, 61% of ICD coded diagnoses in a recent Australian study were confirmed laparoscopically.22 The largest source of underestimation of pelvic inflammatory disease is because up to 90% of cases could be diagnosed in primary care.13,23 Although we were able to include outpatient diagnoses from 1993 we could not count these cases from 1985 to 1992 and we could not include cases managed in primary care. It would, however, be inappropriate to simply multiply the observed rate to account for under‐ascertainment if hospital diagnosed cases differed from those diagnosed in primary care. Although there are similarities between acute and subclinical pelvic inflammatory disease,24 mild cases treated in primary care might be less likely to cause tubal damage. Attributing all diagnosed cases of complications to chlamydia compensates for some under‐reporting. For all three conditions we included all diagnosed cases although clinical diagnosis of pelvic inflammatory disease is known to be imprecise and chlamydia was only one possible cause.9 Another source of under‐ascertainment was that in women with diagnosed chlamydia, ascending infection might have been prevented by antibiotic treatment. We do not think that this led to a substantial underestimate in our study because of infrequent screening. Indeed, only 12% of complications occurred in women with a positive chlamydia test: the remainder were in women who had only negative tests or had never been tested. This indicates that the episode of chlamydia which might have caused the condition was untreated and that the natural history was unaffected by treatment. There is no evidence that single dose antibiotic treatment reduces the progression of lower genital tract chlamydia than multiple dose regimens so this is unlikely to have had an impact.25
Chlamydia test status is a proxy for the true exposure, chlamydia infection. A positive test might not indicate newly acquired infection, women with either no or only negative tests might have had test results that could not be linked or undiagnosed infection, and culture diagnosis will have missed some infections. Such misclassification would attenuate observed associations between chlamydia infection and its complications. This is suggested by our finding of a stronger association between the risk of sequelae and educational level than chlamydia status. The low risk of complications in women who were never screened suggests that this group of women were at low risk of chlamydia. Women who were not screened were different from women who underwent screening: they were more likely to have a university education and to be older and from families with high incomes (data not shown). These characteristics are partly a proxy for sexual behaviour, for which we did not have information. The incomplete coverage of screening also suggests an important contribution of both physicians' and women's perceptions of risk and decisions to offer, and accept, screening. The factors associated with chlamydia screening, and implications for screening programmes are a separate research question that will be addressed in a subsequent publication. These issues do not change the main finding of this study—that the risk of serious hospital diagnosed complications associated with chlamydia observed over a long period of time in the general population is lower than expected from studies in clinical populations.
Comparison with other prospective studies
Population based randomised controlled trials and cohort studies suggest that the overall incidence of hospital diagnosed pelvic inflammatory disease is 5–7 per 1000 woman years,8,10,11 and 17–31 per 1000 woman years including cases diagnosed in primary care (table 1).11,12,13 We estimated an incidence of hospital diagnosed pelvic inflammatory disease of 1.9 (95% CI 1.8 to 2.0) per 1000 woman years. In women with chlamydia, incidence rates of pelvic inflammatory disease diagnosed in all settings,14 and hospital diagnosed ectopic pregnancy,15 were estimated to be 56 and 4 per 1000 women years, respectively. While many of these studies suffer from the limitations outlined above, the estimated incidence rates of complications were consistently lower than from studies in clinical populations. These have found that up to 40% of untreated cervical chlamydial infections result in pelvic inflammatory disease within a few weeks,26,27 and that 20–25% of women with pelvic inflammatory disease from any cause will have an ectopic pregnancy or become infertile.2,3,4 This suggests that clinic based studies included women with more severe disease that was more likely to progress.8
Meaning of the study
A diagnosis of chlamydia makes women fearful about their future fertility.28 Many asymptomatic infections detected during screening will have been present for some time and clinicians need to be able to give reliable advice to infected women about the probability of reproductive damage. Current advice suggests high rates of complications based on studies in clinic and hospital populations,5,6 and higher risks of complications with repeated infection.29 Our study, together with other population based data, provides reassurance that the long term risks of being hospitalised with pelvic inflammatory disease, ectopic pregnancy, and infertility following a positive chlamydia test are lower than previously thought. Ectopic pregnancy and infertility are consequences of pelvic inflammatory disease, rather than the lower genital tract infection. We were unable to estimate these rates directly: our study was an observational cohort and, as observed in clinical practice, most episodes of ectopic pregnancy and infertility (about 85%) were not preceded by a documented episode of pelvic inflammatory disease. Furthermore, few women had repeated episodes of diagnosed chlamydia, probably because of the low frequency of screening, so we could not examine the association with repeated infections. There is now a need for ethical prospective studies to define the prognosis of genital chlamydial infection even better, by incorporating information about sexual behaviour and other risk factors, and about the aetiology of diagnosed complications.
Key messages
Studies of hospital and clinic populations suggest that up to 40% of cases of untreated chlamydia progress to pelvic inflammatory disease within a few weeks of infection, and that 20–25% of women with pelvic inflammatory disease will have an ectopic pregnancy or become infertile
In a long term observational cohort study of young women in Uppsala County, Sweden, the cumulative incidences of hospital diagnosed pelvic inflammatory disease, ectopic pregnancy, and infertility by age 35 were 2–4% overall and 3–7% in those with a history of diagnosed chlamydia
These findings, which are consistent with the findings of other population based studies, should be used to reassure patients
If the incidence of complications associated with chlamydia have been overestimated then the benefits and cost effectiveness of current screening programmes might also have been exaggerated
Implications for research and health policy
Our findings suggest that the cost effectiveness of chlamydia screening might have been overestimated.7,8 Economic evaluations typically assume that annual chlamydia screening uptake is 60–100% and apply estimates of disease progression based on clinical populations to all cases of asymptomatic chlamydia detected by screening.30,31,32,33 An increasing number of studies show that about 50% of asymptomatic prevalent chlamydia infections resolve spontaneously within a year with little risk of pelvic inflammatory disease,34,35,36 so the incidence of major outcomes is probably overestimated. These evaluations are also frequently based on static models that do not account for the effects of re‐infections and spontaneous resolutions of chlamydia on population transmission.37,38 In practice, the uptake of opportunistic chlamydia screening in target groups in the United States is well below 50%,39,40 and our study suggests that complications in the general population are less common than in clinical settings. There is now a need for high quality dynamic economic modelling studies based on realistic assumptions about the uptake and frequency of screening, and the likelihood of severe reproductive tract morbidity. A low incidence of chlamydial complications is good news for individual patients but raises questions about the presumed cost effectiveness of current chlamydia screening programmes.
Acknowledgements
The study was funded by the National Institute of Public Health, Sweden.
Nicola Low was funded by a NHS Career Scientist Award until 31 March 2005.
We would like to thank Gunnar Medin for his help with extraction and management of original data from Uppsala, and Åke Nilssen of Statistics Sweden for undertaking the record linkage.
Contributors
NL, ME, BL, and BH designed the study and obtained funding; BH supervised the data collection; RH and FI did the statistical analyses under the supervision of JS and NL; NL drafted the manuscript; all authors contributed to revising the manuscript.
Footnotes
Conflict of interest: none declared.
Ethical approval: The research ethics committee of the Uppsala University Hospital approved this project.
Some results of this study have been presented at the 16th Biennial Meeting of the ISSTDR; Amsterdam, Netherlands, 10–13 July, 2005; the BASHH/ASTDA Spring Meeting, Bath, UK; 19‐21 May 2004; and the RCOG/BASHH joint meeting, London, UK, 3 Dec 2004.
References
- 1.Cates W, Jr, Wasserheit J N. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol 19911641771–1781. [DOI] [PubMed] [Google Scholar]
- 2.Weström L. Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol 1975121707–713. [DOI] [PubMed] [Google Scholar]
- 3.Weström L, Bengtsson L P, Mårdh P A. Incidence, trends, and risks of ectopic pregnancy in a population of women. BMJ 198128215–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weström L, Joesoef R, Reynolds G.et al Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 199219185–192. [PubMed] [Google Scholar]
- 5.Health Protection Agency Chlamydia. http://www.hpa.org.uk/infections/topics_az/hiv_and_sti/sti‐chlamydia/chlamydia.htm, 2005. Accessed 5 July 2005
- 6.The National Woman's Health Information Center Chlamydia. http://www.4woman.gov/faq/stdchlam.htm 4woman.g ov. US Department of Health and Human Services 2005
- 7.Low N, Egger M. What should we do about screening for genital chlamydia? Int J Epidemiol 200231891–893. [DOI] [PubMed] [Google Scholar]
- 8.Van Valkengoed I G, Morré S A, van den Brule A J.et al Overestimation of complication rates in evaluations of Chlamydia trachomatis screening programmes—implications for cost‐effectiveness analyses. Int J Epidemiol 200433416–425. [DOI] [PubMed] [Google Scholar]
- 9.Simms I, Warburton F, Weström L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sex Transm Infect 200379491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark K L, Howell M R, Li Y.et al Hospitalization rates in female US Army recruits associated with a screening program for Chlamydia trachomatis. Sex Transm Dis 2002291–5. [DOI] [PubMed] [Google Scholar]
- 11.Østergaard L, Andersen B, Møller J K.et al Home sampling versus conventional swab sampling for screening of chlamydia trachomatis in women: a cluster‐randomized 1‐year follow‐up study. Clin Infect Dis 200031951–957. [DOI] [PubMed] [Google Scholar]
- 12.Scholes D, Stergachis A, Heidrich F E.et al Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 19963341362–1366. [DOI] [PubMed] [Google Scholar]
- 13.Simms I, Rogers P, Charlett A. The rate of diagnosis and demography of pelvic inflammatory disease in general practice: England and Wales. Int J STD AIDS 199910448–451. [DOI] [PubMed] [Google Scholar]
- 14.Rothman K J, Lanza L, Lal A.et al Incidence of pelvic inflammatory disease among women treated for gonorrhea or chlamydia. Pharmacoepidemiol Drug Saf 19965409–414. [DOI] [PubMed] [Google Scholar]
- 15.Andersen B, Østergaard L, Puho E.et al Ectopic pregnancies and reproductive capacity after Chlamydia trachomatis positive and negative test results: a historical follow‐up study. Sex Transm Dis 200532377–381. [DOI] [PubMed] [Google Scholar]
- 16.Kamwendo F, Forslin L, Bodin L.et al Programmes to reduce pelvic inflammatory disease—the Swedish experience. Lancet 1998351(Suppl) 325–28. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann B, Egger M. Genital Chlamydia trachomatis infections in Uppsala County, Sweden, 1985–1993: declining rates for how much longer? Sex Transm Dis 199522253–260. [DOI] [PubMed] [Google Scholar]
- 18.Low N. Current status of chlamydia screening in Europe. Eurosurveillance 200485 [Google Scholar]
- 19.Low N, Harbord R M, Egger M.et al Screening for chlamydia. Lancet 20053651539. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R E, Newhall W J, Papp J R.et al Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections—2002. Morb Mort Wkly Rep Recomm Rep 2002511–38. [PubMed] [Google Scholar]
- 21.Parker J, Bisits A, Proietto A M. A systematic review of single‐dose intramuscular methotrexate for the treatment of ectopic pregnancy. Aus N Z J Obstet Gynaecol 199838145–150. [DOI] [PubMed] [Google Scholar]
- 22.Chen M Y, Fairley C K, Donovan B. Discordance between trends in chlamydia notifications and hospital admission rates for chlamydia related diseases in New South Wales, Australia. Sex Transm Infect 200581318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M Y, Pan Y, Britt H.et al Trends in clinical encounters for pelvic inflammatory disease and epididymitis in a national sample of Australian general practices. Int J STD AIDS 2005. (in press) [DOI] [PubMed]
- 24.Wiesenfeld H C, Sweet R L, Ness R B.et al Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis 200532400–405. [DOI] [PubMed] [Google Scholar]
- 25.Low N. Chlamydia (uncomplicated, genital). http://www.clinicalevidence.com/ceweb/conditions/seh/1607/1607.jsp 2005
- 26.Weström L, Eschenbach D. Pelvic inflammatory disease. In: Holmes KK, Mårdh P‐A, Sparling PF, et al, eds. Sexually transmitted diseases. New York: McGraw‐Hill, 1999
- 27.Stamm W E, Guinan M E, Johnson C.et al Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med 1984310545–549. [DOI] [PubMed] [Google Scholar]
- 28.Duncan B, Hart G, Scoular A.et al Qualitative analysis of psychosocial impact of diagnosis of Chlamydia trachomatis: implications for screening. BMJ 2001322195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillis S D, Owens L M, Marchbanks P A.et al Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol 1997176103–107. [DOI] [PubMed] [Google Scholar]
- 30.Paavonen J, Puolakkainen M, Paukku M.et al Cost‐benefit analysis of first‐void urine Chlamydia trachomatis screening program. Obstet Gynecol 199892292–298. [DOI] [PubMed] [Google Scholar]
- 31.Hu D, Hook EW I I I, Goldie S J. Screening for Chlamydia trachomatis in women 15 to 29 years of age: a cost‐effectiveness analysis. Ann Intern Med 2004141501–513. [DOI] [PubMed] [Google Scholar]
- 32.Handsfield H H. Screening asymptomatic women for Chlamydia trachomatis: abstract and commentary. JAMA 19982801800–1801. [DOI] [PubMed] [Google Scholar]
- 33.Washington A E, Arno P S, Brooks M A. The economic cost of pelvic inflammatory disease. JAMA 19862551735–1738. [PubMed] [Google Scholar]
- 34.Golden M R, Schillinger J A, Markowitz L.et al Duration of untreated genital infections with Chlamydia trachomatis: a review of the literature. Sex Transm Dis 200027329–337. [DOI] [PubMed] [Google Scholar]
- 35.Morré S A, van den Brule A J, Rozendaal L.et al The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one‐year follow‐up. Int J STD AIDS 200213(Suppl 2)12–18. [DOI] [PubMed] [Google Scholar]
- 36.Molano M, Meijer C J, Weiderpass E.et al The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5‐year follow‐up study. J Infect Dis 2005191907–916. [DOI] [PubMed] [Google Scholar]
- 37.Roberts T, Robinson S, Barton P.et al The correct approach to modelling and evaluating chlamydia screening. Sex Transm Infect 200480324a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welte R, Postma M, Leidl R.et al Costs and effects of chlamydial screening: dynamic versus static modeling. Sex Transm Dis 200532474–483. [DOI] [PubMed] [Google Scholar]
- 39.Miller W C. Screening for chlamydial infection: are we doing enough? Lancet 2005365456–458. [DOI] [PubMed] [Google Scholar]
- 40.Levine W C, Dicker L W, Devine O.et al Indirect estimation of chlamydia screening coverage using public health surveillance data. Am J Epidemiol 200416091–96. [DOI] [PubMed] [Google Scholar]