Abstract
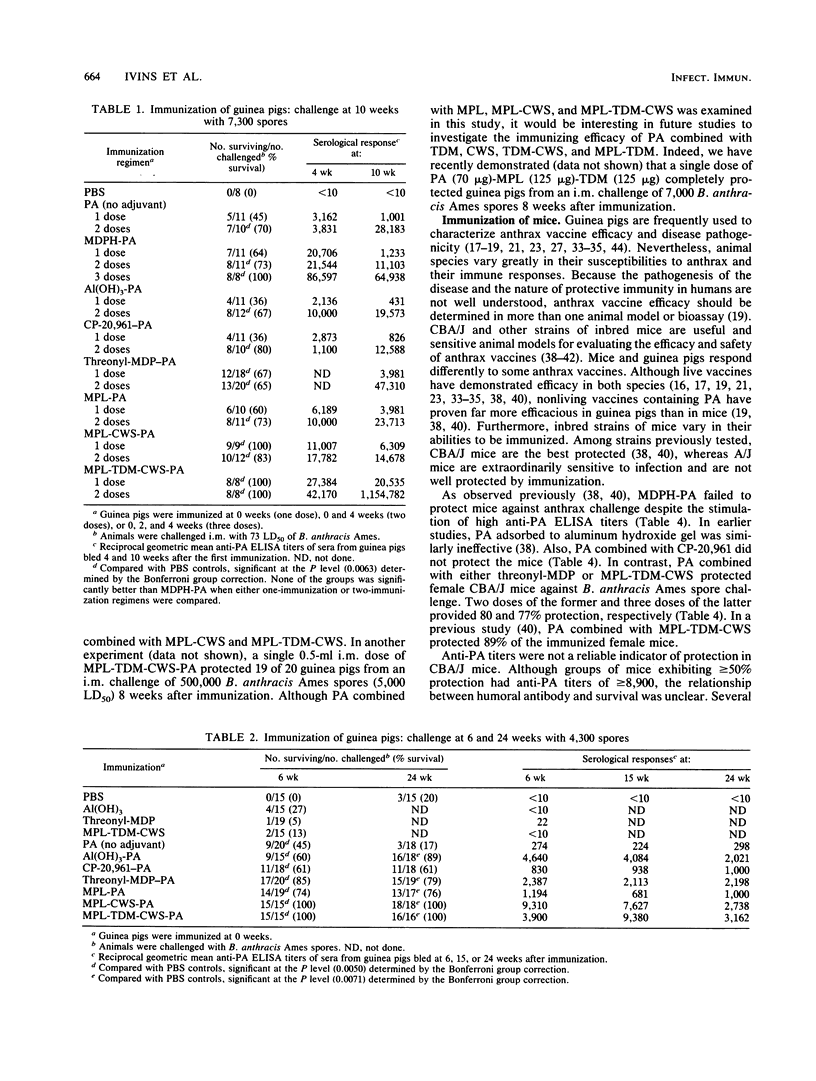
The protective efficacy of immunization against anthrax with Bacillus anthracis protective antigen (PA) combined with different adjuvants was tested in Hartley guinea pigs and CBA/J and A/J mice. Adjuvant components derived from microbial products that were tested included threonyl-muramyl dipeptide (threonyl-MDP); monophosphoryl lipid A (MPL); trehalose dimycolate (TDM); and the delipidated, deproteinized, cell wall skeleton (CWS) from either Mycobacterium phlei or the BCG strain of Mycobacterium bovis. Non-microbially derived adjuvants tested included aluminum hydroxide and the lipid amine CP-20,961. In guinea pigs, all adjuvants and adjuvant mixtures enhanced antibody titers to PA as well as survival after a parenteral challenge of virulent B. anthracis Ames spores. In contrast, PA alone or combined with either aluminum hydroxide or CP-20,961 failed to protect mice. Vaccines containing PA combined with threonyl-MDP or MPL-TDM-CWS protected a majority of female CBA/J mice. Statistical analysis of survival data in the guinea pigs indicated that PA-MPL-CWS and PA-MPL-TDM-CWS were more efficacious than the currently licensed human anthrax vaccine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. O., Reynolds J. A. Adjuvant effects of the lipid amine CP-20,961. J Reticuloendothel Soc. 1979 Dec;26(Suppl):667–680. [PubMed] [Google Scholar]
- Anderson A. O., Rubin D. H. Effect of Avridine on enteric antigen uptake and mucosal immunity to reovirus (1/Lang). Adv Exp Med Biol. 1985;186:579–590. doi: 10.1007/978-1-4613-2463-8_71. [DOI] [PubMed] [Google Scholar]
- Brachman P. S. Anthrax. Ann N Y Acad Sci. 1970 Oct 30;174(2):577–582. doi: 10.1111/j.1749-6632.1970.tb45583.x. [DOI] [PubMed] [Google Scholar]
- Brachman P. S., Gold H., Plotkin S. A., Fekety F. R., Werrin M., Ingraham N. R. Field Evaluation of a Human Anthrax Vaccine. Am J Public Health Nations Health. 1962 Apr;52(4):632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars N. E., Allison A. C. Adjuvant formulation for use in vaccines to elicit both cell-mediated and humoral immunity. Vaccine. 1987 Sep;5(3):223–228. doi: 10.1016/0264-410x(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P., Kaveri S. V., Byars N., Starkey J., Ferrone S., Raychaudhuri S. Human high molecular weight-melanoma associated antigen mimicry by an anti-idiotypic antibody: characterization of the immunogenicity and the immune response to the mouse monoclonal antibody IMel-1. Cancer Res. 1991 Nov 15;51(22):6045–6051. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. D., Battisti L., Koehler T. M., Thorne C. B., Ivins B. E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun. 1985 Aug;49(2):291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt H. C., Rosenstreich D. L. Trypanosoma rhodesiense infection in mice: sex dependence of resistance. Infect Immun. 1984 Jan;43(1):337–340. doi: 10.1128/iai.43.1.337-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono-Connors L. C., Schmaljohn C. S., Dalrymple J. M. Expression of the Bacillus anthracis protective antigen gene by baculovirus and vaccinia virus recombinants. Infect Immun. 1990 Feb;58(2):366–372. doi: 10.1128/iai.58.2.366-372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono-Connors L. C., Welkos S. L., Ivins B. E., Dalrymple J. M. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect Immun. 1991 Jun;59(6):1961–1965. doi: 10.1128/iai.59.6.1961-1965.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Ezzell J. W., Jr, Jemski J., Hedlund K. W., Ristroph J. D., Leppla S. H. Immunization studies with attenuated strains of Bacillus anthracis. Infect Immun. 1986 May;52(2):454–458. doi: 10.1128/iai.52.2.454-458.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Welkos S. L. Cloning and expression of the Bacillus anthracis protective antigen gene in Bacillus subtilis. Infect Immun. 1986 Nov;54(2):537–542. doi: 10.1128/iai.54.2.537-542.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Welkos S. L., Knudson G. B., Leblanc D. J. Transposon Tn916 mutagenesis in Bacillus anthracis. Infect Immun. 1988 Jan;56(1):176–181. doi: 10.1128/iai.56.1.176-181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Welkos S. L., Knudson G. B., Little S. F. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect Immun. 1990 Feb;58(2):303–308. doi: 10.1128/iai.58.2.303-308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Welkos S. L. Recent advances in the development of an improved, human anthrax vaccine. Eur J Epidemiol. 1988 Mar;4(1):12–19. doi: 10.1007/BF00152686. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- Little S. F., Knudson G. B. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun. 1986 May;52(2):509–512. doi: 10.1128/iai.52.2.509-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. S., Kan-Mitchell J., Kempf R. A., Harel W., Shau H. Y., Lind S. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1988 Oct 15;48(20):5883–5893. [PubMed] [Google Scholar]
- PUZISS M., MANNING L. C., LYNCH J. W., BARCLAYE, ABELOW I., WRIGHT G. G. Large-scale production of protective antigen of Bacillus anthracis in anaerobic cultures. Appl Microbiol. 1963 Jul;11:330–334. doi: 10.1128/am.11.4.330-334.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUZISS M., WRIGHT G. G. Studies on immunity in anthrax. X. Gel-adsorbed protective antigen for immunization of man. J Bacteriol. 1963 Jan;85:230–236. doi: 10.1128/jb.85.1.230-236.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman L. S., Gordon D. M., Wistar R., Jr, Krzych U., Gross M., Hollingdale M. R., Egan J. E., Chulay J. D., Hoffman S. L. Use of adjuvant containing mycobacterial cell-wall skeleton, monophosphoryl lipid A, and squalane in malaria circumsporozoite protein vaccine. Lancet. 1991 Apr 27;337(8748):998–1001. doi: 10.1016/0140-6736(91)92659-p. [DOI] [PubMed] [Google Scholar]
- Storch T. G., Chused T. M. Sex and H-2 haplotype control the resistance of CBA-BALB hybrids to the induction of T cell lymphoma by Moloney leukemia virus. J Immunol. 1984 Nov;133(5):2797–2800. [PubMed] [Google Scholar]
- Turnbull P. C., Broster M. G., Carman J. A., Manchee R. J., Melling J. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect Immun. 1986 May;52(2):356–363. doi: 10.1128/iai.52.2.356-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C., Leppla S. H., Broster M. G., Quinn C. P., Melling J. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med Microbiol Immunol. 1988;177(5):293–303. doi: 10.1007/BF00189414. [DOI] [PubMed] [Google Scholar]
- Vosika G. J., Barr C., Gilbertson D. Phase-I study of intravenous modified lipid A. Cancer Immunol Immunother. 1984;18(2):107–112. doi: 10.1007/BF00205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT G. G., GREEN T. W., KANODE R. G., Jr Studies on immunity in anthrax. V. Immunizing activity of alum-precipitated protective antigen. J Immunol. 1954 Dec;73(6):387–391. [PubMed] [Google Scholar]
- Welkos S. L., Friedlander A. M. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb Pathog. 1988 Aug;5(2):127–139. doi: 10.1016/0882-4010(88)90015-0. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Friedlander A. M. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb Pathog. 1988 Jan;4(1):53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Keener T. J., Gibbs P. H. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986 Mar;51(3):795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L., Trotter R. W., Becker D. M., Nelson G. O. Resistance to the Sterne strain of B. anthracis: phagocytic cell responses of resistant and susceptible mice. Microb Pathog. 1989 Jul;7(1):15–35. doi: 10.1016/0882-4010(89)90108-3. [DOI] [PubMed] [Google Scholar]


