Abstract
Objective
Patterns of transmission of HIV are different among different regions of the world and change over time within regions. In order to adapt prevention strategies to changing patterns of risk, we need to understand the behaviours that put people at risk of infection and how new infections are distributed among risk groups.
Methods
A model is described to calculate the expected incidence of HIV infections in the adult population by mode of exposure using the current distribution of prevalent infections and the patterns of risk within different populations. For illustration the model is applied to Thailand and Kenya.
Results
New infections in Kenya were mainly transmitted through heterosexual contact (90%), while a small but significant number were related to injecting drug use (4.8%) and men who have sex with men (4.5%). In Thailand, the epidemic has spread over time to the sexual partners of vulnerable groups and in 2005 the majority of new infections occurred among the low risk heterosexual population (43%). Men having sex with men accounted for 21% and sex work (including sex workers, clients, and partners of clients) for 18% of new infections. Medical interventions did not contribute significantly to new infections in either Kenya or Thailand.
Conclusions
The model provides a simple tool to inform the planning of effective, appropriately targeted, country specific intervention programmes. However, better surveillance systems are needed in countries to obtain more reliable biological and behavioural data in order to improve the estimates of incidence by risk group.
Keywords: HIV, incidence, mode of transmission, Kenya, Thailand
The Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) estimate that at the end of 2005 around 40 million people were living with HIV globally of whom close to 5 million were newly infected with HIV during 2005.1 The epidemic affects different parts of the world differently and even within regions the epidemic progresses at different rates and at different levels of intensity within different exposure groups. HIV infections levels have remained below 1% in North Africa and the Middle East, West and Central Europe, North America, Oceania, and most parts of Asia, where transmission of the virus occurs mainly in concentrated groups of injecting drug users (IDUs), men who have sex with men (MSM), and sex workers and their clients and partners. In contrast, almost 8% of people living in sub‐Saharan Africa, where the major route of HIV transmission is through heterosexual sex in the general population, are infected with HIV, with prevalence levels of around 20% or higher in most southern African countries.1
Low coverage of prevention programmes has limited the impact of national prevention efforts. For example, in south and southeast Asia in 2003, it was estimated that targeted HIV prevention programmes reached only 19% of sex workers, 5% of injecting drug users, and less than 2% of MSM.2 As a result, prevention efforts in many countries have failed because not enough attention has been paid to the modes of transmission and the groups at highest risk of becoming infected with HIV. Prevention efforts are often built on broad classifications of the type of epidemics in a country or region rather than on a careful analysis of the distribution of new infections in a particular country.3
In almost all regions of the world patterns of transmission have changed over time. In order to control HIV epidemics effectively and to reach those most in need, prevention strategies need to be adapted to the changing patterns of HIV risk. However, to target those most in need, it is important to first understand the behaviours that put people at risk of infection and the current distribution of new infections by risk group. In this paper we describe a model that was developed to calculate the expected number of new adult infections in the coming year using the current distribution of prevalent infections and the patterns of risk within different populations. The model serves to identify risk groups among whom information on the number of new infections can help countries and regions to plan and focus appropriate interventions. We illustrate the model by applying it to two countries: Kenya, which has a generalised epidemic, and Thailand, which has an epidemic that is concentrated in nature.
Methods
Model
A model (currently available as an Excel spreadsheet) was developed in collaboration with the UNAIDS Reference Group on Estimates, Modelling and Projections to calculate the expected short term incidence of HIV infections among the adult population by mode of transmission, using as input data the current prevalence of HIV infection, the number of individuals in particular risk groups, and the risk of exposure to infection within each group.4 The model was first applied in 20033 and has subsequently been developed as part of the UNAIDS/WHO set of methods and has been included in regional training courses conducted by UNAIDS and WHO. The model and instructions for application are available at http://www.unaids.org.
In the model it is assumed that the risk of infection in a susceptible individual is a simple binomial function of the number of partners and number of contacts with each partner. The risk per susceptible which depends upon the current prevalence of infection within their contacts is then derived, taking into account the transmission probabilities, either in the presence or absence of sexually transmitted infections (STI). By multiplying this by the number of susceptibles at risk in the population, the expected incidence for the coming year is then
where I is the incidence of HIV in the target population, which depends upon the number of uninfected individuals who are susceptible to becoming infected, U, and the HIV prevalence in the partner population, p. The variable S is the prevalence of STIs in the target or partner population, while β′ and β represent the probability of transmission of HIV during a single contact in the presence or absence of an STI (in the case of transmission by sharing needles, β′ = β). The variable υ is the proportion of acts that are currently protected (for example, through effective condom use or the use of sterile needles), a is the number of contacts per partner, and n is the number of partners.
Data requirements for application of the model
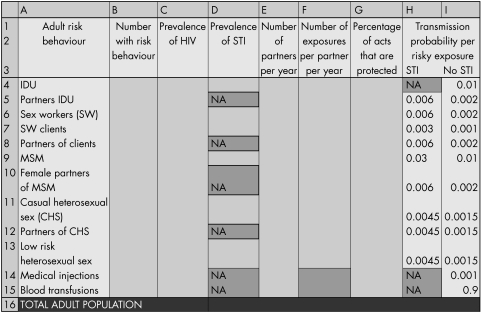
Country specific behavioural and surveillance data should be used for application of the model. The adult population is firstly divided into specific risk groups as indicated in figure 1. Risk groups are mutually exclusive, and individuals with more than one high risk behaviour are assigned to the group that represents the most risky of their behaviours. The “medical injections” group is the exception to this rule: those in the “low risk heterosexual sex” group are also at risk through medical injections (for other groups, medical injections represent a small component of their overall HIV risk). Data required for completing the spreadsheet include the size of each risk group, the prevalence of HIV in the particular risk group, the prevalence of sexually transmitted infections (the probability of transmitting HIV to partners is generally higher among those with an STI than those without an STI), the average number of partners per year, the average number of exposure events per partner per year, and the percentage of those events that are protected. For sexual transmission, this is the average number of sexual partners per year and the average number of sexual acts per partner per year, as well as the average percentage of sex acts that are protected through condom use. For injecting drug users, this is the average number of needle sharing partners per year and the average number of times a needle is shared with each injecting partner per year, as well as the average percentage of times that clean needles are used. In the case of medical injections or blood transfusions, the number of injections or transfusions received per year is required, while the number of exposure events “per partner” is fixed at one. The percentage of protected acts of exposure is estimated through the percentage of times that a sterile needle is used, or the percentage of blood units that are effectively screened.
Figure 1 Data required by risk groups to allow estimation of HIV incidence. Default transmission probability values are shown in columns H and I of the spreadsheet.
Transmission probability
Estimates of transmission probability per contact, shown in columns H and I of the spreadsheet (fig 1), were based on an exhaustive review of the literature on HIV infectivity (per partnership and per contact) by Imperial College in 2004 as part of a UNAIDS report5 for different modes of transmission, including heterosexual and homosexual intercourse, parenteral and blood transfusions. The review included a total of 112 studies providing transmission probabilities and considered factors that affect the per contact infectivity such as sexually transmitted infections, male circumcision status, treatment status, and viral load. The report shows significant heterogeneity in estimates of transmission probabilities. For example, the transmission probabilities per heterosexual contact with an infected partner range from 0.0003 to 0.2 (median 0.001) for male to female transmission and from 0.0003 to 0.082 (median 0.0007) for female to male transmission, with higher estimates for people with sexually transmitted infections and for uncircumcised men, and a slightly wider range of estimates for resource poor settings than for industrialised settings. Estimates of per contact transmission of HIV for anal intercourse vary from 0.004 to 0.183 (partner with AIDS).5 Estimates of risk of HIV‐1 transmission for parenteral exposure and blood transfusion vary between 0.006 and 0.02 (median 0.08) for intravenous drug injection, 0.0 to 0.0046 (median 0.002) for accidental percutaneous injury, and 0.89 to 0.96 (weighted mean 0.925) for contaminated blood transfusions.6 Although these estimates are provided in the spreadsheet as default transmission probabilities, users of the spreadsheet can change these according to country specific estimates of infectivity or new research, or they can adjust the values according to information on risk factors such as circumcision rates.
Application of the model to two example countries
We applied the model to two countries: one with a generalised epidemic (Kenya) and one with an epidemic which is concentrated in nature (Thailand). Results from both published and unpublished studies were used and experts with experience and knowledge of the epidemic in both countries (JS and TB) were asked to complete the cells in the spreadsheet. The data points and assumptions used in the application of the model to Kenya and Thailand are provided in the supporting information in the appendix (see http://www.stijournal.com/supplemental).
Results
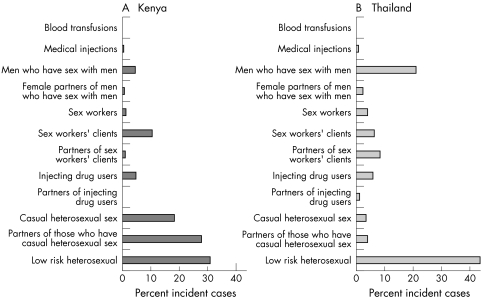
The incidence by mode of transmission is shown in figure 2 for (A) Kenya and (B) Thailand. The bars in the figures represent the percentage contribution of each risk group to the total number of new infections in each country and illustrate the different patterns of infection in the two countries.
Figure 2 Distribution of the percent incident cases by mode of exposure.
Kenya
A total of 82 369 new infections (out of a total 15–49 year adult population of about 16.4 million) were estimated to have occurred in Kenya in 2005. The majority of these infections were among the general, low risk population (30.1%), individuals involved in casual heterosexual sex with non‐regular partners (18.3%), and partners of those involved in casual sex (27.7%). Clients of sex workers accounted for 10.5% and sex workers for 1.3% of all new infections. Although previously thought insignificant, a large number of new infections occurred as a result of injecting drugs (n = 3991, 4.8%) and through men having sex with men (n = 3697, 4.5%). Small numbers of infections occurred as a result of medical injections (0.6%) and blood transfusions (0.2%). The incidence expressed per 100 population per year is given by risk group in table 1.
Table 1 Incidence per 100 population per year by risk group for Kenya and Thailand.
| Adult risk group | Incidence/100/year Kenya | Incidence/100/year Thailand |
|---|---|---|
| Injecting drug users (IDUs) | 16.3 | 2.6 |
| Partners of IDUs | 1.4 | 0.7 |
| Sex workers | 1.9 | 0.5 |
| Clients of sex workers | 3.6 | 0.05 |
| Partners of clients of SW | 0.8 | 0.2 |
| Men who have sex with men (MSM) | 4.5 | 0.7 |
| Females partners of MSM | 1.3 | 0.4 |
| Casual heterosexual sex (CHS) | 0.5 | 0.03 |
| Partners CHS | 0.9 | 0.13 |
| Low risk heterosexual sex | 0.4 | 0.03 |
| Medical injections | 0.003 | 0.0 |
| Blood transfusions | 0.2 | 0.0 |
Thailand
In Thailand, a total of 17 811 new infections (out of a total 15–49 year adult population of about 37 million) were estimated to have occurred in the adult population in 2005. Of these new infections, the majority occurred among the general low risk population (43.4%) and among MSM (20.9%). Other risk groups that contributed significantly to the number of new infections were sex workers (3.9%) and their clients (6.1%) as well as the partners of clients of sex workers (8.4%), injecting drug users (5.7%), and those who engage in casual heterosexual sex (3.4%) and their partners (3.9%). The percentage of new infections related to medical injections and blood transfusions were small, 0.8% and 0% respectively. The estimated incidence per 100 population per year in each risk group is shown in table 1.
Discussion
The model described here provides a simple tool to inform the planning of effective, appropriately targeted, country specific intervention programmes. It allows the user to identify those risk groups among whom most of the new HIV infections will occur and the relative orders of magnitude of the incident infections between the different risk groups, which in turn will help countries to focus intervention strategies and to explore current coverage of interventions. The model does not take into consideration the distribution of all behaviours within the risk groups, overlapping risk behaviours, the patterns of mixing of demographic, social, geographic, and economic variables and the influence of specific sexually transmitted infections, and therefore cannot be used to generate accurate predictions. However, it should be pointed out that most countries lack the detailed data that accurate predictions require.
The model was applied to two countries to illustrate its application. Results indicate and confirm that patterns of transmission of HIV vary widely between countries.
The estimated numbers of new infections in Kenya and Thailand based on our model outputs have been confirmed and calibrated by other models. The total number of new infections is estimated to be around 82 400 for Kenya and 17 800 for Thailand. These compare well with the estimates of 86 300 for Kenya from the Spectrum model7 and 16 500 for Thailand from the Asian Epidemic model.8 In addition, incidence estimates in some of the risk groups obtained from our model have been confirmed by empirical data. Although incidence obtained from cohort studies on sex workers in Kenya show large variation, estimates over recent years (1998–2003) vary from 1.7% to 8.5% per year with lower estimates in more recent years.9 According to the US Census Bureau, new infections in this population group peaked in the early 1990s, and our model estimate of 1.9% among sex workers in 2005 is plausible. Similarly, our model estimate of incidence among IDUs in Thailand (2.6%) falls within the range of estimates obtained from cohort studies among IDUs in Thailand (0.5% to 10.2% per year) in recent years (1999–2002).9
Results for Kenya show that the majority of new infections in 2005 occurred through heterosexual contact (90% of new infections), of which the majority were in the low risk population and among individuals engaging in casual sex and their partners. The model confirms that sex workers and their clients are extremely vulnerable groups, not only for the acquisition of HIV, but also for the transmission of sexually transmitted infections and HIV.10 Although the relative contribution of sex workers to the total number of new infections was small (1.3%) because the sex worker population is small relative to the total population, the incidence rate in this group was high at 1.9 per 100 per year. Clients of sex workers accounted for 10.5% of all new infections with an incidence rate of 3.6% per year. The model further shows that MSM accounted for 4.5% of new infections, with a high incidence rate of 4.5 per 100 per year. This calculation was based on the assumption that 1% of men in the general population have sex with other men and may be an underestimate of the true MSM situation in Kenya. Statistics on MSM are hard to obtain because homosexuality in Kenya is a criminal offence.11 It is recognised however, that networks of homosexuals are found throughout Africa, although many adopt a heterosexual lifestyle in order to fit in.12 Reports that male homosexuality is fashionable among young men and is practiced in prisons, boarding schools, and colleges,12 as well as studies suggesting homosexual activity among truck drivers, especially between older men and young boys,13 have provided evidence to suggest that sex between men in Kenya is more common than generally believed. Similarly, while sub‐Saharan Africa has generally been considered largely free of injecting drug use, a review of studies from East Africa has shown an increase in injecting drug use in Kenya.14 The study shows that heroin is freely available on the Kenya coast, that 45% of heroin users in Nairobi are injectors, that heroin injectors share injecting equipment and have sex with each other and with non‐users, and that 50% of injecting drug users interviewed in Nairobi were HIV positive.14 Our model shows that while the overall percentage of people injecting drugs in the adult population might be small (estimated to be around 0.3% of the male population), the incidence rate in this risk group is very high (16.3 per 100 per year) and IDU accounted for 4.8% of all new infections.
Many interventions in Kenya, such as the strengthening STI/HIV control project, have implemented community and clinical interventions among selected vulnerable groups to reduce transmission of STIs and HIV infections. While most of these interventions have targeted female sex workers, some projects have also been designed to target both female sex workers and their clients.13 Studies in Kenya have reported high levels of sexual interaction with casual or non‐regular partners13,15 which in addition to low condom use during these contacts lead to high transmission rates13 and should be a priority for targeting interventions. The taboo surrounding homosexuality has impeded the provision of AIDS education and support for these men and there has been no official recognition of the role homosexuals play in transmitting the virus.12 The increasing number of IDUs in Kenya together with the very high HIV infection rate among them, as well as the lack of information about the dangers of injecting, sharing needles, and unprotected sex,14 call for an urgent response and introduction of harm reduction methods in these groups.
The epidemic in Thailand has evolved through different stages over time. The main routes of HIV transmission in the late 1980s and early 1990s in Thailand were injecting drug use and sex work,16,17,18 from which HIV spread in the 1990s to the partners of clients of sex workers.19 However, the government was quick to respond and successfully implemented comprehensive national strategies including practicing safe injection techniques, public AIDS education messages, a 100% condom use campaign, and mobilising all sectors of Thai society.16,20,21,22,23 Thailand was therefore one of the first countries to reduce HIV prevalence by the mid 1990s.24 Our model estimates that the general, low risk heterosexual population accounted for 43% of all new infections in 2005 although the incidence rate in this population was low (0.03 per 100 per year). MSM also accounted for a large proportion of new infections (21%) while sex workers, clients, and partners of clients of sex workers explained a further 18% of new infections. Injecting drug users and their partners accounted for a total of 7% of new infections. The incidence rate among IDUs was estimated to be high at 2.6 per 100 per year.
The HIV epidemic in Thailand has spread beyond vulnerable groups to the general population, while it also appears to be on the rise again in MSM. In addition, the epidemic threatens to regain momentum in communities where complacency has set in (for example, among young people).23 Prevention strategies must therefore be adapted to the changing patterns of risk behaviour and situations involving MSM, IDUs, sex workers, and their clients. More attention should be given to prevention strategies aimed at reducing HIV transmission between regular partners (in particular in young people), one of whom may have been exposed to HIV through buying or selling sex, while sustaining existing prevention efforts targeting sex work.
In many countries with low level or concentrated epidemics the HIV epidemic is initially limited to vulnerable population such as IDUs, sex workers and their clients, and MSM.25 These groups are often hard to reach because of local laws and social stigma and interventions required to reach them will differ. However, as the epidemic progresses, the virus will spread to the sexual partners of vulnerable groups and the size and the composition of the populations to be targeted for effective intervention and care will change, as will the resources that are needed to control the epidemic.25 The spreadsheet model presented here can help countries to assess the change in epidemics, to prioritise target groups for interventions, and to plan more effectively the resources required to implement these interventions.
Attempts to apply this model to specific countries during regional training workshops conducted by UNAIDS and WHO in 2005 showed that country specific data on HIV prevalence or risk behaviour are often lacking, hence limiting the use of the model. Although more countries with generalised epidemics in sub‐Saharan Africa are conducting Demographic and Health Surveys (DHS) that can provide useful information on sexual behaviour in the general population, data on groups that are particularly vulnerable, such as sex workers, MSM, and IDUs, are often limited. The example from Kenya has shown that these groups cannot be ignored when planning and targeting interventions. Similarly, in countries with concentrated or low level epidemics, information may be available from studies targeting specific groups with higher behavioural risk, while there is less information on behaviour among the general population.
In addition to limited availability of data, the quality of the data collection as well as measurement procedures can affect the accuracy of the estimates. For example, women saying that they had received money, goods, or favours in exchange for sex in population based surveys cannot be assumed to have engaged in commercial sex. When assessing studies of behaviour, attention needs to be paid to the measurement of key parameters and to the quality of data collection. Care should also be taken when extrapolating information from a study conducted in a specific part of the country to the rest of the country, for example the reported number of sex work or IDU partners in the capital city of a country would be different from that in more remote parts of the country.
There is an urgent need for improved biological and behavioural surveillance systems to provide more reliable data for planning effective interventions. Given the availability of relevant data, the model presented here provides a simple tool for estimating who are most likely to be infected with HIV in the coming year and what behaviours put them at risk of infection, which will provide governments and national AIDS programmes with the information needed to plan and focus intervention and prevention efforts so as to effectively address the epidemics in their countries.
Supplementary Material
Acknowledgements
We thank Geoff Garnett, Neff Walker, Peter Ghys, and Elizabeth Pisani for contributing to the initial development of the spreadsheet model, and Wiwat Peerapatanapokin for checking the data in the Thailand spreadsheet.
Authors' contributions
All authors were involved in the project and contributed to writing the paper. PJW, JS, and TB contributed to the initial development of the spreadsheet and PJW and EG to the revision in 2005. JS and TB completed the model spreadsheets for Kenya and Thailand.
Abbreviations
IDU - injecting drug user
MSM - men who have sex with men
UNAIDS - Joint United Nations Programme on HIV/AIDS
WHO - World Health Organization
Footnotes
Competing interests: none.
References
- 1.UNAIDS/WHO AIDS Epidemic Update: December 2005. Geneva, Switzerland
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) A scaled‐up response to AIDS in Asia and the Pacific. UNAIDS, Bangkok. UNAIDS/05. 15E 2005
- 3.Pisani E, Garnett G P, Brown T.et al Back to basics in HIV prevention: focus on exposure. BMJ 20033261384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS Reference Group on Estimates, Modelling and Projections Modelling the expected short term distribution of incidence of HIV infections by exposure group. Available at http://www.unaids.org (accessed April 2006)
- 5.Baggaley R, Boily M C, White R G.et alReport of Systematic review of HIV‐1 transmission probabilities in absence of antiretroviral therapy. 2004 Imperial College, London. (Supporting document for the UNAIDS report by White R, et al. The proportion of HIV incidence due to unsafe injections, unsafe blood transfusions and mother to child transmission in rural Masaka, Uganda. London: London School of Hygiene and Tropical Medicine, 2004, )
- 6.Baggaley R F, Boily M C, White R G.et al Risk of HIV‐1 transmission for parenteral exposure and blood transfusion: a systematic review and meta‐analysis. AIDS 200620805–812. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS 2006 Global Report. (in press)
- 8. Integrated Analysis and Advocacy (A2 Thailand). Preliminary results of HIV/AIDS projection 2005–2025. HIV/AIDS Projection meeting, December 2005. Ministry of Public Health, Nonthaburi, Thailand.
- 9.US Census Bureau, Population Division, International Programs Center HIV/AIDS surveillance data base. September 2005 Release. Available at http://www.census.gov/ipc/www/hivaidsd.html (accessed April 2006)
- 10.Hawken M P, Melis R D, Ngombo D T.et al Part time female sex workers in a suburban community in Kenya: a vulnerable hidden population. Sex Transm Infect 200278271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anonymous HIV and Kenya's homosexuals. African Health 19982048. [PubMed] [Google Scholar]
- 12.Kiama W. Where are Kenya's homosexuals? AIDS Anal Afr 199999–10. [PubMed] [Google Scholar]
- 13.Ferguson A, Pere M, Morris C.et al Sexual patterning and condom use among a group of HIV vulnerable men in Thika, Kenya. Sex Transm Infect 200480435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckerleg S, Telfer M, Hundt G L. The rise of injecting drug use in east Africa: a case study from Kenya. Harm Reduction Journal. 2005;2, 12 (available from http://www.harmreductionjournal.com/content/2/1/12 ) [DOI] [PMC free article] [PubMed]
- 15.Central Bureau of Statistics (CBS) [Kenya], Ministry of Health (MOH) [Kenya], and ORC Macro Kenya Demographic and Health Survey. Calverton, Maryland: CBS, MOH, and ORC Macro, 2004
- 16.Tanne J H. AIDS spreads eastward. BMJ 19913021557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastro T D, Satten G A, Nopkesorn T.et al Probability of female‐to‐male transmission of HIV‐1 in Thailand. Lancet 1994343204–207. [DOI] [PubMed] [Google Scholar]
- 18.Dore G J, Kaldor J M, Ungchusak K.et al Epidemiology of HIV and AIDS in the Asia‐Pacific region. Med J Aust 1996165494–498. [PubMed] [Google Scholar]
- 19.Joint United Nations Programme on HIV/AIDS (UNAI DS) HIV/AIDS prevention and control: an experience of the Royal Thai Army in Thailand. 2004 UNAIDS, Geneva UNAIDS/04.36E
- 20.Kaldor J M, Sittirai W, John T J.et al The emerging epidemic of HIV infection and AIDS in Asia and the Pacific. AIDS 19948(Suppl 2)S1–S2. [PubMed] [Google Scholar]
- 21.Dwyer J M, Mahathir M, Nath L M. Challenge and response: HIV in Asia and the Pacific. Med J Aust 1996165489–493. [DOI] [PubMed] [Google Scholar]
- 22.Rojanapithayakorn W. Evoluation of the response to AIDS in Thailand. Aidscaptions 199419–12. [PubMed] [Google Scholar]
- 23.Ministry of Public Health, Thailand, and the World Health Organization, Regional Office for South‐East Asia External review of the health sector response to HIV/AIDS in Thailand. India: WHO, August, 2005
- 24.World Health Organization Western Pacific and South‐East Asia HIV/AIDS in Asia and the Pacific Region 2003. Geneva: WHO, 2004
- 25.Joint United Nations Programme on HIV/AIDS (UNAIDS) and Asian Development Bank Costing guidelines for HIV/AIDS intervention strategies. February 2004.UNAIDS, Geneva.UNAIDS/04.41E
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.