Abstract
Neuropeptide Y (NPY) is an important modulatory neuropeptide that regulates several physiological systems, including the activity of sensory neurons. We evaluated whether activation of the NPY Y1 receptor could modulate the activity of capsaicin-sensitive nociceptors in trigeminal ganglia and dental pulp. We tested this hypothesis by measuring capsaicin-stimulated calcitonin gene-related peptide release (CGRP) as a measure of nociceptor activity. Capsaicin-evoked CGRP release was inhibited by 50% (p < 0.05) in trigeminal ganglia and by 26% (p < 0.05) in dental pulp when tissues were pre-treated with [Leu31,Pro34]NPY. The Y1 receptor was found to co-localize with the capsaicin receptor TRPV1 in trigeminal ganglia. These results demonstrate that activation of the Y1 receptor results in the inhibition of the activity of capsaicin-sensitive nociceptors in the trigeminal ganglia and dental pulp. These findings are relevant to the physiological modulation of dental nociceptors by endogenous NPY and demonstrate an important novel analgesic target for the treatment of dental pain.
Keywords: pain, inflammation, sympathetic, NPY, Y1, capsaicin
Introduction
Neuropeptide Y (NPY) is a neurotransmitter originally identified in the brain that modulates numerous physiological activities, including feeding, metabolism, anxiety, immunity, vascular tone, and nociception (Lin et al., 2004; Brain and Cox, 2006; Brumovsky et al., 2007; Wheway et al., 2007). NPY is widely expressed in the central and peripheral nervous systems and can be released from sensory neurons, sympathetic neurons, and enteric neurons. NPY has also been identified in non-neural structures such as glia and platelets (Ericsson et al., 1991; Ubink and Hokfelt, 2000). NPY expression in sensory neurons is substantially up-regulated after neural injury and inflammation (Wakisaka et al., 1991; Mark et al., 1998; Honore et al., 2000; Ossipov et al., 2002). Dental injuries—including extraction, pulp exposure, and inferior alveolar nerve transection—induce an increase in NPY expression in the trigeminal ganglion (Itotagawa et al., 1993; Fristad et al., 1996). Even though the up-regulation of NPY after injury has been well-described, comparatively little is known about the effects of NPY on trigeminal nociceptors.
The receptors for NPY are G-protein-coupled receptors that exhibit dynamic alterations in signaling pathways, leading to neuronal excitatory or inhibitory effects after receptor activation (Holliday et al., 2004; Moran et al., 2004; Gibbs et al., 2007). There are 4 receptors for NPY that have been identified in humans: Y1, Y2, Y4, and Y5 (Larhammar, 1996). The Y1 and Y2 receptors are the most prevalent receptors for NPY and have been the most-studied in relation to modulation of sensory and nociceptive processes (Zhang et al., 1994, 1997). These receptors are present on distinct populations of sensory neurons, and activation of these receptors can modulate the activity of pain fibers.
Previous studies in our laboratory have demonstrated that activation of the NPY Y1 receptor can inhibit capsaicin-sensitive nociceptors, or pain fibers, terminating in either the spinal cord or hind paw tissue in rats (Gibbs et al., 2004, 2006); however, it is not known if NPY has similar effects on pulpal neurons. Capsaicin is the pungent ingredient in chili peppers and is used to study an important class of pain-sensing fibers that express TRPV1 (thermo-sensitive transient receptor potential vanilloid receptor type 1), an important molecular integrator of inflammatory pain. The quantification of evoked CGRP is a measure of the activity of peptidergic nociceptors that is well-established, and analgesics such as morphine inhibit capsaicin-evoked CGRP (Pohl et al., 1989; Ulrich-Lai et al., 2001; Hargreaves et al., 2003). The purpose of these studies is to determine whether activation of the Y1 receptor modulates capsaicin-stimulated CGRP release in the dental pulp and trigeminal ganglia.
Materials & Methods
In vitro Superfusion
Male Sprague-Dawley rats (175–200 g; Harlan, Indianapolis, IN, USA) were used in all experiments. Animals were housed on a 12:12 hr light-dark cycle with free access to food and water. These experiments were performed in concert with others utilizing other tissues from the animals, to minimize the overall usage of animals. All procedures were approved by the University of Texas Animal Care and Use Committee.
The superfusion method was performed as previously described (Hargreaves et al., 1992). Animals were decapitated, and trigeminal ganglia were quickly removed. After removal, the ganglia were placed in ice-cold, oxygenated Krebs buffer (NaCl 135 mM, KCl 3.5 mM, MgCl 1 mM, NaH2PO4 1 mM, CaCl2 2.5 mM, BSA 0.1%, dextrose 3.3 mM, ascorbic acid 0.1 mM, HEPES 10 mM, thiorphan 16 μM; pH 7.4) until a group of 4 ganglia was collected. Tissue from each group was then cut into slices (McIlwain Tissue Chopper, Gomshall, Surrey, UK), weighed, and placed in a 1.5-ml superfusion chamber, which was pumped (Brandel P20 Peristaltic pump, Gaithersburg, MD, USA) with Krebs buffer (36°C, pH 7.4). After equilibration, superfusate was collected in seven-minute fractions (Gilson FC203B Fraction collector, Middleton, WI, USA) and assayed for iCGRP content via radioimmunoassay.
Similar methodology was used in dental pulp superfusion experiments. After decapitation, pulpal tissue was removed from maxillary and mandibular incisor and pooled until tissue from 4 animals was collected. Tissue was then chopped and placed in a superfusion chamber. After tissue equilibration for 1 hr, superfusate was collected in ten-minute fractions.
The iCGRP radioimmunoassay (RIA) consisted of pre-incubating samples for 48 hrs at 4°C with 100 μL of CGRP antisera (kindly donated by Dr M. Iadarola, NIDCR, NIH, Bethesda, MD, USA). Then, 100 μL of [125I-Tyr]CGRP (approximately 20,000 cpm) and 50 μL of goat anti-rabbit antisera coupled to ferric beads (PerSeptive Diagnostics, Cambridge, MA, USA) were added and allowed to incubate for an additional 48 hrs at 4°C. Bound and free tracers were separated by immunomagnetic separation. All drugs were tested for interference in the RIA, and no cross-reactivity was detected.
Experimental Design
We collected several fractions to determine the basal level of CGRP released from trigeminal ganglia and dental pulp (trigeminal ganglia = 3 fractions, dental pulp = 6 fractions). Tissues were then pre-treated with either vehicle (Krebs) or the Y1 agonist [Leu31,Pro34]NPY (30 nM) for 1 fraction, and then stimulated with capsaicin (30 μM) in the presence of vehicle or the Y1 agonist. We collected several fractions after stimulation to evaluate the time-course for CGRP to return to basal level.
Immunohistochemistry
After decapitation, fresh trigeminal ganglia were carefully dissected, then embedded in Tissue-Tek O.C.T. (Sakura Finetek, Torrence, CA, USA) and frozen and stored at -80°C until being sectioned. Ganglia were cut into 20-μm sections and thaw-mounted onto Superfrost plus glass slides. Sections were fixed for 1 hr in ice-cold 3.7% formalin. Sections were permeabilized for 1 hr with 0.2% Triton X-100 and blocked for 30 min with 10% normal goat serum (NGS) in phosphate-buffered saline. Tissues were then incubated for 18 hrs at 4°C with polyclonal guinea-pig anti-TRPV1 serum (Neuromics, Minneapolis, MN, USA) diluted 1:5000 in 10% NGS containing 0.1% NaN3, followed by incubation with a FITC-labeled goat anti-guinea-pig secondary antibody at 25°C for 1 hr (Vector Laboratories, Burlingame, CA, USA). The tissue was then incubated for 18 hrs at 4°C with polyclonal rabbit anti-Y1 serum (Dia Soren, Stillwater, MN, USA), diluted 1:200 as above, followed by biotinylated goat anti-rabbit antibody for 1 hr at 25°C (Vector Laboratories) and Avidin-Texas Red for 1 hr at 25°C (Vector Laboratories). Appropriate control experiments were performed, including incubation of tissue with primary antibody that was pre-adsorbed with blocking peptide, or by substitution of primary antibody with normal rabbit serum. Images were acquired by means of a Nikon E600 microscope (Melville, NY, USA), equipped with appropriate filters, and by a Photometrics SenSys CCD-cooled digital camera (Roper Scientific, Tuscan, AZ, USA) connected to a computer equipped with Metamorph V4.1 image analysis software (Universal Image Corporation, Downingtown, PA, USA).
Statistics
Superfusion time-course data were analyzed with two-way analysis of variance (ANOVA) followed by the Bonferroni post-test with GraphPad Prism software (San Diego, CA, USA). Data were presented as fold increase over baseline (mean baseline values of iCGRP in fmol/mL are given in the Fig. legends). Peak CGRP release values were compared by an unpaired two-tailed t test. Results were considered significant when the probability that they occurred due to chance alone was less than 5% (i.e., P < 0.05). Data are reported as mean ± SEM.
Results
Capsaicin evoked a three-fold increase in iCGRP release from trigeminal ganglia (Fig. 1). The peak release was observed in fraction 6 and returned to baseline levels by fraction 8. The two-way ANOVA for repeated measures indicated that the experimental group treated with [Leu31,Pro34]NPY was significantly different from the group treated with Vehicle (Fig. 1). Pre-treatment of trigeminal ganglia with the Y1 receptor agonist [Leu31,Pro34]NPY significantly (p < 0.001 by the Bonferroni post hoc test after two-way ANOVA in Fig. 1 and p < 0.05 by t test in Fig. 2) inhibited capsaicin-evoked iCGRP release (Figs. 1, 2A) by 50%. This indicates that activation of the Y1 receptor inhibits the activation of capsaicin-sensitive trigeminal sensory neurons.
Figure 1.
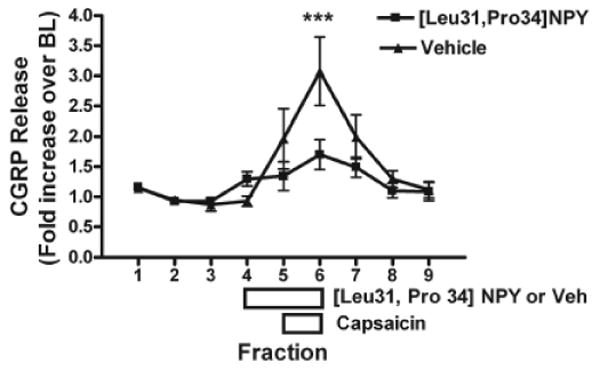
Effect of [Leu31,Pro34]NPY on capsaicin-stimulated CGRP release from trigeminal ganglia. Freshly isolated ganglia were chopped and placed in perfusion chambers. Tissues were pre-treated with the Y1 agonist [Leu31,Pro34]NPY (30 nM) for 1 fraction (7 min). Tissues were then co-treated with [Leu31,Pro34]NPY (30 nM) and capsaicin (30 μM) for 1 fraction (7 min). Data are presented as fold-increase over baseline (BL), where the baseline represents the average CGRP release from the first 3 fractions (mean BL = 53.1 ± 4.0 fmol/mL). Statistical analysis by two-way ANOVA demonstrated a significant effect of drug treatment (F = 5.35, p < 0.05) and time (F = 8.21, p < 0.0001) on the measured outcome of CGRP release. A Bonferroni post hoc test demonstrates that the agonist-treated tissues released significantly less CGRP when stimulated by capsaicin in fraction 6 compared with vehicle-treated (veh) tissues (p < 0.001). Error bars = SEM. N = 15.
Figure 2.
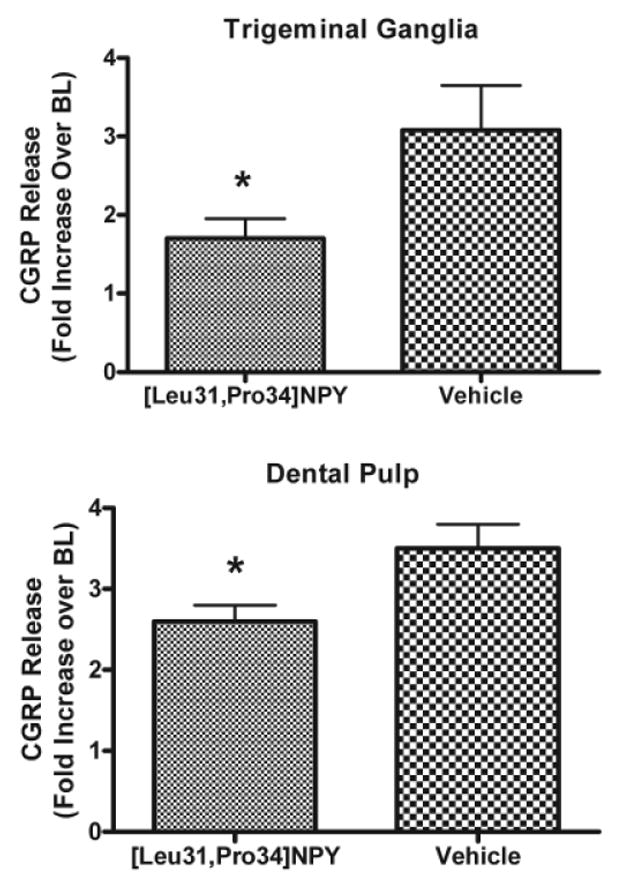
Effect of [Leu31,Pro34]NPY on peak capsaicin-stimulated CGRP release from trigeminal ganglia and dental pulp. Data are presented as fold-increase over baseline (BL), where baseline represents the average CGRP release prior to drug treatment (3 fractions in trigeminal ganglia and 5 fractions in dental pulp; BL = 53.1 ± 4.0 fmol/mL for trigeminal ganglia and 4.6 ± 0.3 fmol/mL for dental pulp). Statistical analysis by an unpaired two-tailed t test demonstrated that tissues pre-treated with [Leu31,Pro34]NPY (30 nM) released significantly less CGRP when stimulated with capsaicin (30 μM in trigeminal ganglia and dental pulp) (p < 0.05 for trigeminal ganglia and dental pulp). Error bars = SEM. N = 13 for trigeminal ganglia; n = 18 for dental pulp.
To test the hypothesis that activation of the Y1 receptor could inhibit peripheral terminals of capsaicin-sensitive nociceptors, we performed a similar experiment using dental pulp as the test tissue. Capsaicin treatment resulted in a 3.5-fold increase in CGRP release from peripheral terminals in dental pulp (Fig 2b). This release was significantly (p < 0.05) inhibited by pre-treatment with [Leu31,Pro34]NPY, by 26%. This indicates that activation of the Y1 receptor can inhibit the activity of capsaicin-sensitive neurons in peripheral tissues, specifically dental pulp.
We next evaluated whether the Y1 receptor potentially regulates capsaicin-sensitive neurons by a direct mechanism (i.e., in the same cell). Using immunohistochemistry, we determined whether the capsaicin receptor TRPV1 and the Y1 receptor were co-expressed in the same trigeminal neurons (Fig. 3). Demonstration of receptor co-expression provides a cellular basis by which activation of the Y1 receptor could initiate an intracellular signal transduction cascade, resulting in the inhibition of TRPV1 activity.
Figure 3.
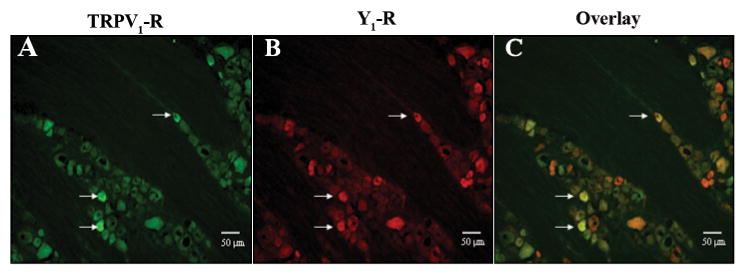
Co-localization of TRPV1 receptor immunoreactivity (green) with Y1 receptor immunoreactivity (red) in rat trigeminal ganglia (magnification = 20X). White arrows indicate examples of cells demonstrating co-localization. The co-localization of the Y1 receptor with the TRPV1 receptor indicates that Y1 receptors are localized on capsaicin-sensitive nociceptors.
Discussion
In these studies, we describe for the first time an inhibitory function of the NPY Y1 receptor on pro-inflammatory neurotransmitter release in the dental pulp. This was demonstrated by evaluation of the effects of treating dental pulp and trigeminal ganglia with a specific agonist to the Y1 receptor ([Leu31,Pro34]NPY), and evaluation of the effect of this drug on capsaicin-evoked CGRP release, which is a well-established measure of nociceptor exocytotic activity. We found that pre-treatment of both isolated dental pulp and isolated trigeminal ganglia with this Y1 agonist significantly inhibited CGRP release. Finally, we report anatomical evidence documenting the co-localization of the NPY Y1 receptor with the capsaicin receptor TRPV1 in the cell body of trigeminal sensory neurons. These findings demonstrate that activation of the Y1 receptor can inhibit the activation of nociceptors by capsaicin and therefore could be a target for future analgesic development.
NPY Expression in Dental Pulp and Effects of Injury
In addition to its potential as a pharmacotherapeutic target, the Y1 receptor is likely involved in physiologic modulation of pulpal nociceptor activity via activation by its endogenous ligand, NPY. Under normal or uninjured conditions, NPY is expressed at a very low level in sensory neurons. This is consistent with findings from histological studies, which demonstrated that NPY expression in normal dental pulp is primarily localized in sympathetic neurons approximating blood vessels and not sensory neurons (Uddman et al., 1984; Jacobsen et al., 1998). After pulp exposure in rodents, which produces inflammation and degeneration of peripheral neurons, an increase in NPY expression is observed in sensory neurons of the dental pulp (Itotagawa et al., 1993; Oswald and Byers, 1993; Wakisaka et al., 1996). A similar mechanism appears to exist in human dental pulp, since an increase in NPY expression is observed in dental pulp in carious vs. non-carious teeth (Caviedes-Bucheli et al., 2006; El Karim et al., 2006). Given the findings presented in this manuscript, it is likely that endogenous NPY could act on Y1 receptors to inhibit neuronal activity of pulpal afferent terminals. If Y1 receptors were the primary NPY receptor subtype expressed in the dental pulp, then NPY would likely act as an endogenous analgesic substance. In contrast, if Y2 receptors were more prevalent on sensory neurons, then NPY could either inhibit or activate sensory neurons (Gibbs et al., 2007). It is unknown at this time whether Y1 is the most highly expressed receptor in the dental pulp, or what happens to Y1 or Y2 expression after pulpal injury. This is an important area for future study.
Y1 Receptor Mechanisms for Inhibiting Nociceptor Activity
The majority of existing evidence demonstrates an inhibitory effect of Y1 receptor activation (Brumovsky et al., 2007). We have previously demonstrated that Y1 receptor activation inhibits capsaicin-evoked CGRP release from hindpaw skin as well as capsaicin-evoked mechanical allodynia. These earlier findings are consistent with the results presented here. Thus, we can conclude that the NPY Y1 receptor is capable of inhibiting peripheral capsaicin-sensitive nociceptor activity.
There are several potential mechanisms whereby NPY could inhibit peripheral nociceptors by activating the Y1 receptor. First, activation of Y1 receptors could inhibit the release of neurotransmitters such as CGRP and SP by the activation of intracellular signaling pathways. For example, NPY activates G-protein-activated inwardly-rectifying potassium channels and inhibits N- and P/Q-type Ca++ channels, which in turn can inhibit neurotransmitter release from nerve terminals. Although this hypothesis was not tested in these studies, this is a likely mechanism whereby the Y1 receptor could inhibit nociceptor activity. In addition, it is possible that Y1 could activate a phosphatase such as calcineurin, leading to dephosphorylation and desensitization of TRPV1 (Patwardhan et al., 2005). However, it is not known if Y1 activation leads to increased calcineurin activity. Other possibilities are that the Y1 receptor agonist was able to modulate capsaicin-evoked CGRP release from sensory neurons indirectly, by acting on vasculature or immune cells. NPY has demonstrated peripheral vasopressor effects and immunomodulatory effects that are mediated by Y1 receptors, and it is possible that these could influence the activity of sensory neurons indirectly (Malmström, 1997; Wheway et al., 2007). However, given the in vitro experimental approach utilized in these studies with dissociated tissues, it is unlikely that complex neuro-immune and neurovascular interactions are intact. Furthermore, the anatomical demonstration of TRPV1 and Y1 receptor co-localization in the cell body of trigeminal neurons supports the hypothesis that a direct interaction of the signal transduction pathways between these receptors exists. In conclusion, we demonstrated inhibitory effects of Y1 receptor activation in dental pulp and in trigeminal ganglia. These findings indicate that the Y1 receptor is a novel target for future analgesic development and could also be an important endogenous mechanism regulating pain due to pulpal inflammation.
Acknowledgments
This work was supported by funding from the NIH: R01 NS45186 (KMH) and F30DE14326 (JG).
References
- Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;147(Suppl 1):202–211. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol Sci. 2007;28:93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Caviedes-Bucheli J, Lombana N, Azuero-Holguin MM, Munoz HR. Quantification of neuropeptides (calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and vasoactive intestinal polypeptide) expressed in healthy and inflamed human dental pulp. Int Endod J. 2006;39:394–400. doi: 10.1111/j.1365-2591.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Lamey PJ, Linden GJ, Awawdeh LA, Lundy FT. Caries-induced changes in the expression of pulpal neuropeptide Y. Eur J Oral Sci. 2006;114:133–137. doi: 10.1111/j.1600-0722.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Hemsen A, Lundberg JM, Persson H. Detection of neuropeptide Y-like immunoreactivity and messenger RNA in rat platelets: the effects of vinblastine, reserpine, and dexamethasone on NPY expression in blood cells. Exp Cell Res. 1991;192:604–611. doi: 10.1016/0014-4827(91)90082-6. [DOI] [PubMed] [Google Scholar]
- Fristad I, Heyeraas KJ, Kvinnsland IH. Neuropeptide Y expression in the trigeminal ganglion and mandibular division of the trigeminal nerve after inferior alveolar nerve axotomy in young rats. Exp Neurol. 1996;142:276–286. doi: 10.1006/exnr.1996.0197. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Flores CM, Hargreaves KM. Neuropeptide Y inhibits capsaicin-sensitive nociceptors via a Y1-receptor-mediated mechanism. Neuroscience. 2004;125:703–709. doi: 10.1016/j.neuroscience.2004.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Flores CM, Hargreaves KM. Attenuation of capsaicin-evoked mechanical allodynia by peripheral neuropeptide Y Y1 receptors. Pain. 2006;124:167–174. doi: 10.1016/j.pain.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gibbs JL, Diogenes A, Hargreaves KM. Neuropeptide Y modulates effects of bradykinin and prostaglandin E2 on trigeminal nociceptors via activation of the Y1 and Y2 receptors. Br J Pharmacol. 2007;150:72–79. doi: 10.1038/sj.bjp.0706967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves KM, Bowles WR, Garry MG. An in vitro method to evaluate regulation of neuropeptide release from dental pulp. J Endod. 1992;18:597–600. doi: 10.1016/S0099-2399(06)81329-4. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Bowles WR, Jackson DL. Intrinsic regulation of CGRP release by dental pulp sympathetic fibers. J Dent Res. 2003;82:398–401. doi: 10.1177/154405910308200514. [DOI] [PubMed] [Google Scholar]
- Holliday NM, Michel MC, Cox HM. NPY receptor subtypes and their signal transduction. In: Michel MC, editor. Neuropeptide Y & related peptides. Berlin: Springer-Verlag; 2004. pp. 45–74. [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Itotagawa T, Yamanaka H, Wakisaka S, Sasaki Y, Kato J, Kurisu K, et al. Appearance of neuropeptide Y-like immunoreactive cells in the rat trigeminal ganglion following dental injuries. Arch Oral Biol. 1993;38:725–728. doi: 10.1016/0003-9969(93)90013-c. [DOI] [PubMed] [Google Scholar]
- Jacobsen EB, Fristad I, Heyeraas KJ. Nerve fibers immunoreactive to calcitonin gene-related peptide, substance P, neuropeptide Y, and dopamine beta-hydroxylase in innervated and denervated oral tissues in ferrets. Acta Odontol Scand. 1998;56:220–228. doi: 10.1080/00016359850142835. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Malmström RE. Neuropeptide Y Y1 receptor mechanisms in sympathetic vascular control. Acta Physiol Scand Suppl. 1997;636:1–55. [PubMed] [Google Scholar]
- Mark MA, Colvin LA, Duggan AW. Spontaneous release of immunoreactive neuropeptide Y from the central terminals of large diameter primary afferents of rats with peripheral nerve injury. Neuroscience. 1998;83:581–589. doi: 10.1016/s0306-4522(97)00402-8. [DOI] [PubMed] [Google Scholar]
- Moran TD, Colmers WF, Smith PA. Opioid-like actions of neuropeptide Y in rat substantia gelatinosa: Y1 suppression of inhibition and Y2 suppression of excitation. J Neurophysiol. 2004;92:3266–3275. doi: 10.1152/jn.00096.2004. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, et al. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald RJ, Byers MR. The injury response of pulpal NPY-IR sympathetic fibers differs from that of sensory afferent fibers. Neurosci Lett. 1993;164:190–194. doi: 10.1016/0304-3940(93)90889-s. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, et al. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl M, Lombard MC, Bourgoin S, Carayon A, Benoliel JJ, Mauborgne A, et al. Opioid control of the in vitro release of calcitonin generelated peptide from primary afferent fibres projecting in the rat cervical cord. Neuropeptides. 1989;14:151–159. doi: 10.1016/0143-4179(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Ubink R, Hokfelt T. Neuropeptide Y expression in Schwann cell precursors. Glia. 2000;32:71–83. [PubMed] [Google Scholar]
- Uddman R, Grunditz T, Sundler F. Neuropeptide Y: occurrence and distribution in dental pulps. Acta Odontol Scand. 1984;42:361–365. doi: 10.3109/00016358409033616. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Flores CM, Harding-Rose CA, Goodis HE, Hargreaves KM. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from rat trigeminal ganglion: evidence for intraganglionic neurotransmission. Pain. 2001;91:219–226. doi: 10.1016/S0304-3959(00)00439-5. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Youn SH, Kato J, Takemura M, Kurisu K. Neuropeptide Y-immunoreactive primary afferents in the dental pulp and periodontal ligament following nerve injury to the inferior alveolar nerve in the rat. Brain Res. 1996;712:11–18. doi: 10.1016/0006-8993(95)01421-7. [DOI] [PubMed] [Google Scholar]
- Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem. 2007;7:1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, et al. Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc Natl Acad Sci USA. 1994;91:11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi T, Holmberg K, Landry M, Huang W, Xiao H, et al. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc Natl Acad Sci USA. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]


