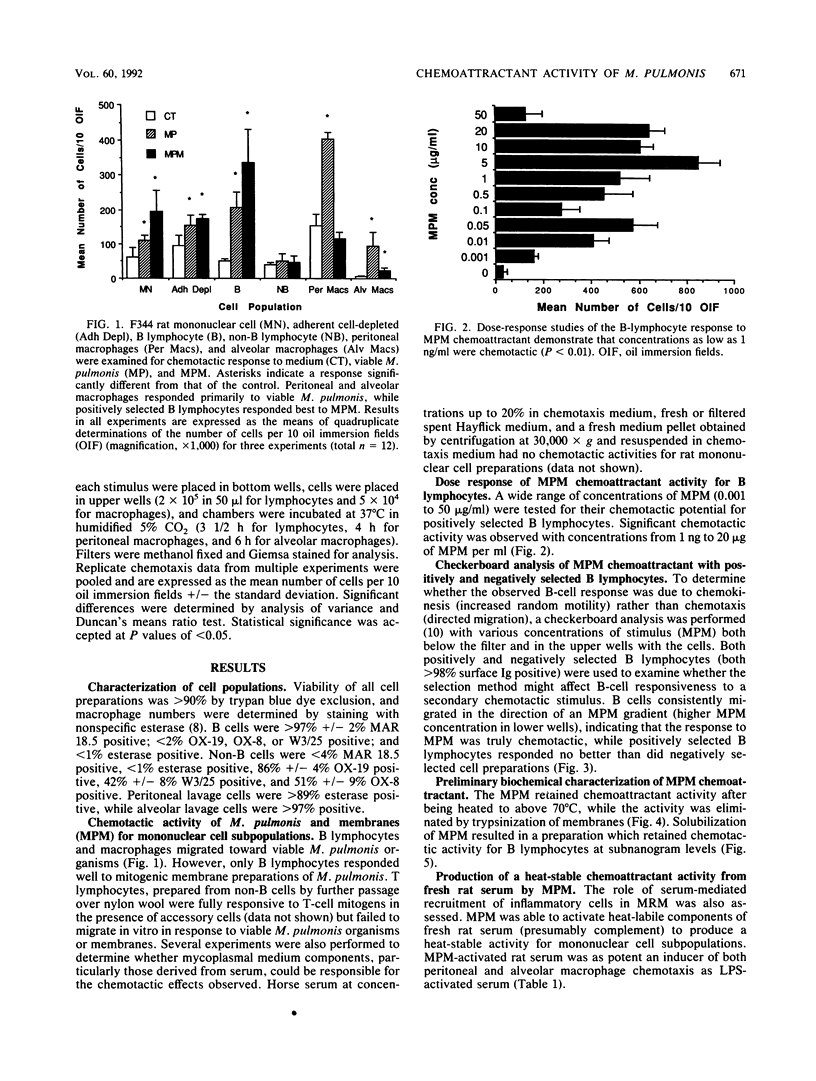
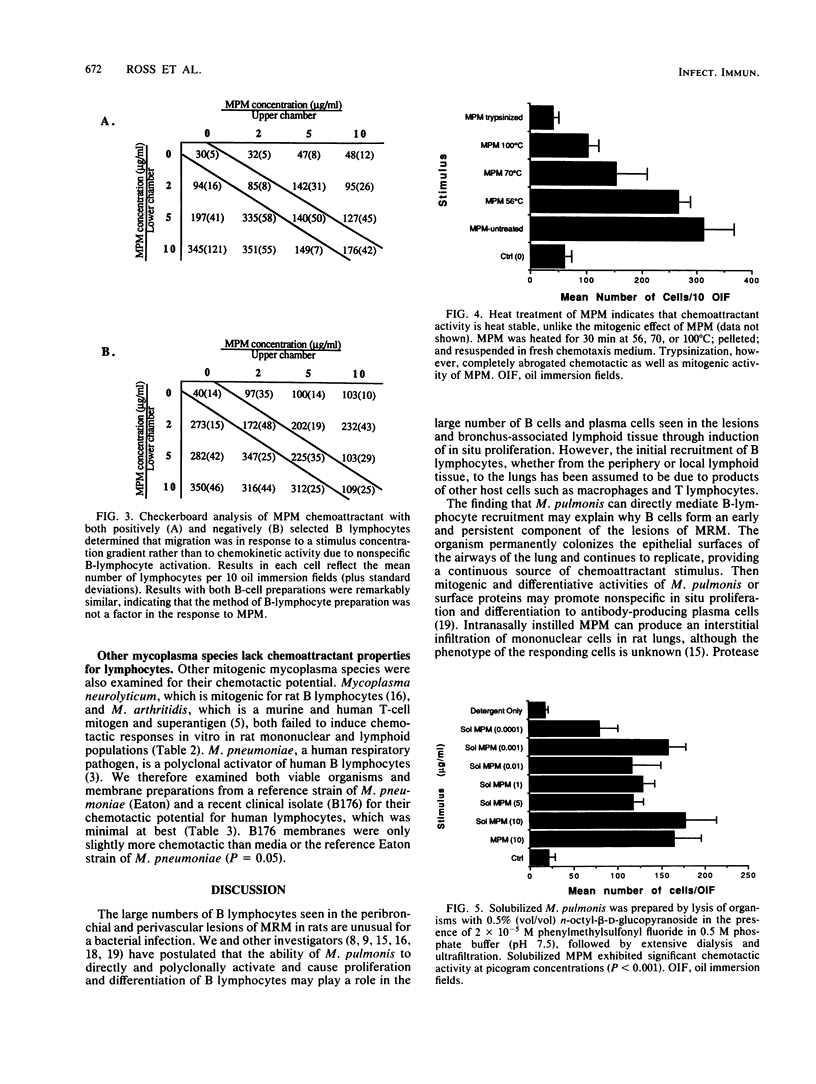
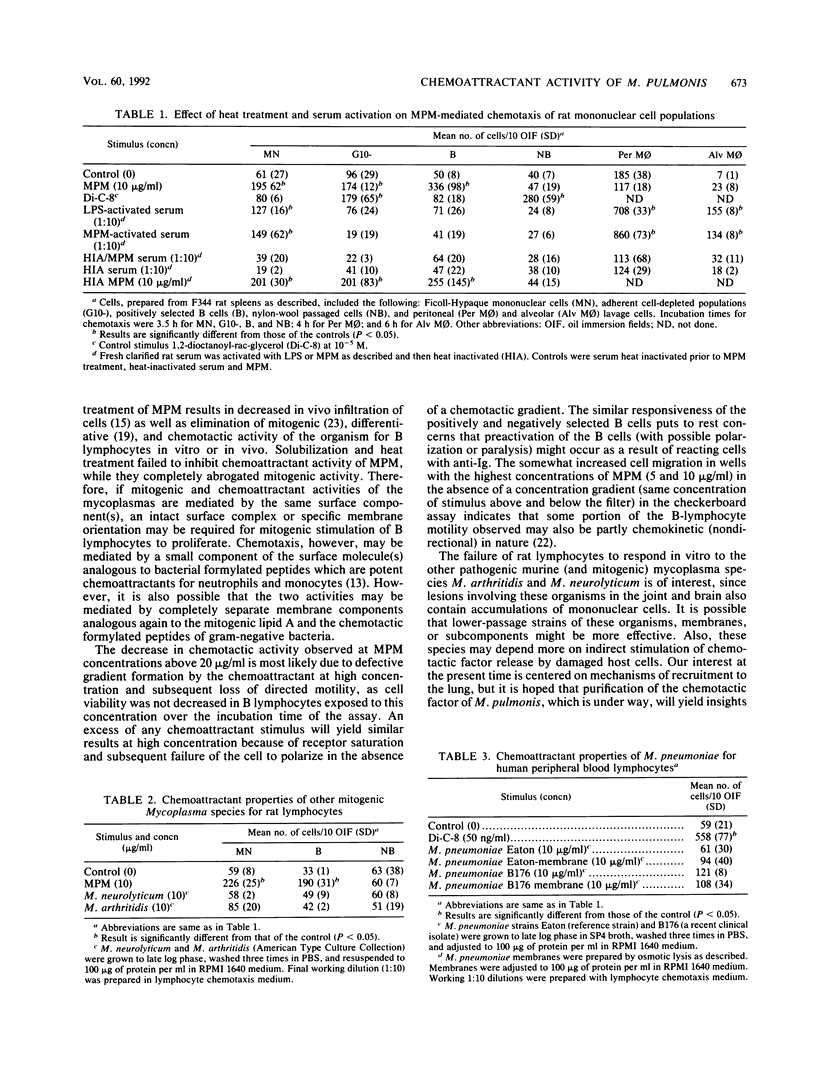
Abstract
Mycoplasma pulmonis causes chronic murine respiratory mycoplasmosis, which is characterized by extensive peribronchial and perivascular infiltration of mononuclear cells, including B lymphocytes. B-lymphocyte recruitment into sites of inflammation is presently poorly understood but must involve directed chemotaxis of these cells in response to some external recruitment stimulus. In these studies, picogram amounts of M. pulmonis membrane protein were found to possess potent chemoattractant activity for resting rat B lymphocytes. This report is the first description of a bacterially derived chemoattractant for B lymphocytes and offers a unique opportunity to study regulation of B-lymphocyte recruitment to a site of chronic pulmonary inflammation. Furthermore, M. pulmonis membrane activation of fresh rat serum was found to produce a potent stimulus for recruitment of peritoneal and alveolar macrophages. M. pulmonis-mediated recruitment of lymphocytes and macrophages may play a significant role in the pathogenesis of murine respiratory mycoplasmosis, a role in which organisms on the bronchiolar epithelial surfaces may release proteins which can directly or indirectly promote chemotaxis of inflammatory cells from the circulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. S., Beer D. J., Theodore A. C., Kornfeld H., Bernardo J., Center D. M. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990 Jul;142(1):238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- Berman J. S., Cruikshank W. W., Beer D. J., Kornfeld H., Bernardo J., Theodore A. C., Center D. M. Lymphocyte motility and lymphocyte chemoattractant factors. Immunol Invest. 1988 Nov-Dec;17(8-9):625–677. doi: 10.3109/08820138809089017. [DOI] [PubMed] [Google Scholar]
- Biberfeld G. Activation of human lymphocyte subpopulations by Mycoplasma pneumoniae. Scand J Immunol. 1977;6(11):1145–1150. doi: 10.1111/j.1365-3083.1977.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis (MAM). VIII. Selective activation of T cells expressing distinct V beta T cell receptors from various strains of mice by the "superantigen" MAM. J Immunol. 1990 Jan 15;144(2):425–431. [PubMed] [Google Scholar]
- Czinn S. J., Lamm M. E. Selective chemotaxis of subsets of B lymphocytes from gut-associated lymphoid tissue and its implications for the recruitment of mucosal plasma cells. J Immunol. 1986 May 15;136(10):3607–3611. [PubMed] [Google Scholar]
- Davis J. K., Maddox P. A., Thorp R. B., Cassell G. H. Immunofluorescent characterization of lymphocytes in lungs of rats infected with Mycoplasma pulmonis. Infect Immun. 1980 Jan;27(1):255–259. doi: 10.1128/iai.27.1.255-259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee A. P. Advantages and limitations of methods for measuring cellular chemotaxis and chemokinesis. Mol Cell Biochem. 1984 Apr;62(1):5–11. doi: 10.1007/BF00230072. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Honda M., Mibu Y., Yamamoto S., Hirashima M. Natural mediators of leukocyte chemotaxis. Methods Enzymol. 1988;162:140–170. doi: 10.1016/0076-6879(88)62072-6. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Chemotaxis. Curr Opin Immunol. 1989 Oct;2(1):19–27. doi: 10.1016/0952-7915(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Lai W. C., Pakes S. P., Lu Y. S., Brayton C. F. Mycoplasma pulmonis infection augments natural killer cell activity in mice. Lab Anim Sci. 1987 Jun;37(3):299–303. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Naot Y., Davidson S., Lindenbaum E. S. Mitogenicity and pathogenicity of Mycoplasma pulmonis in rats. I. Atypical interstitial pneumonia induced by mitogenic myeoplasmal membranes. J Infect Dis. 1981 Jan;143(1):55–62. doi: 10.1093/infdis/143.1.55. [DOI] [PubMed] [Google Scholar]
- Naot Y., Ginsburg H. Activation of B lymphocytes by mycoplasma mitogen(s). Immunology. 1978 Apr;34(4):715–720. [PMC free article] [PubMed] [Google Scholar]
- Pilaro A. M., Sayers T. J., McCormick K. L., Reynolds C. W., Wiltrout R. H. An improved in vitro assay to quantitate chemotaxis of rat peripheral blood large granular lymphocytes (LGL). J Immunol Methods. 1990 Dec 31;135(1-2):213–223. doi: 10.1016/0022-1759(90)90275-z. [DOI] [PubMed] [Google Scholar]
- Ruuth E., Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989 Dec;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Cassell G. H., Woodward J. G. Induction of class II MHC antigen expression in macrophages by Mycoplasma species. J Immunol. 1989 May 15;142(10):3392–3399. [PubMed] [Google Scholar]
- Wilkinson P. C. Relation between locomotion, chemotaxis and clustering of immune cells. Immunology. 1990 Jan;69(1):127–133. [PMC free article] [PubMed] [Google Scholar]
- Williamson J. S., Davis J. K., Cassell G. H. Polyclonal activation of rat splenic lymphocytes after in vivo administration of Mycoplasma pulmonis and its relation to in vitro response. Infect Immun. 1986 May;52(2):594–599. doi: 10.1128/iai.52.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]



