Abstract
The suprachiasmatic nucleus (SCN) controls circadian behavior, and neurons in the SCN are intrinsic oscillators. Meredith et al. now identify the BK potassium channel as a key modulator of spontaneous firing of the SCN.
You are lying in your bed, staring at the ceiling, waiting for sleep to come your way and wondering why it has become so difficult to find. Among other reasons, these sleepless nights are caused by our circadian timing system turning on arousal centers in our brain at inappropriate times. This problem may be particularly common during the summer travel season when we are jetting off to attend a conference or perhaps to catch a World Cup match. Would it not be nice to be able to turn down this biological timing signal, if only for a few days while we adjust to new time zones? New research in this issue by Meredith and colleagues into the ionic mechanisms underlying circadian oscillations may well open up the prospect for such manipulations in the future1.
Humans and other organisms have daily rhythms in their behavior and physiology. In mammals, the part of the nervous system responsible for most circadian behavior is a bilaterally paired structure in the hypothalamus, the SCN. Many neurons in the SCN are intrinsic oscillators that continue to generate near 24-hour rhythms in electrical activity, secretion and gene expression when isolated from the rest of the organism. Individual SCN neurons contain a molecular feedback loop that drives these rhythms. However, membrane excitability and/or synaptic transmission may also be required for generation of the molecular oscillations. For example, disruption of electrical activity with tetrodotoxin (TTX) damps molecular circadian rhythms of mPer1 levels in SCN tissue2. A similar loss of function at the molecular level is observed in mice deficient in receptors for the neuropeptide transmitter vasoactive intestinal polypeptide3. Thus, clarifying the ionic mechanisms responsible for the generation of rhythms in electrical activity in SCN neurons is an important step for understanding the generation and output of circadian oscillations.
The new study by Meredith and colleagues takes a step in this direction by examining the role of the large-conductance calcium-activated potassium (BK) channels in circadian behavior1. With this work, the authors have identified a specific channel that seems to be responsible for the output of the SCN without an obvious role in other aspects of the circadian system. The BK channels seem not to be important for the generation of daily rhythms or for their synchronization by light.
To explore the role of this channel in circadian function, Meredith and colleagues took advantage of mice carrying a deletion of the Kcnma1 (Slo−/−) gene that encodes the core-forming subunit of the BK channel. The authors found that BK-deficient mice have lower amplitude circadian rhythms in wheel running and home cage activity as well as in core body temperature. The mice still showed circadian rhythms, but the magnitude of the day/night variation was greatly reduced. Notably, the frequency (or period) of the biological rhythm did not seem to be altered by the loss of the BK channels. Furthermore, the light response of the circadian system as measured by the onset of the activity rhythms was not altered in the mutants. This analysis suggests that the output of the circadian system is selectively damped by the loss of the BK channels.
Previous work used oligonucleotide arrays to show that transcription of the Kcnma1 gene cycles in SCN tissue4. Here the authors determined that the BK protein is rhythmically expressed in the SCN of mice maintained in constant conditions—that is, the levels of the protein vary with the circadian cycle. The peak expression of the BK channel is during the night, when the magnitude of the BK current also peaks5. Functionally, the pharmacological blockage of BK channels decreases the current flow that occurs between action potentials6 and decreases the amplitude of the day/night difference in firing rate in SCN neurons5. Meredith et al. explored the electrical activity of the SCN in the mutant mice. One of the characteristic features of neurons in the SCN is that they show circadian rhythms in electrical discharge with more action potentials generated in the day than in the night. The peak rate of action potential generation during the day was not altered in the BK-deficient mice; however, the SCN neurons from the mutant mice showed more electrical activity during the night. Given the close association between SCN electrical activity and rhythmic behavior, the lower-amplitude rhythms in the SCN may be responsible for the reduction in the robustness of the behavioral rhythmicity.
Finally, the authors looked at gene expression in the SCN of the mutant mice. A few years ago, this would have seemed like a superfluous experiment. However, work in Drosophila7 and mice8 suggests that treatments that alter the membrane potential can disrupt the rhythms in the expression of clock genes such as Period. In this case, microarray data indicates that overall gene expression profiles were not systemically different between mutant and wild-type hypothalamus. The only transcript that was consistently different between genotypes was a transcript of the Kcnma1 gene itself. Notably, a more detailed analysis of Arntl (Bmal1) expression by in situ hybridization indicates that the rhythm in expression of this clock gene was not altered by the loss of the BK channel. So the BK channel does not seem to be important in the rhythmic expression of clock genes that lie at the heart of the generation of circadian oscillations. Overall, the authors conclude that BK channels are essential for the amplitude or ‘robustness’ of circadian rhythms.
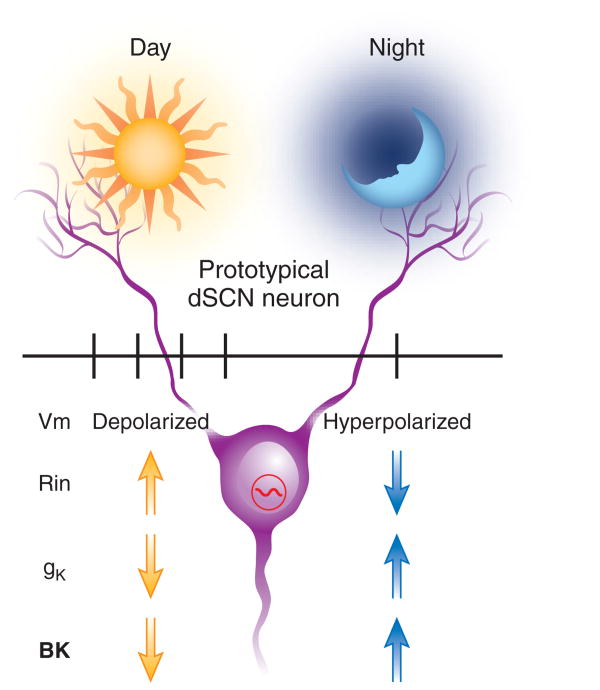
This set of experiments adds to our growing understanding of the ionic mechanisms responsible for the generation of rhythms in electrical activity in SCN neurons. It is likely that a set of intrinsic voltage-sensitive currents are responsible for the circadian variation in firing rate in SCN neurons (Fig. 1). One possibility is that these cells fluctuate between two subthreshold values, a hyperpolarized downstate and a depolarized upstate. During the upstate (day), neurons are spontaneously active and can fire action potentials. The largest source of the excitation driving the cell to generate spontaneous action potentials seems to be TTX-sensitive Na+ currents that open at subthreshold voltages9. The amplitude of the L-type Ca2+ current also shows a diurnal rhythm in the SCN and may well contribute to the excitatory drive during the day10. In addition, SCN neurons express a K+ current known as the fast delayed rectifier (fDR). This current is active in the times between the generation of action potentials during the day. In the SCN, the fDR is thought to be critical for translating membrane depolarization into a regular pattern of action potential firing11. During the downstate (night), the SCN neurons are electively inactive due to a membrane hyperpolarization associated with an increase in total membrane conductance12. The nightly upregulation of the BK current may be essential for the inactivity of SCN neurons during the night.
Figure 1.
Working model of the bimodal state of SCN neurons. The dSCN neurons exist in two stable electrical configurations. During the upstate (day), the cells show spontaneous activity; during the downstate (night), the cells are silent. A set of currents are responsible for each of these two states. At night, the SCN neurons are hyperpolarized due to a net increase in potassium conductance. The BK current may be responsible for this nightly silencing of SCN neurons.
Of course, like many good scientific studies, this new work1 raises more questions than it answers. It would have been good to see actual measurements of the BK currents in SCN neurons and to determine the impact of the loss of this channel on the daily rhythms in membrane potential and conductance that characterize SCN neurons. Future studies will need to go beyond demonstrating a correlation between the decrease in amplitude in the electrical rhythms of SCN neurons and the downstream behavior. There also remain some tricky questions about the mechanisms underlying how the molecular clock drives the rhythms in the BK protein and current. Another puzzle is that SCN neurons have circadian rhythms in calcium levels that peak during the day13,14, whereas the calcium-sensitive BK currents peak at night. Nonetheless, these studies are beginning to provide the information needed to understand the physiological underpinnings of the generation of spontaneous firing in SCN neurons. From a circadian perspective, it is important not only to identify those currents that regulate spontaneous firing but also to understand how those currents change from day to night to drive the neural activity rhythm. With some luck, this work will ultimately allow the manipulation of our circadian timing system, and fewer sleepless nights.
References
- 1.Meredith AL, et al. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaguchi S, et al. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 3.Maywood ES, et al. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Panda S, et al. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 5.Pitts GR, Ohta H, McMahon DG. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 6.Cloues RK, Sather WA. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitabach MN, Blau J, Holmes TC. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 8.Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. J Neurosci. 2005;25:762–766. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson AC, Yao GL, Bean BP. J Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- 11.Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhlman SJ, McMahon DG. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- 13.Colwell CS. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda M, et al. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]