Abstract
Based upon a prior cross-sectional study, we hypothesized that an aerobic exercise intervention in sedentary older adults would improve a primary T cell-dependent immune response. Participants were a subset of older subjects from a large, ongoing exercise intervention study who were randomly assigned to either an aerobic exercise (Cardio, n=30, 68.9 ± 0.8 yrs) or flexibility/balance (Flex, n=20, 69.9 ± 1.2 yrs) intervention. The intervention consisted of either 3 aerobic sessions for 30–60 min at 55–70% VO2 max or two 60 min flexibility/balance sessions weekly for 10 months. Eight months into the intervention, samples were collected before intramuscular administration of KLH (125 µg), followed by sampling at 2, 3, and 6 wks post-KLH. Serum anti-KLH IgM, IgG1, and IgG2 was measured by ELISA. Physiological and psychosocial measures were also assessed pre-and post-intervention. While there was no difference in the anti-KLH IgG2 response between groups, Cardio displayed significantly (p ≤ 0.05) higher anti-KLH IgG1 (at wks 2, 3, and 6 post) and IgM responses when compared to Flex. Despite cardiovascular intervention-induced improvement in physical fitness (~11% vs. 1% change in VO2peak in Cardio vs. Flex, respectively), we found no relationship between improved fitness and enhanced anti-KLH antibody responses. Optimism, perceived stress, and affect were all associated with enhanced immune response. We have shown for the first time that cardiovascular training in previously sedentary elderly results in significantly higher primary IgG1 and IgM antibody responses, while having no effect on IgG2 production.
Keywords: exercise, aging, elderly, primary antibody response, vaccination, immunity
Introduction
In the year 2000 there were 35 million people over the age of 65 and this is expected to increase to 54.6 million by the year 2020 (DHHS, 2006). Unfortunately, longevity is associated with a number of new medical problems, many of which have an underlying immunological component. It is well known that aging leads to a state of immunosenescence characterized by derangements of the immune system that increase the susceptibility of the elderly to infections, cancer and autoimmune diseases (Pawelec et al., 2006).
One of the consequences of this state of immunosenescence is the relative inability to respond to vaccination with a vigorous antibody or cell-mediated immune response (Bernstein et al., 1999; McElhaney et al., 2006). This results in a lack of protection and/or an early loss of the protective effects of vaccines (Burns et al., 1993; Goodwin et al., 2006; Targonski et al., 2007). Adequate responses to vaccination are important in protecting older adults from infectious diseases such as influenza virus, pneumococcal disease, varicella zoster virus, and Clostridium tetani. Cellular changes associated with the age-related loss of vaccine efficacy include; deficits in antigen presentation by dendritic cells (Agrawal et al., 2007), thymic involution and decreasing numbers of naïve CD4+ T cells (Goronzy & Weyand, 2005), and increasing numbers of both memory CD4+ and CD8+ T cells and memory B cells (Effros, 2007; Lazuardi et al., 2005; Saurwein-Teissl et al., 2002). Interestingly, several independent studies have demonstrated that high proportions of CD8+ T lymphocytes that lack expression of the important costimulatory molecule CD28 predict poor vaccine responses (Vallejo, 2005). These associations highlight the wide ranging age-related decrements in cell mediated immunity and humoral immunity and the need to search for ways to improve vaccine responses.
The use of exercise to augment vaccine responses has been explored with positive results. Moderate aerobic exercise training (Kohut et al., 2004) in older adults and muscle damaging eccentric contractions in younger adults (Edwards et al., 2007) have both been shown to increase immune responses to influenza vaccination. In addition, several cross-sectional studies have found that highly physically fit (Keylock et al., 2007) or active (Kohut et al., 2002) elderly exhibit elevated antibody responses to recall vaccinations. Although relevant for protection and recovery from these pathogens, interpretation of antibody response data to recall vaccinations such as influenza or tetanus toxoid can be limited due to complications arising from differences in pre-vaccination antibody levels and previous exposure history, both of which strongly influence the cellular requirements and nature of the subsequent antibody response (Beyer et al., 1996). Examination of the influence of exercise on primary immune responses in the elderly is important in understanding its influence on emerging infectious diseases.
Additionally, a number of psychosocial variables, that are known to be improved with exercise training (McAuley & Katula, 1999), have also been linked with immune function in older adults. For example, the production of proinflammatory cytokines that influence a number of conditions associated with aging can be driven, in part, by negative emotions (Kiecolt-Glaser et al., 2002). Critical reviews also suggest an inverse relationship between psychological stress and antibody responses to immunizations (Cohen et al., 2001). Further, Pressman et al. (2005) found that elevated levels of loneliness and small social networks were independently associated with weaker antibody response to influenza vaccination. Moreover, relatively stable psychological constructs such as dispositional optimism have been associated with changes in immune function (Kohut et al., 2002; Segerstrom, 2005).
Keyhole limpet hemocyanin (KLH) is a protein derived from the giant keyhole limpet that elicits a primary immune response in humans (Smith et al., 2004a; Smith et al., 2004b) thereby offering insight into the body’s response to novel antigens and overcoming the confounding influence of previous exposure history. The in vivo KLH immunoglobulin response is good measure of immune function that has both experimental advantages, as well as clinical relevance. This is supported by the following: 1) the cells involved with the generation of this response remain in the hormonal milieu of the organism; 2) the kinetics of the developing response can be easily monitored; 3) measurement of the antigen specific antibody response more accurately reflects the function of acquired immunity; 4) the cells involved with this response are T cells and B cells, two primary players in acquired immune responses; 5) the antibody response generated against KLH is similar to the immunological response generated after vaccination with other vaccines; 6) a reduction in specific antibodies to bacteria, virus, or soluble toxin could render the organism more susceptible to disease caused by these pathogens; and 7) KLH is clinically relevant because it is used as a immunotherapeutic in the treatment of cancer (Jurincic-Winkler et al., 1996; Livingston, 1995).
In a cross-sectional study, Smith et al. (2004a) have previously demonstrated that antibody responses to KLH in elderly physically active people were greater than their sedentary counterparts and comparable to that of younger adults. Unfortunately, in cross-sectional designs, many other factors (e.g. better diet, fewer co-morbidities etc.) might confound this association (Keylock et al., 2007). Therefore, the purpose of this study was to examine the antibody response to KLH in a group of previously sedentary older adults randomized to either a cardiovascular exercise or flexibility/balance intervention and determine whether changes in fitness and/or psychosocial functioning could explain changes in antibody responses. We hypothesized that the cardiovascular exercise group would exhibit higher antibody responses to this novel challenge. Given the associations previously reported between psychosocial functioning and immune responses, we further hypothesized that higher antibody responses would be associated with less loneliness and perceived stress and greater optimism and positive affect.
Materials and Methods
Subjects
All procedures were approved by the University of Illinois Institutional Review Board and all subjects signed an approved informed consent form before being enrolled in the study. We recruited 50 subjects from the 2nd and 3rd waves (2004 and 2005, n = 109) of a larger ongoing randomized clinical exercise intervention trial (The Immune Function Intervention Trial [ImFIT]; 3 annual waves from 2003–2005; clinical trials identifier NCT00548990) examining the effects of aerobic exercise training on responses to influenza and tetanus vaccines (which were administered ~5 months prior to the KLH inoculation). The CONSORT diagram for this sub-study can be found in Figure 1. Screening took place in two stages, including a telephone interview (e.g. pre-screen) and blood and exercise testing. Sedentary older adults aged 62–82 were recruited from the Champaign/Urbana area by means of local media, senior citizen center and other public places. For inclusion in the study, subjects had to be sedentary for 6 months prior to beginning the study. Subjects were excluded if they had a recent history of cancer or inflammatory disease, chronic obstructive pulmonary disorder, uncontrolled diabetes mellitus, congestive heart failure, recent illness or vaccination, smoked, or were taking any medications that interfered with immune responses (e.g. corticosteroids). If potential subjects passed telephone pre-screening, they were invited to the lab for a fasting blood test and graded exercise test. Potential participants were excluded if they exhibited abnormal results for complete blood cell counts (CBC’s) or comprehensive metabolic panel (CMP) testing (e.g. glucose, hemoglobin, albumin, electrolytes, liver enzymes, glomerular filtration rate calculated from serum creatinine).
Figure 1.
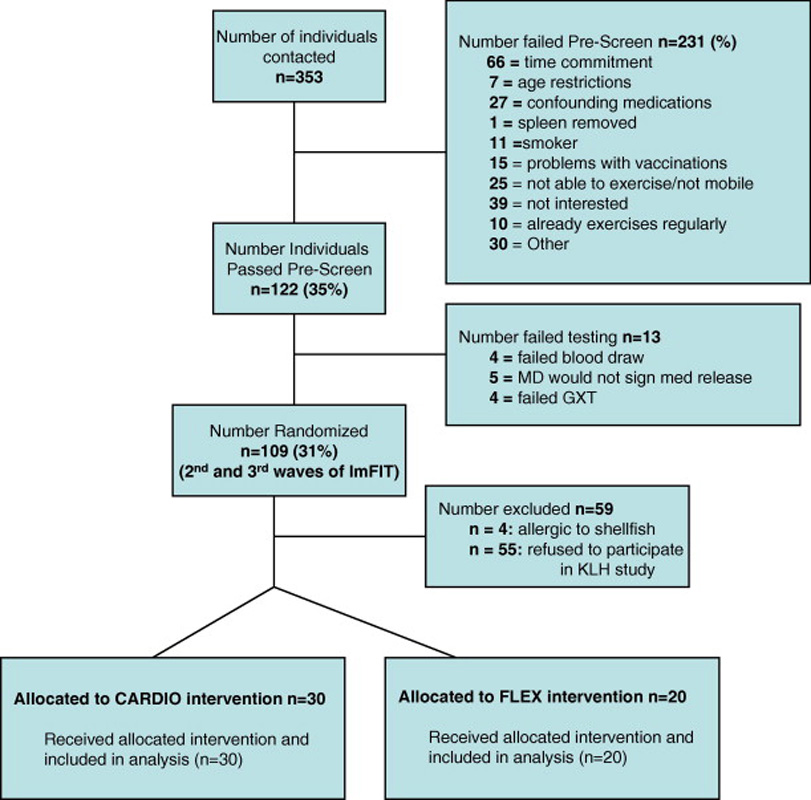
CONSORT diagram for trial on effects of exercise intervention on primary antibody response to KLH.
After randomization, we found no statistically significant differences (p > 0.05) between groups at the pre-intervention time point in CBC or CMP tests with the exception of results for the absolute number of monocytes (0.58 ± 0.03 vs. 0.49 ± 0.03 cells/µl for Flex and Cardio, respectively; p = 0.05), serum sodium (138 ± 0.35 vs. 139.6 ± 0.31 mmol/l for Flex and Cardio, respectively; p = 0.006), and potassium (4.33 ± 0.07 vs. 4.16 ± 0.05 mmol/l for Flex and Cardio, respectively; p = 0.04). Despite pre-intervention treatment groups differences, these variables were all within the normal reference range and there was no relationship between these variables and antibody response data (data not shown).
Qualifying subjects were adaptively randomized (Begg and Iglewicz, 1980) based upon age, gender, and cardiovascular disease and diabetic status. One hundred and nine of the 353 contacted people (31%) were randomized (Figure 1). Of these, 54 people were excluded because they were either allergic to shellfish (n=4) or refused participation (n = 55) resulting in a final subject number of 25 in the Flex and 30 in the Cardio groups. The average number of prescribed medications taken daily by each participant was 2.1 ± 0.4 and 2.0 ± 0.3 for the Flex and Cardio groups (P > 0.05), respectively and there were no group differences (data not shown) in the classes of medications taken between the groups (e.g. aspirin, cardiovascular and hypertensive medications, cholesterol medications, hormonal replacement). For this sub-study, exercise sessions began 8 months prior to KLH injection and continued during the 6 weeks after KLH administration.
Exercise Testing
All subjects from the ImFIT trial performed pre-and post-intervention graded exercise tests for screening and data collection purposes. Subjects were asked to refrain from vigorous activity 24 hours before the exercise test. The initial treadmill speed was subject specific (2–4 mph), a comfortable walk. At each stage of the test, the grade was increased and this was sometimes accompanied by an increase in speed. During the exercise test oxygen consumption (VO2) was measured from expired air until the subject reached volitional fatigue. A ParvoMedics® metabolic system and software were used to measure expired oxygen, carbon dioxide, ventilation, and respiratory exchange ratio. During each stage blood pressure was monitored and heart rate was measured via direct 12-lead electrocardiographic monitoring. The highest VO2 value attained during the test was determined to be the VO2peak and was used as an indication of cardiovascular fitness. Each subject also performed a 1-mile time walked according to the Rockport Test protocol (Kline et al., 1987) as an additional indicator of cardiovascular fitness.
Exercise Intervention
We hypothesized that cardiovascular exercise training and the resultant gain in physical fitness would result in an improvement in the primary antibody response. As such, we randomized our subjects into either a cardiovascular exercise group (Cardio) or a flexibility/balance attention control group (Flex). As subjects were sedentary at study onset, the prescribed exercise intensity of the Cardio group began at a light level (45–55% VO2peak), progressing to a moderately vigorous level (60–70% VO2peak), within 3 months from the start of the 10-month intervention. Frequent assessment of heart rate (both by palpation and telemetry) and rating of perceived exertion ensured that the exercise intensities were performed at the prescribed level. Supervised sessions were conducted 3 times per week (M, W, F) and the duration of each session gradually increased (based on the subject’s individual fitness level) from 10–15 min to ~45–60 min per session by the 4th month. The mode of exercise (walking, cycling, swimming, stair climbing, rowing) varied depending on subject preference, but employed brisk walking at least 2 times per wk. Our rationale for a moderate intensity exercise prescription of reasonable duration was guided by the literature that suggests that intense (>80% VO2max), prolonged (>1 hr), or over training levels of exercise may suppress many immune function measures (Nieman, 1997).
The Flex group participated in 2 supervised sessions per wk with each session lasting ~75 min. They engaged in an array of large muscle group stretching exercises employing various low level resistance devices such as Thera-bands®; they also worked on balance with Dynadiscs®, foam rollers, balance balls, and other pertinent equipment. The low level of resistance employed and the intermittent nature of the activities were selected to minimize any cardiovascular or muscular adaptations (e.g. hypertrophy). Indeed, assessment of intensity based upon heart rate and VO2 measures indicated low (e.g. <20% VO2peak) exercise intensity in this group. Attendance at the intervention sessions was 87.9 ± 1.2% and 84.1 ± 2.1% for the Flex and Cardio groups, respectively (t48 = 1.3; p = 0.20).
Dietary Assessment
Participants in the study were excluded in the pre-screening if their goal in participating in the study was to lose significant body weight by changing their dietary intake in conjunction with study the exercise intervention. As such, we performed 3 separate dietary intake assessments prior to, at the mid-point (5 mo), and end (10 mo) of the intervention. Subjects were instructed on completion of dietary intake records, and asked to complete a 1-day food record before coming to the lab. A registered dietitian reviewed the recall with each participant, clarifying food terms, ingredients, brands, and portion sizes. Information about supplement use was obtained at the time of diet record review as well. If the record represented a non-typical day, a usual day’s intake was also recorded during the interview.
Psychosocial Variables
To assess perceived stress, we used the Perceived Stress Scale (Cohen et al., 1983). We used the Memorial University of Newfoundland Scale of Happiness (MUNSH; Kozma & Stones, 1980) to measure positive affect. This measure was developed specifically for older adults and has been validated in a variety of settings (Kozma & Stones, 1983; Webster, 1998). The UCLA Loneliness Scale (Russell et al., 1980) assessed loneliness. Cutrona et al. (1986) have shown the scale to be valid and reliable in the elderly. Internal consistencies for the UCLA Scale were excellent (α’s > .92 at both timepoints). Finally, we used the Life Orientation Test-Revised (LOT-R; Scheier et al., 1994) to measure optimism and pessimism. All measures had adequate to excellent internal consistencies at all measurement points (α’s = .70–.91). Psychosocial variables were assessed at baseline and at five months into the exercise program.
KLH Administration
Each participant was intramuscularly (deltoid muscle) inoculated with 125 µg of KLH (BCI-ImmuneActivator™, Intracel Resources LLC, Rockville, MD) in 0.1 mL sterile saline 8 months after the start of the intervention. The KLH in BCI-ImmuneActivator™ is isolated using a process that preserves the high molecular weight structure while minimizing low molecular weight degradation products and purity is >95% as determined via high pressure liquid chromatography.
Blood Sampling
Blood was collected in serum separator tubes before KLH injection (pre), and at 2, 3, and 6 weeks post-injection after an overnight fast. After blood was drawn, it was centrifuged at 1,200 x g for 15 minutes at 4°C and serum was stored at −80°C until analyzed.
Anti-KLH Antibody Measurement
Serum concentrations of anti-KLH antibodies were determined using a method similar to that used by Smith et al. (2004a). Ninety-six well microtiter plates were coated with 1 µg of Imject mcKLH (Pierce Biotechnology, Rockford, IL) per 100 µl of carbonate coating buffer per well and refrigerated overnight. Plates were washed four times with PBS-tween 20 and then blocked with 200 µl of 1% dry milk in PBS incubated at room temperature for 30 min. They were then washed four times and subject sera was added at a dilution of 1:64 in 1% dry milk in PBS based upon prior titration experiments. The plates were incubated at 37°C for 45 min and then washed 6 times followed by addition of 100 µl of biotin-conjugated anti-human IgM (Sigma, St. Louis, MO), or IgG1, or IgG2 (BD Biosciences, San Jose CA) at dilutions of 1:51,200, 1:200, and 1:200 respectively in 1% dry milk, based upon prior titration experiments. Plates were then incubated for 45 min at 37°C and washed an additional six times and incubated with 100 µl of a 1:200 dilution of avidin-peroxidase (Sigma, St. Louis, MO) for 30 min. Plates were then washed six more times and 50 µl of substrate A (hydrogen peroxide) and 50 µl substrate B (tetramethylbenzidine; BD Biosciences, San Jose CA) was added to each well. The plates were incubated in the dark for 20 min and 50 µl of 1M Phosphoric acid was added to halt the reaction. Well absorbance was read at 405 nm on a microplate spectrophotometer (Labsystems Multiskan Plus). Due to relatively high (~17%) inter-plate coefficients of variation (COV), anti-KLH antibody values are presented as corrected optical density (OD) by dividing raw absorbance’s by a standard positive control sample that was run on each plate in each assay (De Savigny & Voller, 1980). Intra-plate COV’s were <5% and the technician was blinded to treatment.
Statistical Analysis
All statistical tests were performed using SPSS version 14.0. Chi-square analyses and independent group’s t-tests were performed to examine group differences in pre-intervention categorical and continuous variables, respectively. Antibody data were log10-transformed due to skewed distribution. Antibody response data were analyzed using a treatment (Cardio or Flex)× time (pre-, 2wk post-, 3wk post-, 6 wk post-vaccination) analysis of variance (ANOVA) with repeated measures. Statistical significance was determined at p ≤ 0.05. When significant differences were found, Bonferroni’s post-hoc multiple comparisons were used to determine group differences. Greenhouse-Geisser adjustment in degrees of freedom was made if Mauchly’s test of sphericity was significant. Pearson’s bivariate correlations examined relationships among potential correlates of improved antibody responses. Multiple regression analyses were conducted to create standardized residual change scores for the psychosocial variables and correlational analyses examined the associations between exercise-induced psychosocial change and change in primary antibody responses. As directional hypotheses were proposed for the psychosocial and immune function relationships, one-tailed tests were employed. All data are expressed as mean ± SE.
Results
Subject characteristics and physiological responses to intervention
Mean age of the study participants was 69.9 ± 1.2 and 68.9 ± 0.81 years for the Flex and Cardio groups, respectively (t48 = 0.75; p = 0.45). There were 8 males and 12 females in the Flex group and 11 males and 19 females in the Cardio group (χ2 = 0.06; p = 0.81). There were no gender differences in antibody responses to KLH administration or psychosocial responses (or gender × treatment interactions, data not shown) therefore gender was collapsed for all analyses.
Table 1 contains data of physiological variables measured prior to and after the intervention. There were no significant pre-intervention differences in any of the measures except for body weight and BMI; where the Flex group was ~8 Kg heavier (p = 0.05) on average than the Cardio group. The effectiveness of the Cardio intervention is evidenced by the significant increase (~12%) in VO2peak in the Cardio when compared to the Flex group that exhibited no appreciable change in VO2peak. Moreover, the Cardio intervention resulted in improved exercise performance both in maximal METS on the graded exercise test (13%) and improved (8%) Rockport test scores. Despite improvements in fitness and performance, there was little change in other physiological variables such as percentage body fat, resting or maximal heart rate, and resting systolic pressure (Table1) in response to the intervention.
Effects of intervention on primary antibody responses to KLH
We examined the IgM, IgG1 and IgG2 antibody responses to KLH to better understand the influence of exercise intervention on both the magnitude and isotype responses to primary immunization in older adults. It is important to note that the OD associated with the pre values in each figure (2–4) are not pre-existing responses to KLH, but background associated with the ELISA assay as wells without subject sera gave similar OD readings. As can be seen in Figure 2, KLH administration resulted in a significant rise in anti-KLH IgM antibodies in both intervention groups (time main effect F2,74 = 55; p = 0.000). Interestingly, while there was no treatment by time interaction (F2,74 = 1.7; p = 0.19), there was a significant group main effect (F1,48 = 4.1; p = 0.048) such that the Cardio group exhibited, in general, higher IgM concentrations at all time points post-vaccination. Analysis of responders (> 2-fold increase in O.D. post-vaccination at any time point) vs. non-responders revealed that 11 of 20 Flex vs. 23 of 30 Cardio participants responded to the vaccine with an IgM response (χ2 = 2.6; p = 0.11). The most salient finding of the present study was the significant (treatment × time F2,98 = 5.2; p = 0.007) effect of Cardio intervention on the anti-KLH IgG1 response (Figure 3). Indeed, 22 of 30 (73%) Cardio participants vs. only 7 of 20 (35%) Flex participants exhibited a significant rise in anti-KLH IgG1 antibodies (γ2 = 7.3; p = 0.007). Cardio exhibited significantly higher anti-KLH IgG1 levels at 2, 3, and 6 wk post-administration. There was also a tendency for Cardio participants to exhibit higher anti-KLH IgG2 levels (Figure 4) after KLH administration, however, there was no statistically significant interaction effect (F1,65 = 3.0; p = 0.08). Eight of 20 Flex (40%) vs. 18 of 30 (60%) Cardio participants responded with a 2-fold increase in O.D. (χ2 = 1.9; p = 0.17).
Figure 2.
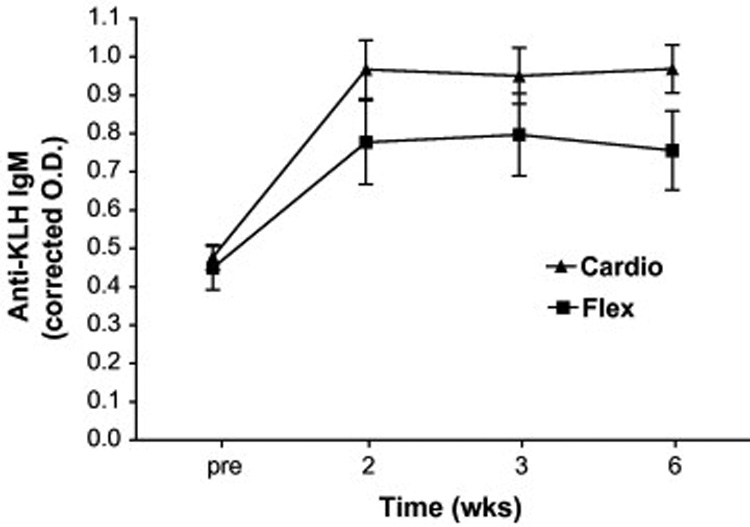
Effects of cardiovascular exercise (Cardio) or flexibility/balance (Flex) intervention on the primary anti-KLH IgM response. Analysis of variance (ANOVA) with repeated measures revealed no significant treatment × time interaction (F2,74=1.73; p=0.19), but there were significant time (F2,74=54.6; p=0.000) and treatment (F1,48=4.1; p=0.04) main effects indicating a significant immune response to KLH in both groups and a higher response (across all times) in the Cardio when compared to the Flex group.
Figure 4.
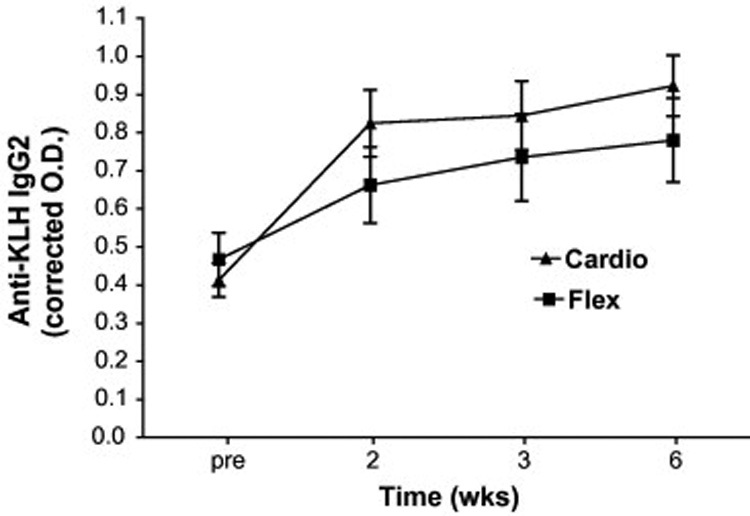
Effects of cardiovascular exercise (Cardio) or flexibility/balance (Flex) intervention on the primary anti-KLH IgG2 response. Repeated measures ANOVA revealed a non-significant (F1,64=3.0; p=0.08) treatment by time interaction and no treatment main effect (F1,48=0.83; p=0.37).
Figure 3.
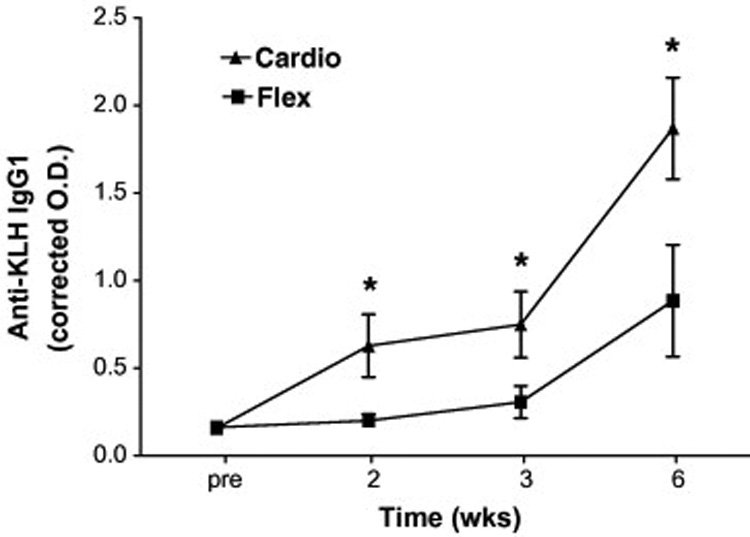
Effects of cardiovascular exercise (Cardio) or flexibility/balance (Flex) intervention on the primary anti-KLH IgG1 response. Repeated measures ANOVA revealed a statistically significant treatment × time interaction (F2,98=5.2; p=0.007), indicating that the groups differed in their response over time. *Post hoc analysis using Bonferroni’s multiple comparisons tests found significant differences between groups at each time point (2, 3, and 6 wks) post-vaccination.
Relationships between intervention-induced changes in physiological and psychosocial variables and primary immune responses
In an attempt to gain insight as to potential correlates of cardiovascular exercise training-induced improvement in anti-KLH IgM and IgG1 antibody responses, we performed a series of analyses examining changes in, and relationships between physical fitness, body composition, dietary, and psychosocial outcomes and anti-KLH antibody responses.
Although treatment groups differed on body mass and tended to differ on BMI (although percentage body fats were similar), we found no relationships between body mass/BMI and anti-KLH IgM or IgG1 antibody responses as measured by change in O.D. or responder status (e.g. 2 fold increase in O.D.)(r’s = −0.15 – 0.08, p’s ≥ 0.05). Moreover, when body mass was included as a covariate in the repeated measures ANOVA, data interpretation was not altered and intervention-induced changes in body composition (even though non-significant) were not related to improvements in antibody responses (data not shown). Fitness, as measured by the gold-standard change in (Δ)VO2peak or by performance on the Rockport test, significantly improved in the Cardio group, so we examined if this improvement was in any way related to improvements in anti-KLH IgM or IgG1 responses. We found no relationships (r’s ranging from −0.09 – 0.18, p > 0.05) between Δ VO2peak or ΔRockport performance and either anti-KLH IgM or IgG1 responses. Subjects were instructed not to change their dietary intake throughout the intervention and verification of this took place in the form of dietary recall interviews pre-intervention and at 5 months into the intervention and post-intervention. We found no time × treatment interactions for dietary intake variables reported to be linked to vaccination responses (Wick & Grubeck-Loebenstein, 1997; Van Loveren et al., 2001) including total caloric or individual macronutrient, vitamin E, iron, zinc, vitamin B12, folate, or selenium intake and there were no significant relationships between these dietary variables and anti-KLH responses (data not shown). Moreover, in no instance were the group means below the current dietary reference intakes indicating a deficiency (data not shown).
Although there were no overall statistically significant mean level changes in psychosocial outcomes, bivariate correlations between the residualized changes in psychosocial variables and primary immune responses reflect the following associations. Increased anti-KLH IgM response was correlated with reductions in loneliness (r =−.27, p =.03) and perceived stress (r =−.22, p =.06) and increases in optimism (r = .26, p =.03) brought about by exercise training. Increases anti-KLH IgG1 were significantly associated with reductions in perceived stress (r =−.24, p=.05) and enhanced optimism (r = .38, p =.003).
Adverse Events
During the 10 months, 2 serious adverse (1 in Cardio and 1 in Flex; broken clavicle as a result of a non-study related fall and surgery for a single arterial stent, respectively) and 10 adverse (6 in Cardio and 4 in Flex) events were documented in this cohort of the larger study. There were no statistically significant differences in events between the groups and all events were deemed either unrelated or probably unrelated to the intervention by the Medical Director.
Discussion
This is the first randomized longitudinal exercise training study to demonstrate a beneficial effect of regular cardiovascular exercise training in previously sedentary older adults on a primary antibody response. Our findings clearly reveal a cardiovascular exercise training-induced improvement in humoral immunity as measured by an increase in antibodies against the novel T-cell dependent immunogen KLH when compared to a flexibility/balance control group. It is important to note that the inclusion of the flexibility/balance intervention group allowed us to control for socialization into a research study. The exercise effect was primarily seen in anti-KLH IgG1 and IgM and not in IgG2. The results of our present study are unique because, unlike previous human studies, we used a novel immunogen that is not confounded by subject’s prior exposure history which has a large bearing on the subsequent antibody response (Beyer et al., 1996). The public health implications of our findings are substantial; in as much as heightened antibody responses to novel vaccinations may protect this at-risk population, who demonstrate reduced vaccine efficacy (Effros, 2007), from emerging diseases such as anti-biotic resistant staphylococcus aureus, HIV or avian influenza virus.
Our results confirm data from the previous cross-sectional study (Smith et al. 2004a) demonstrating that highly physically active and fit older adults responded to novel KLH administration with elevated IgG1 and IgM, but not IgG2, antibody responses when compared to healthy, aged matched, sedentary controls. They also parallel the previous cross-sectional study demonstrating higher IgG1, but not IgG2, antibody responses to recall tetanus toxoid vaccination in highly physically fit compared to low physically fit elderly (Keylock et al., 2007). Other human studies examining exercise-induced alterations in antibody responses to vaccination have also been performed. A 10 month cardiovascular exercise program resulted in a greater mean fold increase in anti-influenza antibody titers in a small group (n=14) of previously sedentary older adults when compared to a sedentary control group (Kohut et al., 2004). Interestingly, the responses to the A influenza virus strains that increased were the ones that were included in the prior year’s vaccine (which all subjects received). The response to the B virus strain was not affected by exercise and was not included in the prior vaccine indicating that, in that study, exercise affected recent recall responses more so than long-term recall responses. In young adults, while heavy or light exercise training loads had no affect on anti-influenza antibody titers 14 post-vaccination, anti-influenza IgG was elevated when measured 12 months following vaccination in the heavy when compared to the light training group (Whitman & Blannin, 2003). Unfortunately, only 3 of the original 11 subjects in the light training group were measured at the 12 month follow-up.
Aside from examining relationships between changes in fitness and psychosocial variables and antibody responses to KLH, we did not address the underlying mechanism(s) for the improved antibody response to cardiovascular exercise intervention in this study. Changes in serum antibody levels could result from an increased production of antibody in response to KLH, a reduction in antibody removal from circulation (e.g. increased half-life), or both. Indeed, based upon previous evidence cited below, one could make an argument for either.
Suzuki & Tagami (2005) demonstrated that voluntary wheel running in mice enhanced antigen-specific antibody-producing splenic B cell responses and prolonged the half-life of IgG in blood; an effect related to liver β2 microglobulin expression (Suzuki et al., 2007), a protein implicated in protecting against IgG catabolism. The significant increase in IgG1 and not IgG2 may have been due to the fact that protein-derived antigens such as KLH result in primarily IgG1 and IgG3 antibody responses (Yount et al., 1968). In addition, the selective improvement in IgG1 may reflect exercise-induced improvements in Th1 cell function and an increase in IgG1 antibody production; as Th1 cytokines such as IL-2 and IFN-γ promote IgG1 production, whereas as Th2 cytokines like IL-4 and IL-10 promote IgG2 production (Snapper & Finkelman, 1999). Aging results in a reduction in Th1 cytokine and reduced IgG1 antibody production (Bernstein et al., 1999). Interestingly, moderate exercise training has been shown to increase IL-2 and IFN-γ production following intranasal herpes simplex virus infection in aged mice (Kohut et al., 2001), granzyme B activity (a marker for augmented cell-mediated immunity and Th1 cytokine production) in aged human cytotoxic T lymphocytes (Kohut et al., 2004), and an increase in ex vivo IFN-γ production following influenza vaccination in the elderly (Kohut et al., 2005). These data, along with ours, suggests that cardiovascular exercise intervention has the potential to augment age-related suppression of Th1 responses. Future studies should examine the influence of exercise training on the cytokine and cell-mediated immune response to primary vaccination in humans, given their importance in regulation of the response (Snapper & Finkelman, 1999) and recovery from infection (McElhaney et al., 2006).
Interestingly, in young adults, muscle damaging eccentric exercise when administered 6 hr before influenza vaccination has been shown to increase IFN-γ production in response to in vitro stimulation with whole vaccine in males and anti-influenza antibody responses in females (Edwards et al., 2007). The authors believed that the damaging exercise created a state of inflammation in the vaccinated muscle and thus acted as an adjuvant increasing antibody production. At the time of the vaccination (4 months into the intervention), it is unlikely that muscle damage (especially of the injected deltoid muscle) was present in our subjects, however an adjuvant effect of exercise, perhaps due to increased blood flow and trafficking/presentation of antigen cannot be ruled out.
Despite the improvements in IgG1 and IgM responses in our study, we were unable to document relationships between intervention-induced improvements in VO2peak or Rockport test performance and changes in antibody response. The reason for this could be the relatively small sample size for such a correlational analysis or the small relative changes in VO2 peak (mean change ~12%) and Rockport test performance (mean change ~ 8%) in our subjects. Alternatively, factors other than fitness may have contributed to the improvement in antibody response. For example, Kohut et al. (2005) reported that depression and sense of coherence partially accounted for an increase in IFN-γ production, but not the increase in antibody production, following vaccination. In the present study, we have shown that reductions in negative states (albeit not statistically improved by exercise, although the trends were in the expected directions) such as perceived stress and loneliness and improvements in optimism were associated with increased anti-KLH IgM and IgG1 responses but not IgG2. Although exploratory in nature, such findings parallel a larger literature that suggest there is a beneficial effect to be conferred on immune response as a function of exercise-induced changes in psychosocial function. Whether psychosocial and fitness change act as independent mediating mechanisms of exercise-induced improvements in antibody responses in the elderly remains to be determined.
Our study has some limitations. First, due to the design, we have no way of determining whether the exercise prior to KLH or after KLH administration was responsible for the improvement in antibody responses. However, it does appear that exercise prior to and post-primary vaccination led to enhanced primary IgG1 and IgM responses. Second, while humoral immunity constitutes one aspect of immune responses, cell-mediated immune responses to vaccination (as measured by delayed-type hypersensitivity [DTH] responses or ex vivo proliferation or cytokine production) also occur and are important in disease protection (McElhaney, 2006). Like in the previous study (Smith et al., 2004a), the DTH response was tested by injecting 5 µg of KLH intradermally 3 wk after intramuscular inoculation with KLH. Unfortunately, unlike the previous study, in no instance could we detect a skin response to the test antigen in this cohort of older adults (data not shown). We believe that the reason for the discrepancy between studies is a technical one, having to do with the source of the KLH antigen and the use of adjuvant. In the previous study, KLH was obtained from Pierce Biotechnology (Rockford, IL) and was conjugated to alum adjuvant, whereas in the present study a highly purified preparation of KLH was obtained from Intracel Corporation and we did not use adjuvant in its administration based upon preliminary studies documenting no significant differences in anti-KLH IgG1 or IgM responses with or without the use of alum. As such, in this study, we were unable to detect a difference in the cell-mediated immune response to KLH. Lastly, despite the fact that our subjects were well-characterized on many counts (e.g. co-morbidities, medications, dietary intake, physical fitness, body composition), we did not assess their cytomegalovirus (CMV) status. Persistent CMV infection is thought to be responsible for oligoclonal expansions of T cells, especially CD8+ cells, in the elderly and related to the reduction in vaccine efficacy (Vasto et al., 2007). CMV status would be an important consideration in future studies of vaccine efficacy in older adults.
In conclusion, this initial longitudinal study demonstrates that moderate cardiovascular exercise augments a primary antibody response in previously sedentary older individuals who exhibit reduced responses to such primary immunizations. This finding is of public health importance because older adults respond less well to immunization when compared to younger people. Moreover, the ever-present risk of emerging diseases (e.g. H5N1), against which the elderly are particularly susceptible due to lack of existing immunity and the inability to mount adequate protective responses, make research focusing on improving vaccine responses in this at-risk group relevant.
Supplementary Material
Acknowledgements
Supported by NIH RO1 AG-18861 to J.A. Woods. The authors would like to thank Ms. Susan Herrel for her instrumental role played in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2007 September; doi: 10.1007/s10875-007-9127-6. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36:81–90. [PubMed] [Google Scholar]
- Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17:82–94. doi: 10.1016/s0264-410x(98)00117-0. [DOI] [PubMed] [Google Scholar]
- Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, van der Water GL, Masurel N, Osterhaus AD. Effects of repeated annual influenza vaccination on vaccine sero-responses in young and elderly adults. Vaccine. 1996;14:1331–1339. doi: 10.1016/s0264-410x(96)00058-8. [DOI] [PubMed] [Google Scholar]
- Burns EA, Lum LG, L'Hommedieu G, Goodwin JS. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. J Gerontol Biol Sci. 1993;48:B231–B236. doi: 10.1093/geronj/48.6.b231. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Miller GE, Rabin BS. Psychological Stress and Antibody Response to Immunization: A Critical Review of the Human Literature. Psychosomatic Medicine. 2001;63:7–18. doi: 10.1097/00006842-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, Russell D, Rose J. Social support and adaptation to stress by the elderly. Psychology and Aging. 1986;1:47–54. doi: 10.1037//0882-7974.1.1.47. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Service. Administration on Aging. Statistics on the Aging Population, A Profile of Older Americans. 2006 http://www.aoa.gov/prof/Statistics/profile/profiles.asp.
- De Savigny D, Voller A. The communication of quantitative ELISA results. In: Malvano R, editor. Immunoenzymatic Techniques. Boston, MA: Nijhoff; 1980. pp. 116–132. [Google Scholar]
- Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, Drayson M, Ring C. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. 2007;21(2):209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- Effros R. Role of T lymphocyte replicative senescense in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17(5):468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Jurincic-Winkler CD, von der Kammer H, Beuth J, Scheit KH, Klippel KF. Antibody response to keyhole limpet hemocyanin (KLH) treatment in patients with superficial bladder carcinoma. Anticancer Research. 1996;16(4A):2105–2110. [PubMed] [Google Scholar]
- Keylock KT, Lowder T, Leifheit K, Cook M, Mariani R, Ross K, Kim K, McAuley E, Woods JA. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J Appl Physiol. 2007;102:1090–1098. doi: 10.1152/japplphysiol.00790.2006. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: Back to the future. Psychosomatic Medicine. 2002;64(1):15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF, Ross J, Rippe JM. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med Sci Spt Exerc. 1987;19(3):253–259. [PubMed] [Google Scholar]
- Kohut ML, Boehm GW, Moynihan JA. Moderate exercise is associated with enhanced antigen-specific cytokine, but not IgM antibody production in aged mice. Mech Age Dev. 2001;122:1135–1150. doi: 10.1016/s0047-6374(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57(9):M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, McElhaney J. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22(17–18):2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Lee W, Martin A, Arnston B, Russell DW, Ekkekakis P, Yoon KJ, Bishop A, Cunnick JE. The exercise-induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain Behav Immun. 2005;19(4):357–366. doi: 10.1016/j.bbi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kozma A, Stones MJ. The measurement of happiness: Development of the Memorial University of Newfoundland Scale of Happiness (MUNSH) J Gerontol. 1980;35:906–912. doi: 10.1093/geronj/35.6.906. [DOI] [PubMed] [Google Scholar]
- Kozma A, Stones MJ. Predictors of happiness. J Gerontol. 1983;38(5):626–628. doi: 10.1093/geronj/38.5.626. [DOI] [PubMed] [Google Scholar]
- Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114(1):37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol Rev. 1995;145:147–166. doi: 10.1111/j.1600-065x.1995.tb00080.x. [DOI] [PubMed] [Google Scholar]
- McAuley E, Katula J. Physical activity interventions in the elderly: Influence on physical health and psychological function. In: Schulz R, Maddox G, Lawton MP, editors. Annual Review of Gerontology and Geriatrics. Vol. 18. New York, NY: Springer publishing; 1998. pp. 115–154. [Google Scholar]
- McElhaney JE, Xie D, David Hager W, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- Nieman DC. Immune response to heavy exertion. J. Appl. Physiol. 1997;82:1385–1394. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Immunity and aging in man. Exp Gerontol. 2006;41(12):1239–1242. doi: 10.1016/j.exger.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, Social Network Size, and Immune Response to Influenza Vaccination in College Freshmen. Health Psychology. 2005;24(3):297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality & Social Psychology. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism and immunity: Do positive thoughts always lead to positive effects? Brain, Behavior, and Immunity. 2005;19:195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004a;97(2):491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- Smith A, Vollmer-Conna U, Bennett B, Wakefield D, Hickie I, Lloyd A. The relationship between distress and the development of a primary immune response to a novel antigen. Brain Behav Immun. 2004b;18(1):65–75. doi: 10.1016/s0889-1591(03)00107-7. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Finkelman FD. Immunoglobulin class-switching. In: Paul WE, editor. Fundamental Immunology. 4th Ed. Philadelphia, PA: Lippincott-Raven Pub.; 1999. pp. 831–861. [Google Scholar]
- Suzuki K, Tagami K. Voluntary wheel-running exercise enhances antigen-specific antibody-producing splenic B cell response and prolongs IgG half-life in the blood. Eur J Appl Physiol. 2005;94(5–6):514–519. doi: 10.1007/s00421-005-1378-4. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Suk PJ, Hong C, Imaizumi S, Tagami K. Exercise-induced liver beta2-microglobulin expression is related to lower IgG clearance in the blood. Brain Behav Immun. 2007;21(7):946–952. doi: 10.1016/j.bbi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;16:3066–3069. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Van Loveren H, Van Amsterdam JGC, Vandebriel RJ, Kimman TG, Rumke HC, Steerenberg PS, Vos JG. Vaccine-induced antibody responses as parameters of the influence of endogenous and environmental factors. Env Health Perspect. 2001;190(8):757–764. doi: 10.1289/ehp.01109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawele G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing. 2007;4:2. doi: 10.1186/1742-4933-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JD. Attachment styles, reminiscence function, and happiness in young and elderly adults. Journal of Aging Studies. 1998;12:315–330. [Google Scholar]
- Wick G, Grubeck-Loebenstein B. Primary and secondary alterations of immune reactivity in the elderly: impact of dietary factors and disease. Immunol. Rev. 1997;160:171–184. doi: 10.1111/j.1600-065x.1997.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Whitham M, Blannin AK. The effect of exercise training on the kinetics of the antibody response to influenza vaccination. J Sports Sci. 2003;21(12):991–1000. doi: 10.1080/0264041031000140464. [DOI] [PubMed] [Google Scholar]
- Yount WJ, Dorner MM, Kunkel HG, Kabat EA. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968;127:633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


