Abstract
You were a patient at Hospital A several years ago when you were suffering from disease X, which has long since resolved. You have just arrived home from a long day's work when the phone rings. When you answer, a soothing voice says, “I am a scientist at Research Institution B two time zones away. I was examining your DNA and found a variant associated with Disease Y that may be really important for your health. Do you want to know about it?” If the scientist were particularly thoughtful, she might ask, “Can you come here for genetic counseling?” You wonder, What is DNA? How did she get mine? What is a variant? What is Disease Y? What is genetic counseling? Who is going to pay for me to go to Research Institution B? Most important, you think, What choice do I have?
There are countless variations on this theme. The call can come from one of your own physicians who was called by the investigator. Your physician may or may not be well informed on what the reported finding about Disease Y means or how to respond. DNA testing can reveal more than susceptibility to disease. People can learn that they do not have the biological connections — parentage or evidence of ethnic origin — that they thought they did.
Colleagues who serve on the Institutional Review Board (IRB) in my institution tell me that they currently do not permit “cold calls” of the type portrayed in the opening paragraph. Such direct contacts have, however, occurred in the past, with or without the blessing of an IRB. Sharing findings with the individual's physician, who is then supposed to serve as a learned and wise intermediary, is not without problems either, given that many physicians understand little about complex genetics. Yet the very existence of this project on managing incidental findings in research demonstrates that some people believe that some research findings ought to be available to participants.
Vignettes such as the one above only begin to identify issues that must be considered in developing a policy on how to manage incidental findings in research, that is, findings that were not the direct object of the study. One of the most salient issues is the potential for discovering incidental findings in analysis of archived genetic data or samples. DNA, which is present in almost all cells, can be obtained and stored in a variety of ways, and for numerous reasons. Pathology laboratories are typically required to retain residual surgical specimens for years and even decades. DNA can be extracted from leftover blood samples before they are disposed of, which typically occurs a few days after collection. Blood spots containing DNA are obtained for screening from virtually every newborn in the country; some states even store these samples for decades. Large repositories of biological materials have been created for a wide array of disorders, and are held by federal and state agencies, universities, and private companies. Researchers throughout the world have collected over the years freezers full of blood and tissue samples. Cell lines can be created and maintained as sources of DNA indefinitely.
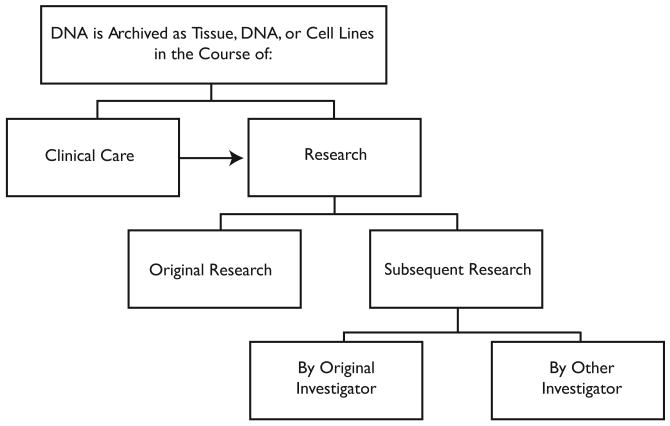
Such samples are often collected for one purpose and subsequently used for another. A surgical specimen may be used for teaching, test validation, or research. An investigator may collect a sample to explore the impact of a particular genetic variant on one disease, only to learn later that variants in different genes may also be relevant to the disease she is interested in or that the original variant may be relevant to a completely different disorder. Investigators often share stored samples and clinical information with other scientists hoping to advance our understanding of health and disease. This kind of sharing has long occurred in research, though less frequently in genetics research.1 Broader sharing in genetics and genomics research is now encouraged and may soon be required by funders2 and publishers.3 A highly simplifed representation of these archiving and sharing relationships is shown in Figure 1.
Figure 1. Archiving and Sharing DNA.
In light of this complex flow and use of samples, it is unlikely that people from whom DNA samples are obtained fully understand the ways in which those samples can be shared and used. While patients who seek care at teaching hospitals are often aware that research is conducted there,4 forms for consent to treatment often make only general statements that pathologic samples may be used for research. People who knowingly enroll in research protocols have greater opportunities to learn about how DNA can be used for scientific investigation. Many commentators have suggested that research participants should be given choices about subsequent uses of DNA5 or at least informed about possible future research and given the choice to opt out.6 Such disclosures and options, however, have not become common practice,7 nor is there much evidence about how fully research participants understand and exercise the choices they are offered. Moreover, a great deal of research is conducted without seeking individual consent, either because the IRB waives that requirement or because identifiers are removed so that the samples are no longer deemed to involve “human subjects.”8
That patients and research participants may be a little hazy about the use of DNA for research is potentially problematic, since every use of clinical specimens for research and any repurposing or sharing of research samples and results is an opportunity for incidental findings as defined in this project. Although incidental findings are not new in genetics, the likelihood of such discoveries has increased dramatically as a result of advances in technology and the expansion of knowledge. In the past, most genetics studies examined a limited number of genes or sites of genetic variation. The investigator would focus on a few candidate genes that were thought to affect the trait or disease of interest. The scope of inquiry was narrow and hence the likelihood of unsought findings was relatively low.
The commercial availability of “chips” that can assay hundreds of thousands or more single nucleotide polymorphisms (SNPs) at a time has changed the face of genomics research, permitting genome-wide association studies. New studies demonstrating correlations between common diseases and specific sites of genetic variation are appearing with ever-increasing frequency. It is already possible, for example, to ask whether an individual has genetic variants associated with an increased risk of age-related macular degeneration, diabetes, Crohn's disease, cardiovascular disease, schizophrenia, and bipolar disorder, to name just a few.9 Thus, every time one of these genome-wide association studies is conducted, the researcher theoretically has the opportunity to look in each individual's DNA not only for SNPs that correlate with the disorder in which she is interested, but also for any other SNPs that other investigators have identified as correlated with other disorders. SNPs are not the only sources of genetic variation that potentially can be assessed across the genome. Insertions, deletions, copy number variations, all of which affect more than one base pair, and epigenetic modifications, which occur after DNA has been replicated, are some others that will be important. Thus, the genomic scientist may ask, Do I look for all the other variants that my method detects, even if it requires special software? If so, what do I do with my findings? “Chip” makers face a similar set of questions: what SNPs that are known to be associated with disease risk do we include on our product?
A growing number of people argue that research participants are entitled to receive personal research results.10 Daryl Pullman and Kathy Hodgkinson, for example, maintain that investigators are morally obligated to return results of “genetic studies [that reveal risks of] serious diseases with high recurrence risks, particularly those for which potential ameliorative interventions exist.”11 Mary Kay Pelias states that research participants are entitled to receive even provisional results.12 David Shalowitz and Franklin Miller urge that respect for persons requires that research participants have access to all individual research results, particularly if the participants ask.13 These commentators specifically reject proposals to limit disclosures to results that are clinically useful. They argue instead that investigators should provide even provisional results with explanations of the limitations of the data. In their view, researchers always bear the burden of justifying nondisclosure. Isaac Kohane and his collaborators recently proposed creating a computer system in which research participants, by defining their own preferences for information, would have complete control over their access to those research results that the researchers have judged to be sufficiently valid.14 Their reasoning, though focused on research results, is broad enough in most cases to support a claim by research participants to include incidental findings as well.
A great deal of research is conducted without seeking individual consent, either because the IRB waives that requirement or because identifiers are removed so that the samples are no longer deemed to involve “human subjects.”
Investigators' decisions not to obtain the software needed to examine individual results beyond those under study and manufacturers' choices not to put on chips disease-associated SNPs with those needed for the research are not likely to be viewed with favor by those research participants who want to receive incidental findings. The experience with newborn screening using tandem mass spectrometry (MS/MS), though not completely analogous to research uncovering incidental findings, powerfully demonstrates the demand for information and impatience with decisions not to look for and report all ascertainable findings. Unlike most previous newborn screening methods, which could detect only one disorder at a time, MS/MS can detect dozens and even hundreds of metabolic abnormalities at the same time. The rapid move toward reporting all the abnormalities detectable by MS/MS, not just those of known clinical utility, was driven in large part by the view that everything that can be revealed by a technology must be sought and disclosed.15
Yet prior experiences with similar disclosure dilemmas in genetics suggest that telling everything may not always be the best option and certainly is not universally practiced. By far the most common incidental finding in genetics is misattributed paternity, which is typically estimated to occur in 1-10 percent of pregnancies.16 Although clinical and forensic testing for the purpose of ascertaining paternity is common, in genetics research, non-parentage is detected only as a consequence of looking for contributions of genetic variation to disease. In that context, then, demonstrating non-parentage is an incidental finding. For years, partial or complete non-disclosure of such findings has been the most common practice in both the clinical and research settings.17 For example, if the husband is found not to carry the mutation that affects a child with an autosomal recessive disorder born to his wife, he is frequently told only that the recurrence risk is very low. The wife, however, may be told individually about the finding of misattributed paternity, which she can then deal with as she sees fit. Non-disclosure is probably even more common in the research setting. The foundations of this practice of partial disclosure or nondisclosure lie in the clinician's and investigator's concern that revelation of misattributed paternity will disrupt the family, perhaps leading to domestic violence or abandonment, as well as in a more reflexive desire to avoid getting involved in sticky situations. Partial disclosure or nondisclosure, however, has been criticized for undermining the man's and the child's rights to know about their biological connections, their heritage, and the truth of their family relations.18
To avoid these dilemmas, clinicians are advised to tell women prior to testing about the risk of uncovering misattributed paternity, giving them the option of not going forward or at least knowledge of what can happen.19 No data exist on the frequency with which such advice is actually given, but raising the possibility of infidelity is not easy, particularly if the woman's partner is in the room. IRBs increasingly require that the possibility of discovering misattributed paternity be included in research consent forms,20 but the efficacy of such warnings is questionable given participants' incomplete retention of the content of consent forms.21
Pleiotropy — when a particular gene has more than one function — is another potential source of unexpected findings, as genetic testing for one purpose may yield undesired results about a different problem.22 The classic example of this is testing for alleles of the ApoE gene. In the past, clinicians considered offering testing to individuals seeking to reduce their cardiovascular risk factors in order to determine whether they had the ε2 allele of this gene, which confers increased risk. The ε4 allele, however, is more complex than the ε2 allele because it is associated not only with elevated cardiovascular risk but also with increased risk of Alzheimer disease, a topic that individuals often avoid.23 Great distress can occur when there is no discussion before testing about the possibility of learning about Alzheimer risk, a topic explored in “A Question of Genes: Inherited Risks,” a show produced by Noel Schwerin and shown on public television in 1997.24 Once this problem was appreciated, counseling about the chance of learning ApoEε4 status was widely recommended when considering testing ApoE to assess cardiovascular risk as well as when conducting research.25 Interestingly, ApoE testing never became very common, not because of these thorny ethical concerns, but because statins, which address cardiovascular risk in part by reducing cholesterol levels, made ApoE status almost completely irrelevant clinically.
A group convened in 1994 by the Centers for Disease Control and Prevention (CDC) and the National Center for Human Genome Research (NCHGR) (predecessor to the National Human Genome Research Institute) was confronted by two more challenges.26 One was that the CDC in the third National Health and Nutrition Examination Survey (NHANES – III) had stated in their consent form that they were collecting DNA as part of the extensive medical evaluation required for that study and that they would return all results to the participants. The CDC subsequently wondered whether they had provided the participants with enough information to consent knowingly to receive genetic test results, some of which had unclear significance. The other challenge emerged from a series of anecdotal reports that other investigators had tested residual surgical specimens from cancer patients for germ-line mutations in genes such as BRCA1 and BRCA2 and HNPCC and then called the patients with the results, much as in the opening vignette above. Some of these patients in the early 1990s were angered and upset by these calls, as they had no idea their samples were involved in research, the calls came from researchers they did not know, and in some cases the calls came years after the patients had been treated. Some of them called Dr. Francis Collins, the Director of the NCHGR, who recounted their complaints to the Working Group in open session. After extensive deliberations, the group convened by the CDC and NCHGR recommended obtaining full informed consent from research subjects and patients whose samples could be used in research involving potentially identifiable samples; this full informed consent form would specifically address the circumstances under which research results would be shared.
The National Bioethics Advisory Commission subsequently endorsed the notion that informed consent documents should address disclosure of individual research results before research is conducted and added that
IRBs should develop general guidelines for the disclosure of the results of research to subjects and require investigators to address these issues explicitly in their research plans. In general, these guidelines should reflect the presumption that the disclosure of research results to subjects represents an exceptional circumstance. Such disclosure should occur only when all of the following apply:
the findings are scientifically valid and confirmed,
the findings have significant implications for the subject's health concerns, and
a course of action to ameliorate or treat these concerns is readily available.27
It is difficult to imagine what research results regarding complex phenotypes could meet this threshold. As noted above, however, there are those who argue that these criteria are too stringent or that some results fulfill these requirements. Still other commentators have recommended that investigators who wish to return research findings undergo review by an IRB or other body to ensure that the requirements of validity and utility are met.28
The Clinical Laboratories Improvement Amendments (CLIA), which mandate that laboratory results to be used diagnostically meet stringent quality assurance requirements, represent another hurdle.29 Most research laboratories do not adhere to CLIA requirements, so that providing individual results on the grounds that the participants will value them and may act upon them arguably violates the statute. Nonetheless, a working group of the National Heart, Lung, and Blood Institute (NHLBI) recommended that certain individual research results could be returned to participants if labeled as “research results” even if they were not obtained in a CLIA-approved laboratory, so long as no CLIA-approved laboratory was available and the test was “run by two different methods and/or under the…direct supervision of a CLIA certified laboratory to confirm results.”30 While these recommendations clearly reflect the tension at the heart of this project, how they can be squared with CLIA is by no means clear.
So where does this leave me? I recognize that some scholars' arguments that people should get all research results support a claim to incidental findings as well. I also believe that some patients and research participants want to receive incidental findings. However, I remain reluctant to endorse low barriers to disclosure.
Certainly, the possibility of incidental findings in research should be addressed in consent forms. It is impossible to anticipate everything that might be discovered, but something can be said about the difference between monogenic fully penetrant disorders and the kinds of relative risks that are more likely to be found. Most people, for example, would see a difference between incidental detection of a mutation in the APC gene that will cause them to develop colon cancer if their colon is not removed and a mutation that mildly increases their risk of developing hypertension, a disorder that would ordinarily be detected in the course of routine care.
Yet the inability to educate research participants fully in the consent process about the array of possible incidental findings means that oversight of investigators' decisions to disclose and processes of conveying information is crucial. Although the conservative criteria proposed by NBAC and many others regarding the disclosure of research findings appear to me to be appropriate for incidental findings as well, the heat of the current debate about access to all results obtained in research, whether intended or incidental, means more discussion about the criteria for disclosure is warranted.
The inability to educate research participants fully in the consent process about the array of possible incidental findings means that oversight of investigators' decisions to disclose and processes of conveying information is crucial.
But by far the most important reason for my hesitancy is that most people do not know how their DNA is being used in research, what conditions are being investigated, or even that research is going on at all. This is not like research on functional magnetic resonance imaging (fMRI) or computed tomography (CT) colonography in which people at least know they have been in a scanner. In most cases of genomics research using archived samples, the problem of the “cold call” and the question that often follows — How can I say I do not want to know when someone says they have important information about me? — is unavoidable. It may be appropriate or even desirable to offer people choices about incidental findings before the research begins. But especially since so many people who are at known genetic risk opt not to be tested when given a choice beforehand,31 a general policy of offering incidental findings to unsuspecting people who had not previously thought about the issue just does not seem right.
Acknowledgments
I would like to thank Jeff Canter, M.D., M.P.H., Lainie Friedman Ross, M.D., Ph.D., and Jim Clayton for their advice and Charlisse Caga-anan, Suzanne Sobotka, and Lindsey Yock for their excellent research assistance, supported by National Human Genome Research Institute (NHGRI) grant # R01 HG003178-01A1 on “Managing Incidental Findings in Human Subjects Research” (PI, S. M. Wolf; Co-Is, J. P. Kahn, F. Lawrenz, C. A. Nelson). The contents of this article are solely the responsibility of the author and do not necessarily represent the official views of NHGRI.
References
- 1.Campbell EG, et al. Data Withholding in Academic Genetics: Evidence from a National Survey. JAMA. 2002;287(4):473–480. doi: 10.1001/jama.287.4.473. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Request for Information (RFI): Proposed Policy for Sharing of Data Obtained in NIH Supported or Conducted Genome-Wide Association Studies (GWAS) 2006 available at < http://grants.nih.gov/grants/guide/notice-files/NOT-OD-06-094.html > (last visited February 12, 2008)
- 3.NIH Pharmacogenetics Research Network. Pharmacogenetics and Pharmacogenomics Knowledge Base. 2007 available at < http://www.nigms.nih.gov/Initiatives/PGRN/> (last visited February 12, 2008)
- 4.Pulley JM, et al. Attitudes and Perceptions of Patients towards Methods of Establishing a DNA Biobank. Cell & Tissue Banking. 2007;8(3):233–241. doi: 10.1007/s10561-007-9051-2. [DOI] [PubMed] [Google Scholar]
- 5.National Action Plan for Breast Cancer. Consent Form for Use of Tissue for Research. 1997 available at < www.4woman.gov/napbc/catalog.wci/napbc/consent.htm > (last visited February 12, 2008);; National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. Vol. 1. Rockville, MD: 1999. [Google Scholar]
- 6.Beskow LM, et al. Informed Consent for Population-Based Research Involving Genetics. JAMA. 2001;286(18):2315–2321. doi: 10.1001/jama.286.18.2315. [DOI] [PubMed] [Google Scholar]
- 7.Weir RF, Horton JR. DNA Banking and Informed Consent: Part 1. IRB. 1995;17(4):1–4. [PubMed] [Google Scholar]
- 8.Office for Human Research Protections, Department of Health and Human Services. Guidance on Research Involving Coded Private Information or Biological Specimens. 2004 available at < http://www.hhs.gov/ohrp/humansubjects/guidance/cdebiol.pdf> (last visited February 12, 2008)
- 9.Wellcome Trust Case Control Consortium. Genome-Wide Association Study of 14,000 Cases of Seven Common Diseases and 3,000 Shared Controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller BP, et al. Privacy in Genetics Research. Science. 1999;285(5432):1359–1361. doi: 10.1126/science.285.5432.1359. [DOI] [PubMed] [Google Scholar]
- 11.Pullman D, Hodgkinson K. Genetic Knowledge and Moral Responsibility: Ambiguity at the Interface of Genetic Research and Clinical Practice. Clinical Genetics. 2006;69(3):199–203. doi: 10.1111/j.1399-0004.2006.00581.x. [DOI] [PubMed] [Google Scholar]
- 12.Pelias MK. Research in Human Genetics: The Tension between Doing No Harm and Personal Autonomy. Clinical Genetics. 2005;67(1):1–5. doi: 10.1111/j.1399-0004.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- 13.Shalowitz DI, Miller FG. Disclosing Individual Results of Clinical Research: Implications of Respect for Participants. JAMA. 2005;294(6):737–740. doi: 10.1001/jama.294.6.737. [DOI] [PubMed] [Google Scholar]
- 14.Kohane IS, et al. Reestablishing the Researcher-Patient Compact. Science. 2007;316(5826):836–837. doi: 10.1126/science.1135489. [DOI] [PubMed] [Google Scholar]
- 15.Alexander D, van Dyck PC. A Vision of the Future of Newborn Screening. Pediatrics. 2006;117(5):S350–S354. doi: 10.1542/peds.2005-2633O. [DOI] [PubMed] [Google Scholar]; Pollitt RJ. Introducing New Screens: Why Are We All Doing Different Things? Journal of Inherited and Metabolic Diseases. 2007;30(4):423–429. doi: 10.1007/s10545-007-0647-2. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KG. How Well Does Paternity Confidence Match Actual Paternity? Evidence from Worldwide Nonpaternity Rates. Current Anthropology. 2006;47(3):513–520. [Google Scholar]
- 17.Wertz DC, Fletcher JC. Ethics and Medical Genetics in the United States: A National Survey. American Journal of Medical Genetics. 1988;29(4):815–827. doi: 10.1002/ajmg.1320290411. [DOI] [PubMed] [Google Scholar]
- 18.Ross LF. Disclosing Misattributed Paternity. Bioethics. 1996;10(2):114–130. doi: 10.1111/j.1467-8519.1996.tb00111.x. [DOI] [PubMed] [Google Scholar]; Turney L. The Incidental Discovery of Nonpaternity through Genetic Carrier Screening: An Exploration of Lay Attitudes. Qualitative Health Research. 2005;15(5):620–634. doi: 10.1177/1049732304273880. [DOI] [PubMed] [Google Scholar]
- 19.Wertz DC, Fletcher JC, Berg K. Review of Ethical Issues in Medical Genetics. World Health Organization; Geneva: 2003. [Google Scholar]
- 20.DeCamp M, Sugarman J. Ethics in Population-Based Genetic Research. Accountability in Research. 2004;11(1):1–26. doi: 10.1080/08989620490280221. [DOI] [PubMed] [Google Scholar]
- 21.Tait AR, Voepel-Lewis T, Malviya S. Do They Understand? (Part I): Parental Consent for Children Participating in Clinical Anesthesia and Surgery Research. Anesthesiology. 2003;98(3):603–608. doi: 10.1097/00000542-200303000-00005. [DOI] [PubMed] [Google Scholar]; Turner P, Williams C. Informed Consent: Patients Listen and Read, but What Information Do They Retain? New Zealand Medical Journal. 2002;115(1164):U218. [PubMed] [Google Scholar]; Pesudovs K, Luscombe CK, Coster DJ. Recall from Informed Consent Counseling for Cataract Surgery. Journal of Law & Medicine. 2006;13(4):496–504. [PubMed] [Google Scholar]
- 22.Wachbroit R. The Question Not Asked: The Challenge of Pleiotropic Genetic Tests. Kennedy Institute of Ethics Journal. 1998;8(2):131–144. doi: 10.1353/ken.1998.0013. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JS, et al. Who Seeks Genetic Susceptibility Testing for Alzheimer's Disease? Findings from a Multisite, Randomized Clinical Trial. Genetics in Medicine. 2004;6(4):197–203. doi: 10.1097/01.gim.0000132688.55591.77. [DOI] [PubMed] [Google Scholar]; Check E. Celebrity Genomes Alarm Researchers. Nature. 2007;447(7143):358–359. doi: 10.1038/447358a. [DOI] [PubMed] [Google Scholar]
- 24.Schwerin N. A Question of Genes. available at < http://www. backbonemedia.org/genes/> (last visited February 12, 2008)
- 25.Scheuner MT. Genetic Evaluation for Coronary Artery Disease. Genetics in Medicine. 2003;5(4):269–285. doi: 10.1097/01.GIM.0000079364.98247.26. [DOI] [PubMed] [Google Scholar]
- 26.Clayton EW, et al. Informed Consent for Genetic Research on Stored Tissue Samples. JAMA. 1995;274(22):1786–1792. [PubMed] [Google Scholar]
- 27.See National Bioethics Advisory Commission, supra note 5.
- 28.Andrews LB, et al., editors. Assessing Genetic Risks: Implications for Health and Social Policy. Washington, D.C.: National Academy Press; 1994. Committee on Assessing Genetic Risks, Division of Health Sciences Policy, Institute of Medicine. [Google Scholar]
- 29.42 U.S.C. § 263a (2007).
- 30.Bookman EB, et al. Reporting Genetic Results in Research Studies: Summary and Recommendations of an NHLBI Working Group. American Journal of Medical Genetics. 2006;140A(10):1033–1040. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]; National Heart Lung and Blood Institute. NHLBI Working Group on Reporting Genetic Results in Research Studies, Meeting Summary. 2004 available at < http://www.nhlbi.nih.gov/meetings/workshops/gene-results.htm > (last visited February 12, 2008)
- 31.Biesecker BB, et al. Psychosocial Factors Predicting BRCA1/BRCA2 Testing Decisions in Members of Hereditary Breast and Ovarian Cancer Families. American Journal of Medical Genetics. 2000;93(4):257–263. doi: 10.1002/1096-8628(20000814)93:4<257::aid-ajmg1>3.0.co;2-8.Foster C, et al. Non-Uptake of Predictive Genetic Testing for BRCA1/2 Among Relatives of Known Carriers: Attributes, Cancer Worry, and Barriers to Testing in a Multicenter Clinical Cohort. Genetic Testing. 2004;8(1):23–29. doi: 10.1089/109065704323016003.; see Roberts et al., supra note 23; Quaid KA, Morris M. Reluctance to Undergo Predictive Testing: The Case of Huntington Disease. American Journal of Medical Genetics. 1993;45(1):41–45. doi: 10.1002/ajmg.1320450112.Hayden MR. Predictive Testing for Huntington's Disease: The Calm After the Storm. The Lancet. 2000;356(9246):1944–1945. doi: 10.1016/S0140-6736(00)03301-8.