Abstract
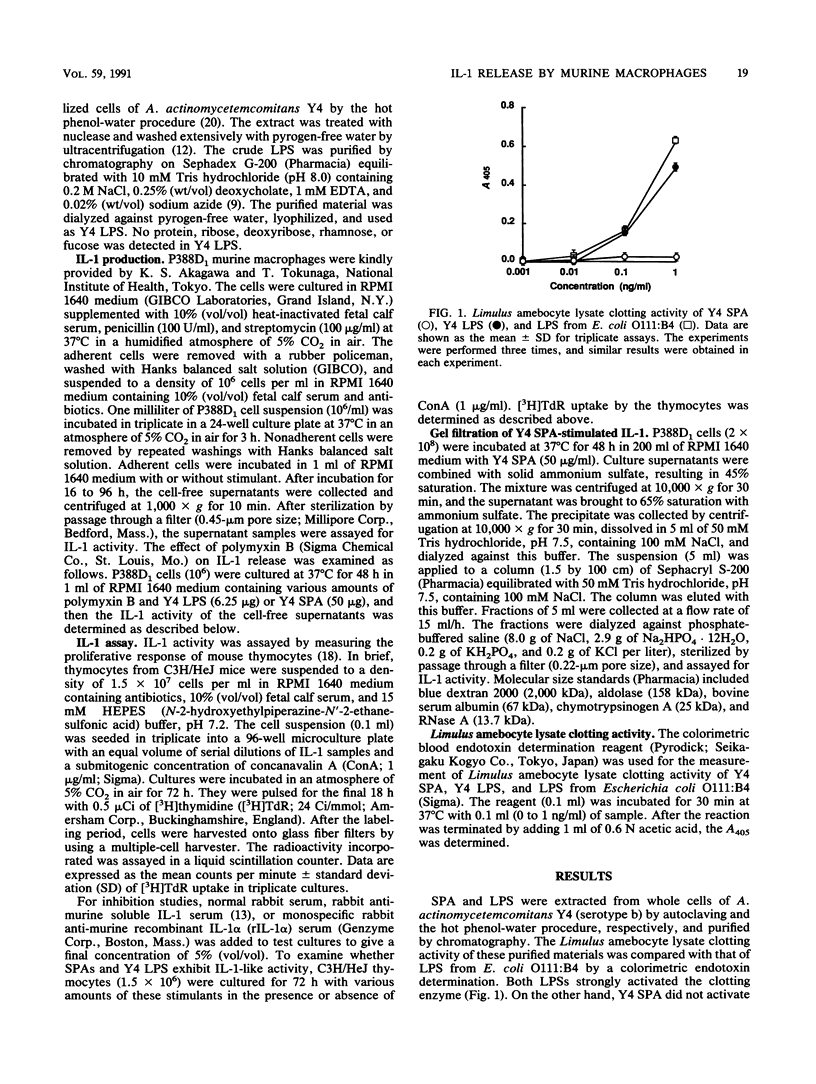
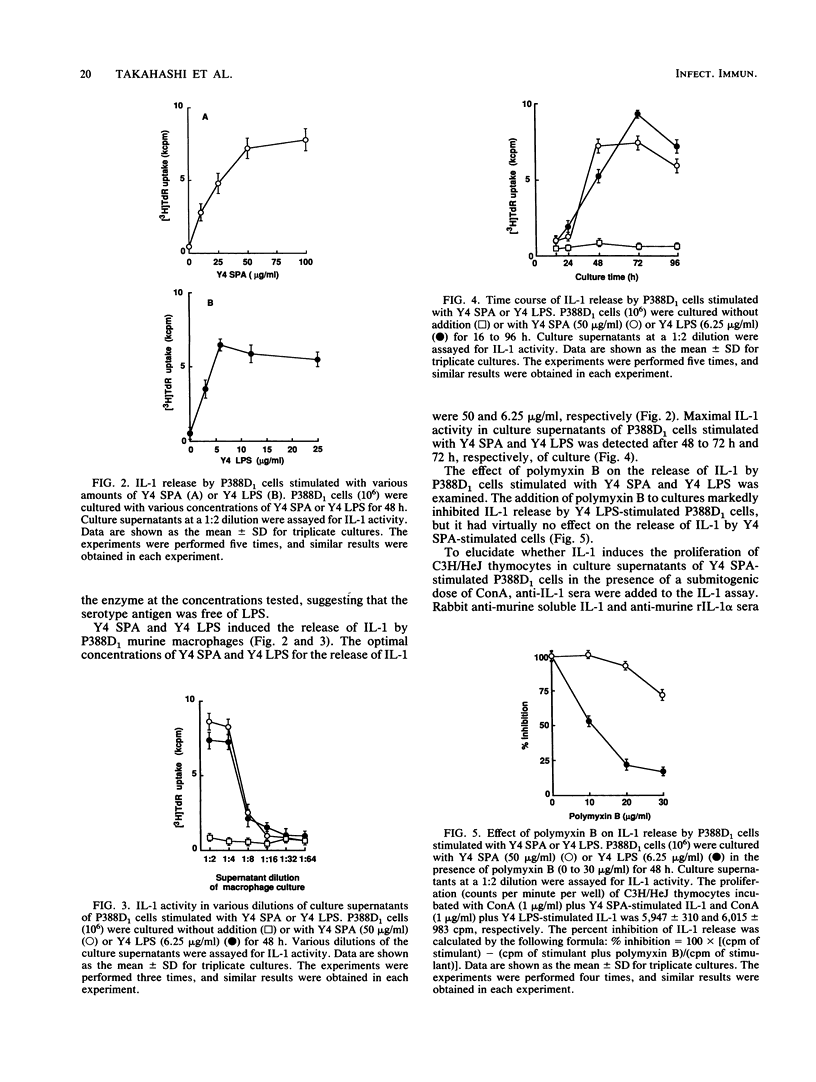
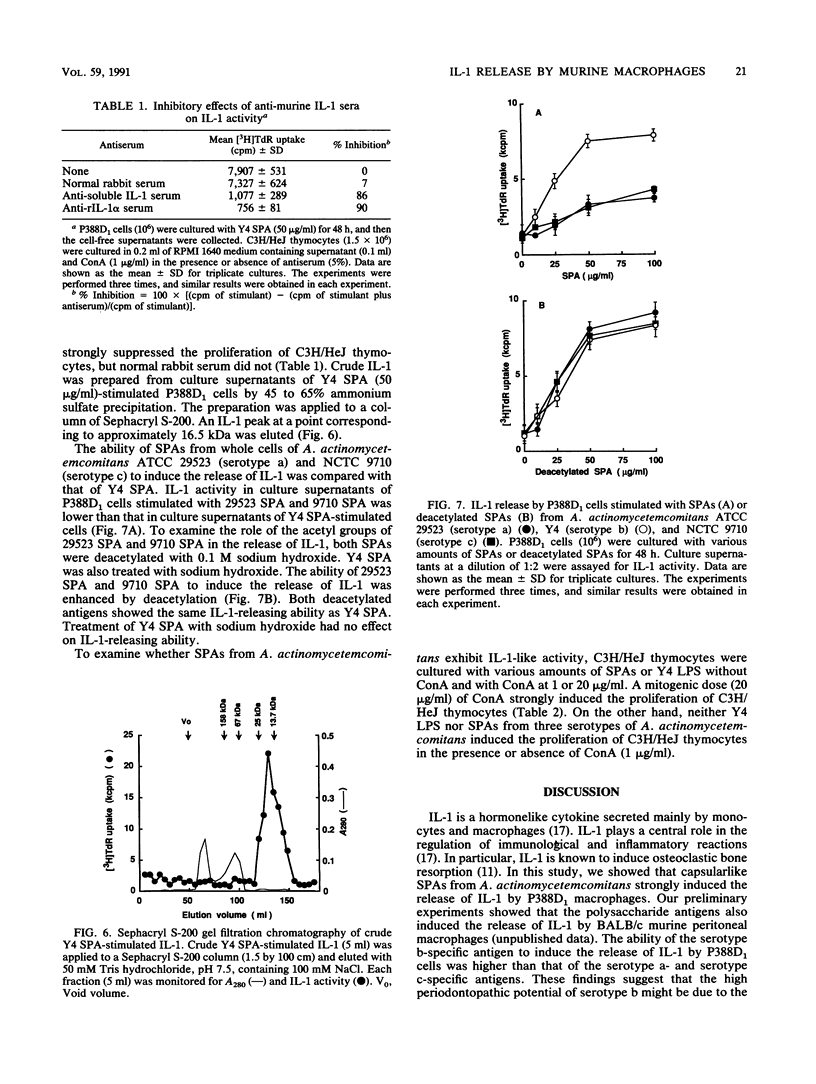
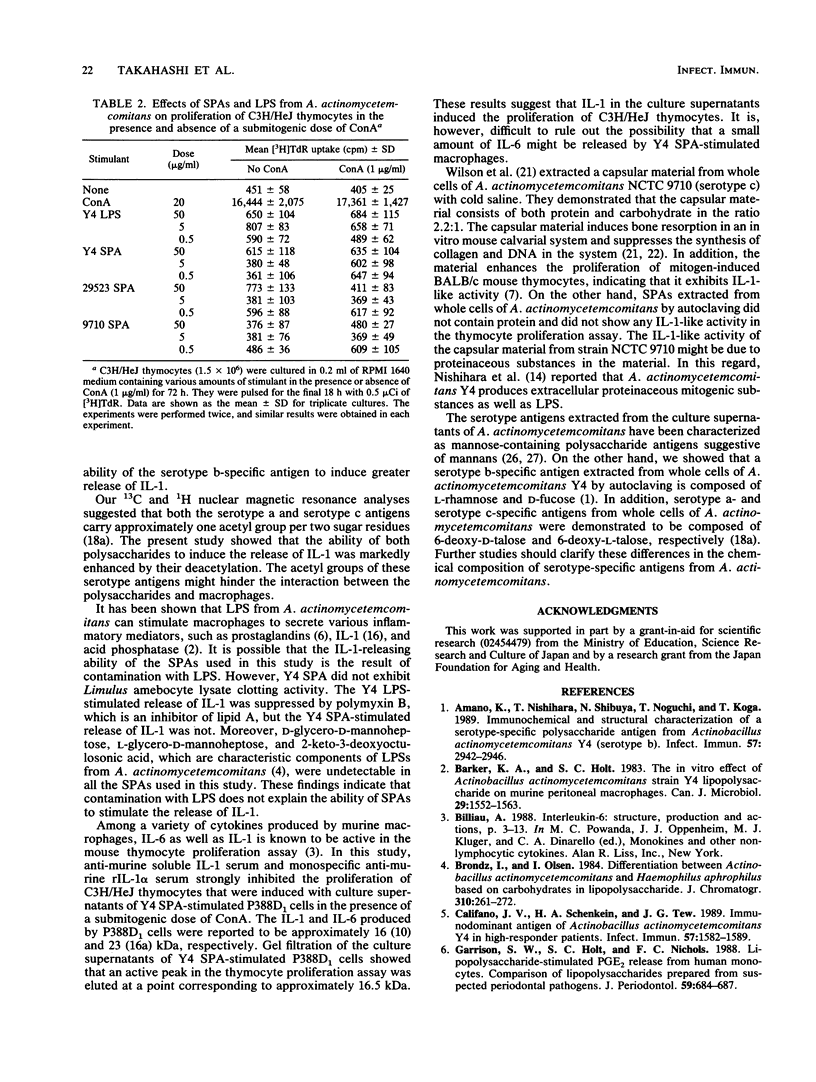
Serotype-specific polysaccharide antigens (SPAs) were extracted from whole cells of Actinobacillus actinomycetemcomitans ATCC 29523 (serotype a), Y4 (serotype b), and NCTC 9710 (serotype c) by autoclaving and purified by chromatography on DEAE-Sephadex A-25 and Sephacryl S-300 columns. Y4 SPA induced interleukin-1 (IL-1) release by P388D1 murine macrophages. Polymyxin B had virtually no effect on the release of IL-1. Rabbit anti-murine IL-1 serum strongly suppressed the proliferation of C3H/HeJ mouse thymocytes induced with the culture supernatants of Y4 SPA-stimulated P388D1 cells and a submitogenic dose of concanavalin A. Gel filtration of the culture supernatants of Y4 SPA-stimulated macrophages on Sephacryl S-200 showed that an IL-1 peak at a point corresponding to approximately 16.5 kDa was eluted. The ability of SPAs from strains ATCC 29523 and NCTC 9710 to induce the release of IL-1 was lower than that of Y4 SPA. The IL-1-releasing ability of serotype a and c antigens was enhanced by deacetylation of both polysaccharides, suggesting that acetyl groups of these antigens might hinder the interaction between the antigens and macrophages.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Nishihara T., Shibuya N., Noguchi T., Koga T. Immunochemical and structural characterization of a serotype-specific polysaccharide antigen from Actinobacillus actinomycetemcomitans Y4 (serotype b). Infect Immun. 1989 Oct;57(10):2942–2946. doi: 10.1128/iai.57.10.2942-2946.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K. A., Holt S. C. The in vitro effect of Actinobacillus actinomycetemcomitans strain Y4 lipopolysaccharide on murine peritoneal macrophages. Can J Microbiol. 1983 Nov;29(11):1552–1563. doi: 10.1139/m83-238. [DOI] [PubMed] [Google Scholar]
- Brondz I., Olsen I. Differentiation between Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus based on carbohydrates in lipopolysaccharide. J Chromatogr. 1984 Oct 12;310(2):261–272. doi: 10.1016/0378-4347(84)80091-2. [DOI] [PubMed] [Google Scholar]
- Califano J. V., Schenkein H. A., Tew J. G. Immunodominant antigen of Actinobacillus actinomycetemcomitans Y4 in high-responder patients. Infect Immun. 1989 May;57(5):1582–1589. doi: 10.1128/iai.57.5.1582-1589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison S. W., Holt S. C., Nichols F. C. Lipopolysaccharide-stimulated PGE2 release from human monocytes. Comparison of lipopolysaccharides prepared from suspected periodontal pathogens. J Periodontol. 1988 Oct;59(10):684–687. doi: 10.1902/jop.1988.59.10.684. [DOI] [PubMed] [Google Scholar]
- Harvey W., Kamin S., Meghji S., Wilson M. Interleukin-1-like activity in capsular material from Haemophilus actinomycetemcomitans. Immunology. 1987 Mar;60(3):415–418. [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Weber D. J., Oddone E. Z., Perfect J. R. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev Infect Dis. 1989 Jan-Feb;11(1):46–63. doi: 10.1093/clinids/11.1.46. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., Lucken R., Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Apr;52(1):76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosentreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by a macrophage cell line, P388D1. II. Biochemical characterization of LAF induced by activated T cells and LPS. J Immunol. 1978 May;120(5):1504–1508. [PubMed] [Google Scholar]
- Nishihara T., Fujiwara T., Koga T., Hamada S. Chemical composition and immunobiological properties of lipopolysaccharide and lipid-associated proteoglycan from Actinobacillus actinomycetemcomitans. J Periodontal Res. 1986 Sep;21(5):521–530. doi: 10.1111/j.1600-0765.1986.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Nishihara T., Ishihara Y., Noguchi T., Koga T. Membrane IL-1 induces bone resorption in organ culture. J Immunol. 1989 Sep 15;143(6):1881–1886. [PubMed] [Google Scholar]
- Nishihara T., Koga T., Hamada S. Extracellular proteinaceous substances from Haemophilus actinomycetemcomitans induce mitogenic responses in murine lymphocytes. Oral Microbiol Immunol. 1987 Mar;2(1):48–52. doi: 10.1111/j.1399-302x.1987.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Nishihara T., Koga T., Hamada S. Production of an interleukin-1 inhibitor by cell line P388D1 murine macrophages stimulated with Haemophilus actinomycetemcomitans lipopolysaccharide. Infect Immun. 1988 Nov;56(11):2801–2807. doi: 10.1128/iai.56.11.2801-2807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara T., Koga T., Hamada S. Suppression of murine macrophage interleukin-1 release by the polysaccharide portion of Haemophilus actinomycetemcomitans lipopolysaccharide. Infect Immun. 1988 Mar;56(3):619–625. doi: 10.1128/iai.56.3.619-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordan R. P., Pumphrey J. G., Rudikoff S. Purification and NH2-terminal sequence of a plasmacytoma growth factor derived from the murine macrophage cell line P388D1. J Immunol. 1987 Aug 1;139(3):813–817. [PubMed] [Google Scholar]
- Oppenheim J. J., Shneyour A., Kook A. I. Enhancement of DNA synthesis and cAMP content of mouse thymocytes by mediator(s) derived from adherent cells. J Immunol. 1976 May;116(5):1466–1472. [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Wilson M., Kamin S., Harvey W. Bone resorbing activity of purified capsular material from Actinobacillus actinomycetemcomitans. J Periodontal Res. 1985 Sep;20(5):484–491. doi: 10.1111/j.1600-0765.1985.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Wilson M., Meghji S., Harvey W. Effect of capsular material from Haemophilus actinomycetemcomitans on bone collagen synthesis in vitro. Microbios. 1988;54(220-221):181–185. [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Christersson L. A., Genco R. J. Diagnosis and treatment of localized juvenile periodontitis. J Am Dent Assoc. 1986 Aug;113(2):295–299. doi: 10.14219/jada.archive.1986.0152. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Slots J., Genco R. J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983 Jul;41(1):19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Slots J., Miyasaki K., Linzer R., Cohen R., Levine M., Genco R. J. Purification and characterization of the serotype c antigen from Actinobacillus actinomycetemcomitans. Infect Immun. 1984 Apr;44(1):22–27. doi: 10.1128/iai.44.1.22-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Umemoto T., De Nardin E., Nakazawa F., Christersson L. A., Genco R. J. Actinobacillus actinomycetemcomitans in the pathogenesis of human periodontal disease. Adv Dent Res. 1988 Nov;2(2):269–274. doi: 10.1177/08959374880020021101. [DOI] [PubMed] [Google Scholar]



