Abstract
Understanding the development of cortical interneuron phenotypic diversity is critical because interneuron dysfunction has been implicated in several neurodevelopmental disorders. Here, tyrosine hydroxylase (TH)-immunoreactive neurons in the developing and adult rat cortex were characterized in light of findings regarding interneuron neurochemistry and development. Cortical TH-immunoreactive neurons were first observed two weeks postnatally and peaked in number three weeks after birth. At subsequent ages, the number of these cell profiles was gradually reduced, and they were seen less frequently in adults. No DNA fragmentation or active caspase 3 was observed in cortical TH cells at any age examined, eliminating cell death as an explanation for the decrease in cell number. Although cortical TH cells reportedly fail to produce subsequent catecholaminergic enzymes, we found that the majority of these cells at all ages contained phosphorylated TH, suggesting that the enzyme may be active and producing L-DOPA as an end-product. Morphological criteria and colocalization of some TH cells with glutamic acid decarboxylase suggests that these cells are interneurons. Previously, parvalbumin, somatostatin, and calretinin were demonstrated in nonoverlapping subsets of interneurons. Cortical TH neurons colocalized with calretinin but not with parvalbumin or somatostatin. These findings suggest that the transitory increase in TH cell number is not due to cell death but possibly due to alterations in the amount of detectable TH present in these cells, and that at least some cortical TH-producing interneurons belong to the calretinin-containing subset of interneurons that originate developmentally in the caudal ganglionic eminence.
Keywords: Tyrosine hydroxylase, Cerebral cortex, Interneuron, Neurotransmitter, Programmed Cell Death, Calretinin
1. Introduction
Although interneurons comprise approximately 25% of total mammalian cerebral cortical neurons, they play a significant role in cortical processing (DeFelipe, 2002; Markram et al., 2004). The heterogeneity of cortical interneuron morphology and neurochemistry presents a complex, incompletely understood picture of neural development (Wonders and Anderson, 2006). Moreover, cortical interneuron dysfunction has been implicated in several neurological disorders with developmental origins (Levitt et al., 2004; Di Cristo, 2007), including epilepsy (Powell et al., 2003; Garbelli et al., 2006), schizophrenia (Reynolds et al., 2002; Lewis et al., 2005; Daskalakis et al., 2007), and depression (Rajkowska et al., 2007). The current study examined a subpopulation of rat cortical interneurons that produced tyrosine hydroxylase (TH), the first, rate-limiting catecholaminergic enzyme. Little is known about this subset of cortical cells. A more complete understanding of the phenotype and development of cortical TH-producing neurons is warranted because of the potential relevance of these cells to cortical circuitry across species (Benavides-Piccione and DeFelipe, 2007). Degeneration of TH-immunoreactive (IR) neurons in the human cortex, for example, is seen in Parkinson’s disease (Fukuda et al., 1999) and dementia (Marui et al., 2003).
Unlike projection neurons, cortical interneurons arise in subcortical forebrain sites, the medial and caudal ganglionic eminences (MGE and CGE, respectively), and then migrate tangentially into the developing cortex (Anderson et al., 1997; Wichterle et al., 2001; Nery et al., 2002; Flames and Marin, 2005; Wonders and Anderson, 2006). In addition to gamma aminobutyric acid (GABA), the calcium-binding proteins parvalbumin and calretinin and the peptide somatostatin are present in three non-overlapping subsets of inhibitory interneurons (Kubota et al., 1994; Gonchar and Burkhalter, 1997; Xu et al., 2004). Interneurons that express parvalbumin and somatostatin migrate from the MGE, whereas those producing calretinin arise in the CGE (Wichterle et al., 2001; Nery et al., 2002; Xu et al., 2004).
A subset of cortical neurons also expresses TH in the rodent (Berger et al., 1985; Kosaka et al., 1987a; 1987b; Satoh and Suzuki, 1990), non-human primate (Weihe et al., 2006), and human (Gaspar et al., 1987; Hornung et al., 1989; Kuljis et al., 1989; Trottier et al., 1989; Fukuda et al., 1999; Ikemoto et al., 1999; Benavides-Piccione and DeFelipe, 2003; Marui et al., 2003; Benavides-Piccione and DeFelipe, 2007). Based on their size, morphology, and partial colocalization with GABA, cortical TH-IR cells were hypothesized to be interneurons. These TH cells, however, remain enigmatic because of their species-specific laminar distribution, lack of additional catecholaminergic traits, and developmental characteristics. In the rat cortex, TH-IR neurons were observed mainly in layer II/III (Berger et al., 1985; Kosaka et al., 1987a; 1987b), whereas in the mouse (Satoh and Suzuki, 1990) and human (Gaspar et al., 1987; Hornung et al., 1989; Kuljis et al., 1989; Trottier et al., 1989; Ikemoto et al., 1999; Benavides-Piccione and DeFelipe, 2003; Marui et al., 2003; Benavides-Piccione and DeFelipe, 2007), TH-IR neurons were seen predominantly in layers V and VI. Not only are these TH neurons outside the classically-defined catecholaminergic cell groups (Hokfelt et al., 1984), but their final transmitter phenotype is also uncertain. In all species examined, cortical TH-IR cells failed to express any catecholaminergic enzymes subsequent to TH, including aromatic amino acid decarboxylase (AADC), which synthesizes dopamine (Berger et al., 1985; Gaspar et al., 1987; Ikemoto et al., 1999; Weihe et al., 2006). Although very few TH-IR cells were noted in the adult rat cortex in classical TH distribution studies (Hokfelt et al., 1984), later reports described a population of cortical TH-IR cells that were observed transiently during postnatal development in the rat (Berger et al., 1985) and mouse (Satoh and Suzuki, 1990). Other reports, in contrast, demonstrated TH-IR cells in the adult rat cortex (Kosaka et al., 1987a; 1987b).
The current study characterized TH-IR neurons in the rat cortex with the goal of addressing several unresolved questions. To confirm previous reports describing transitory TH immunoreactivity in the developing rat cortex (Berger et al., 1985), we analyzed the number of cortical TH-IR cells over a range of postnatal ages and found a transient peak followed by a sharp decline in the number of TH-IR cells. One explanation for this decline in TH neuron number is that these cells undergo programmed cell death. Previous studies demonstrated DNA fragmentation, an indicator of apoptosis, in cortical cells at ages partially overlapping those during which the number of TH-IR neurons decreased (Ferrer et al., 1994; Spreafico et al., 1995; Thomaidou et al, 1997). Our assays revealed no DNA fragmentation in cortical TH-IR neurons. Because DNA fragmentation is a relatively late event during apoptosis, we double-labeled for TH and active caspase 3, a key effector enzyme activated during the apoptotic caspase-cascade (Danial and Korsmeyer, 2004). No immunoreactivity for active caspase 3 was observed in cortical TH cells. Because phosphorylation regulates TH enzyme activity (Xu et al., 1998; Lew et al., 1999; Witkovsky et al., 2004; Bobrovskaya et al., 2004), we assessed TH activity and the possibility of L-3,4-dihydroxyphenylalanine (L-DOPA) production in these cells by immunostaining for one of the phosphorylated forms of TH. The majority of cortical TH-IR cells were colabeled by an antibody against phosphorylated TH. Some cortical TH-IR cells coexpressed the GABA-synthetic enzyme glutamic acid decarboxylase (GAD). Finally, to categorize TH neurons into one or more of the three distinct subsets of cortical interneurons, which concomitantly addressed their embryonic origin, we colocalized TH with parvalbumin, somatostatin, and calretinin. A subset of cortical TH-IR neurons colocalized with calretinin but not with parvalbumin or somatostatin, supporting the hypothesis that at least some of these cells originated in the CGE.
2. Results
At all ages examined, TH immunostaining revealed classical catecholaminergic cell bodies and/or fibers in every brain section, providing an internal positive control for immunostaining of cortical neurons. The shape, distribution and transient developmental appearance of TH-IR neurons in the cerebral cortex were similar to characteristics of cells previously reported in the developing rodent cortex (Berger et al., 1985; Satoh and Suzuki, 1990). In brains of all ages studied, cortical TH-IR somata profiles were typically less than 15 µm across their long axis, primarily fusiform and bipolar, occasionally multipolar, but not pyramidal (Fig. 1). These neurons were scattered throughout the neocortex, concentrated predominantly in cortical areas dorsal and dorsolateral to the striatum and anterior hippocampus. Isolated TH-IR cells were also present in the cingulate cortex. To examine cytoarchitectural distribution, occasional sections were double-labeled for TH and the neuron-specific protein NeuN (Mullen et al., 1992). Neurons positive for TH were colabeled with NeuN (data not shown) and were observed predominantly in layer II/III, with occasional cells present in deeper layers.
Figure 1.
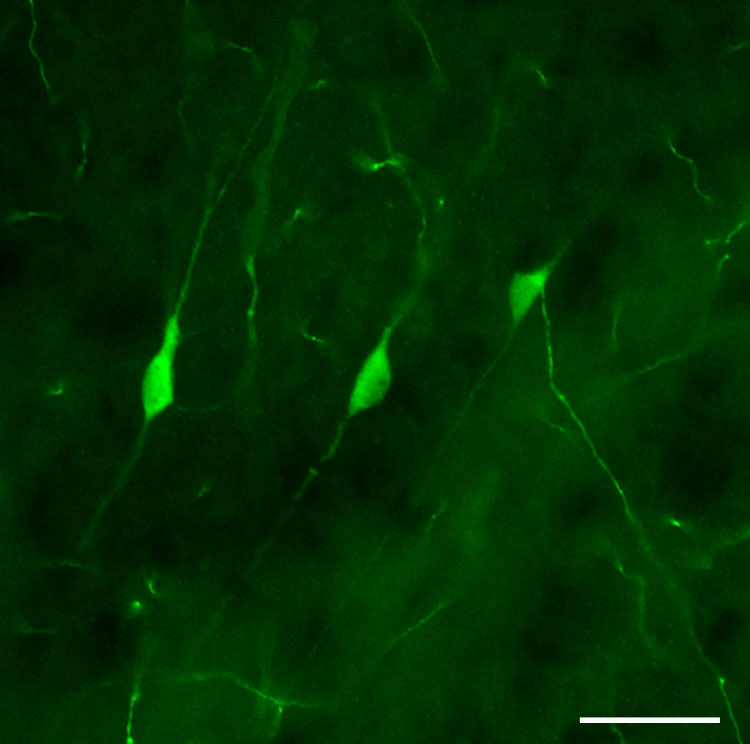
Photomicrograph of nonpyramidal cortical TH-IR neurons in a P16 rat, illustrating their small size (less than 15 µm) and typically fusiform morphology. Scale bar = 20 µm.
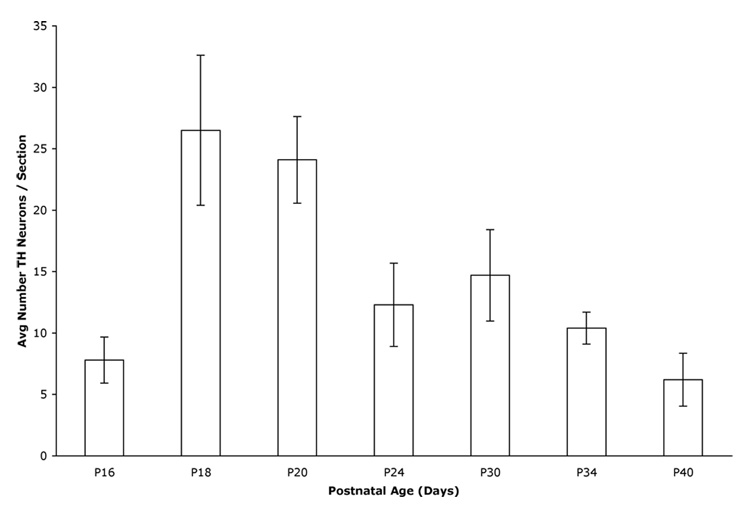
As reported previously (Berger et al., 1985), we observed very few cortical TH-IR neurons prior to postnatal day (P) 12. The analysis of ages from P16 to P40 (adult) revealed a bell-shaped distribution of the number of cortical TH-IR cell profiles, with a peak in cell profile number at P18 and P20 and a subsequent decline through P40 (Fig. 2). Although fewer in number, TH-IR cell profiles were still observed in the adult cortex. Statistical analysis showed significant differences in the relative number of cortical TH-IR cell profiles over time (1-way ANOVA; F(6,27) = 4.85, P = 0.0018). Post-hoc analysis (Tukey's) demonstrated a significant increase in the relative number of TH-IR cell profiles between P16 and P18 and a significant decrease in cell profile number between P18 and P34. To amplify staining intensity, brain sections from two adult rats were immunostained for TH using biotinylated secondary antibodies visualized with fluorochrome-conjugated streptavidin (data not shown). The number of cortical TH-IR cell profiles per section was identical to that observed using secondaries directly conjugated to fluorochrome.
Figure 2.
Graph representing the average number of TH-IR cell profiles per section at each postnatal age. Each bar (with SEM) represents the average number of cortical TH-IR neurons per section per animal at that age. The relative number of cortical TH-IR cell profiles varied significantly over time during postnatal development (1-way ANOVA; F(6,27) = 4.85, P = 0.0018). Post-hoc analysis (Tukey's) showed significant differences between P16 and P18-20, between P18 and P34-40, and between P20 and P40.
To test the hypothesis that the apparent transience of cortical TH-IR neurons is due to programmed cell death, brain sections were labeled for TH and for two markers indicative of cell death, DNA fragmentation and the active form of the proapoptotic enzyme caspase 3 (Danial and Korsmeyer, 2004). DNA fragmentation was assayed using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL), and active caspase 3 was identified immunohistochemically. Control experiments using Chinese hamster ovary (CHO) cells and sections of developing rat paw confirmed that the detection methods used here labeled dying cells in vitro and in situ, respectively (data not shown). Two to four hours after UV light exposure, which induced apoptosis, many CHO cells were TUNEL-positive and immunoreactive for active caspase 3. In sections of rat paw at embryonic day (E) 16.5, the age at which cell death was previously observed in this tissue (Lee et al., 1993), TUNEL-positive and active caspase 3-IR cells were seen in the interdigital mesenchyme where cell death normally occurs.
The developing cortex contained scattered TUNEL-positive and active caspase 3-IR cells. As reported previously (Ferrer et al., 1994; Spreafico et al., 1995; Thomaidou et al, 1997), apoptotic cells were still present three weeks after birth, decreasing in frequency with increasing age. However, double-labeling for TH and TUNEL at P14, P16, P20, and P24 failed to demonstrate DNA fragmentation in cortical TH-IR neurons (data not shown). Likewise, no cortical TH-IR neurons contained active caspase 3 immunoreactivity at any age examined in the current study (Fig. 3 and Table 1). The complete absence of cell death markers suggests that cortical TH-IR neurons are not a transient population of cells.
Figure 3.
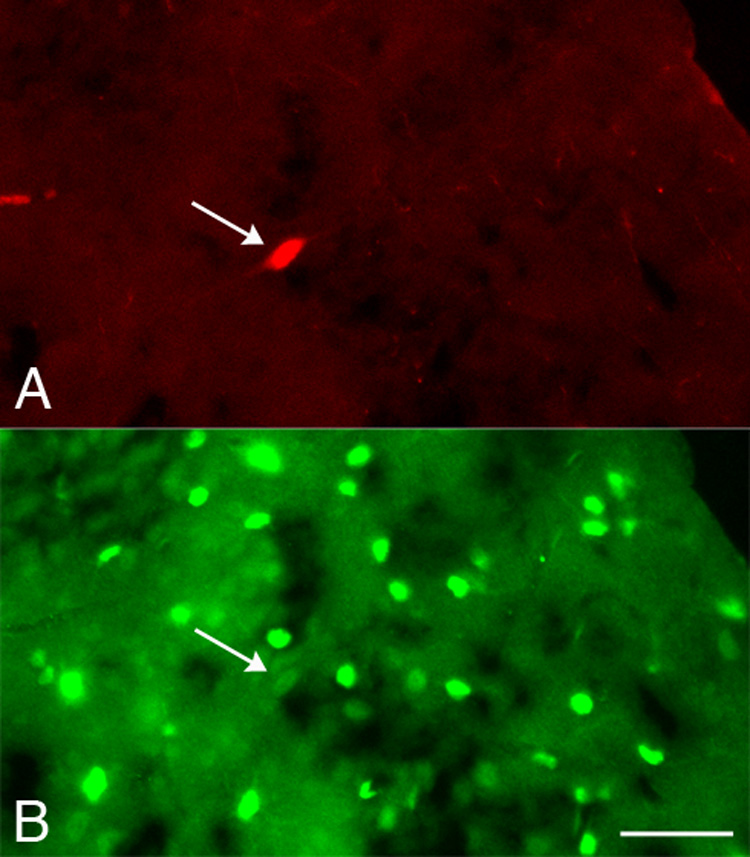
No colocalization of TH and active caspase 3 was observed. Photomicrographs of the same field of cortex at P34 illustrating a TH-IR neuron (A; arrow) that did not contain active caspase 3 immunoreactivity (B). The edge of the cortex can be seen in the upper right corner of each photomicrograph. This photomicrograph shows a section with a relatively higher density of active caspase 3-IR cell profiles than the density seen in other sections of the cortex. Scale bar = 20 µm.
Table 1.
Cortical TH-IR neurons were not double-labeled for active caspase 3 at any age studied.
| Age | Brain Examined | # TH-IR and active caspase 3-IR Cells | Total # TH-IR Cells | % double-labeled |
|---|---|---|---|---|
| P16 | 5 | 0 | 325 | 0 |
| P18 | 4 | 0 | 618 | 0 |
| P20 | 3 | 2 | 1,078 | 0.2 |
| P24 | 4 | 0 | 254 | 0 |
| P30 | 3 | 0 | 193 | 0 |
| P34 | 3 | 0 | 337 | 0 |
| P40 | 2 | 0 | 51 | 0 |
To characterize the phenotype of these cells, we used a variety of markers to identify functional and neurochemical features. Because TH activity is regulated by phosphorylation, we examined whether the TH in cortical neurons was phosphorylated by using an antibody against TH phosphorylated at Ser19 (TH phosphoserine19), one of three serine residues, including Ser31 and Ser40, whose phosphorylation is regulated physiologically (Xu et al., 1998; Lew et al., 1999; Witkovsky et al., 2004; Bobrovskaya et al., 2004). Brains from P16 to P40 rats were examined: P16 (n=3 brains), P18 (n=2), P20 (n=1), P24 (n=2), P34 (n=3) and P40 (n=1). Out of 225 total neurons immunostained with the phosphorylation-independent TH antibody, 173 (77%) were copositive for TH phosphoserine19 (Fig. 4). The percentage of double-labeled cell profiles was similar at all ages examined, suggesting that at ages during which both maximal (P18-20) and minimal (P40) numbers of TH-IR neurons were detected, cortical TH-IR cell profiles contained TH phosphorylated at Ser19.
Figure 4.
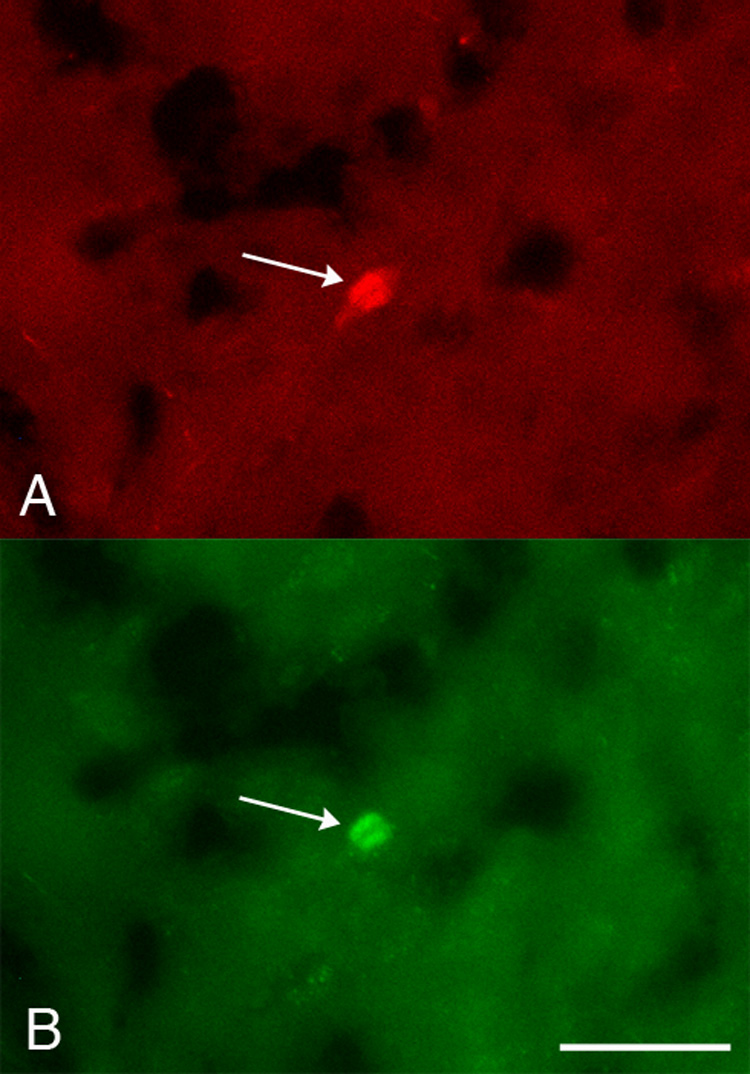
Cortical TH-IR neurons contained phosphorylated TH. Photomicrographs of the same field of cortex at P24 showing a neuron (arrow) immunoreactive for both phosphorylation-independent TH (A) and TH phosphoserine19 (B). Scale bar = 20 µm.
The small size, nonpyramidal morphology, and colocalization with GABA and/or GAD in TH-IR cells in the adult rat cortex suggests that these cells are interneurons (Kosaka et al., 1987a; 1987b). To confirm this phenotype in developing TH-IR neurons, we examined the coproduction of GAD by double-labeled for TH and GAD67, the isoform of GAD prevalent in somata of cortical neurons (Erlander et al., 1991; Feldblum et al., 1993; Esclapez et al., 1994). Neurons and fibers immunoreactive for GAD67 were distributed throughout the cortex as expected. Some cortical TH-IR neurons also contained GAD67 immunoreactivity at all ages studied (Fig. 5 and Table 2). The percentage of cell profiles containing both TH and GAD67 ranged from 13 – 29%. In many colabeled cell profiles, GAD67 immunostaining intensity was faint, suggesting that we were at the threshold of detection. Taken together with morphological criteria, these data suggest that many, if not all, developing and adult cortical TH-IR cells are interneurons.
Figure 5.
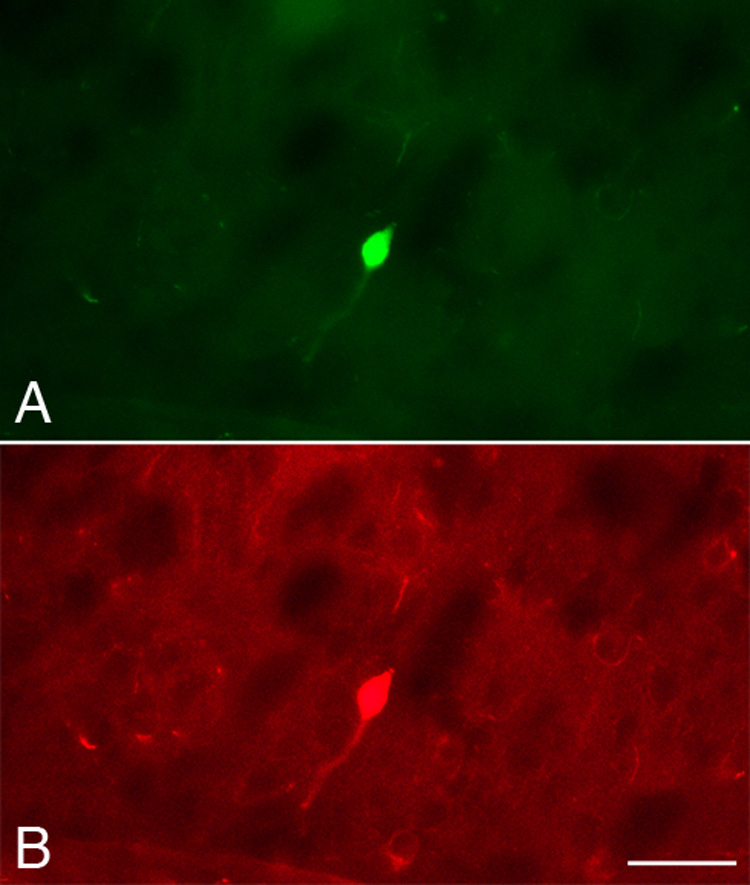
Colocalization of TH and GAD. Photomicrographs of the same field of cortex at P20 showing a neuron double-labeled for TH (A) and GAD (B). Scale bar = 20 µm.
Table 2.
Some cortical TH-IR neurons were double-labeled for GAD.
| Age | Brain Examined | # TH-IR and GAD-IR Cells | Total # TH-IR Cells | % double-labeled |
|---|---|---|---|---|
| P16 | 5 | 124 | 960 | 13 |
| P18 | 5 | 448 | 2424 | 18 |
| P20 | 5 | 342 | 2188 | 16 |
| P24 | 6 | 191 | 1207 | 16 |
| P30 | 4 | 171 | 607 | 28 |
| P34 | 5 | 189 | 899 | 21 |
| P40 | 3 | 47 | 165 | 29 |
To classify cortical TH-IR neurons into one or more of the three nonoverlapping subsets of interneurons as well as to provide indirect evidence for the embryonic origin of TH-IR cells, we examined the colocalization of TH with parvalbumin, somatostatin, or calretinin (Kubota et al., 1994; Gonchar and Burkhalter, 1997; Xu et al., 2004), the expression of which is unique to their site of origin (Wichterle et al., 2001; Nery et al., 2002; Flames and Marin, 2005; Wonders and Anderson, 2006). Parvalbumin-, somatostatin- and calretinin-IR cell profiles were observed in the cortex (Fig. 6B and Fig.7B) as described previously. Very few TH-IR neurons contained immunoreactivity for parvalbumin (0.3-5%; Fig. 6 and Table 3) or somatostatin (0-6%; Table 4). In contrast, a substantial subset of TH-IR neurons (36-53%) colocalized with calretinin at all ages examined (Fig. 7 and Table 5).
Figure 6.
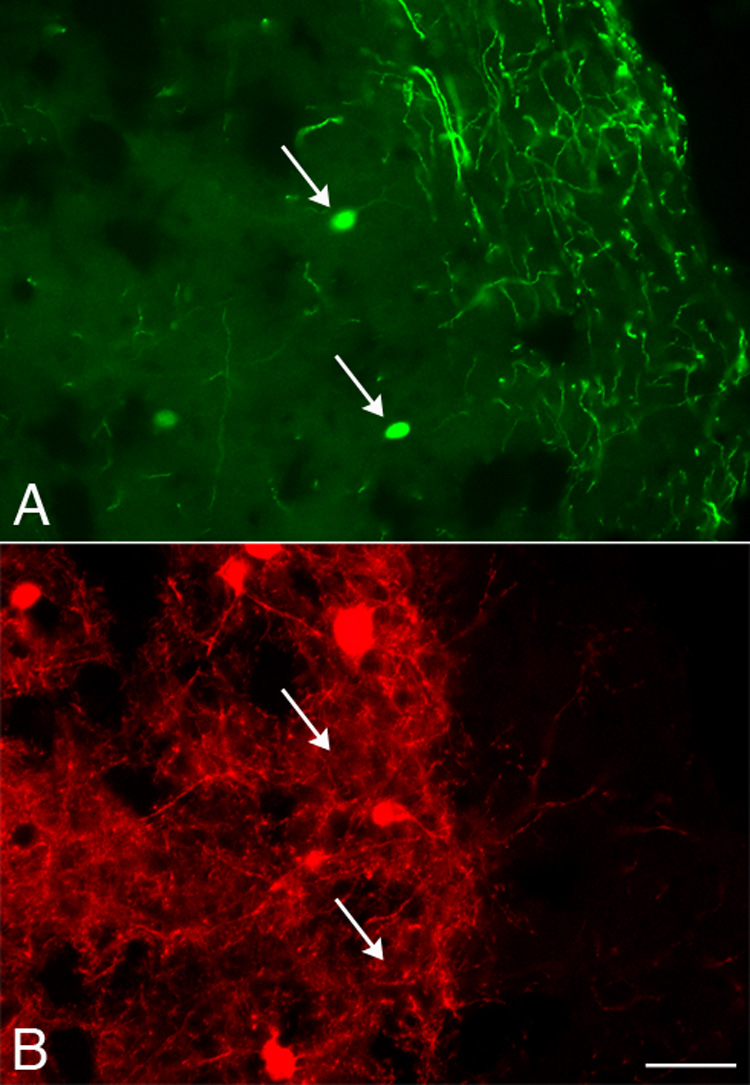
Colocalization of TH and parvalbumin was rarely observed. Photomicrographs of the same field of cortex at P34 showing TH-IR neurons (A; arrows) that did not contain parvalbumin (B). Parvalbumin-IR, TH-negative neurons were visible in this field. Scale bar = 20 µm.
Figure 7.
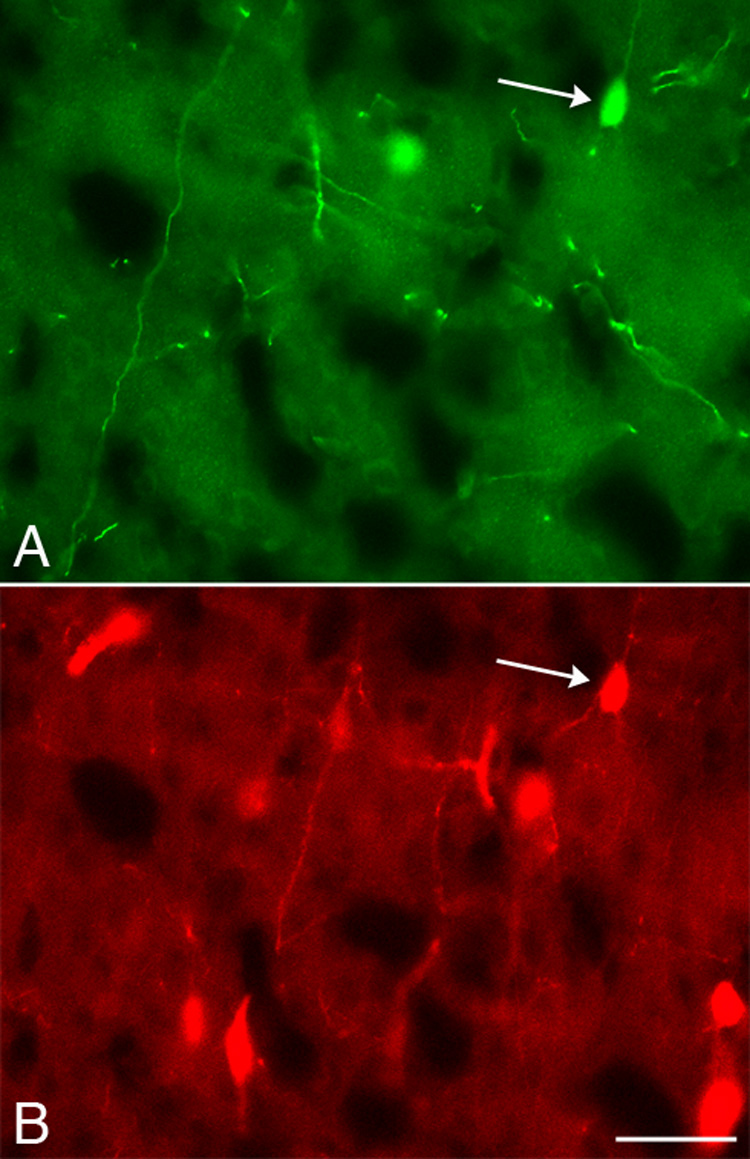
Colocalization of TH and calretinin. Photomicrographs of the same field of cortex at P18 showing a neuron (arrow) double-labeled for TH (A) and calretinin (B). Several calretinin-IR, TH-negative cell profiles were visible in this field. Scale bar = 20 µm.
Table 3.
Few cortical TH-IR neurons were double-labeled for parvalbumin (Parv) at any age studied.
| Age | Brain Examined | # TH-IR and Parv-IR Cells | Total # TH-IR Cells | % double-labeled |
|---|---|---|---|---|
| P16 | 4 | 21 | 603 | 4 |
| P18 | 3 | 29 | 1806 | 2 |
| P20 | 4 | 8 | 707 | 1 |
| P24 | 3 | 34 | 783 | 4 |
| P30 | 4 | 2 | 635 | 0.3 |
| P34 | 5 | 30 | 857 | 4 |
| P40 | 3 | 4 | 78 | 5 |
Table 4.
Few cortical TH-IR neurons were double-labeled for somatostatin (SOM) at any age studied.
| Age | Brain Examined | # TH-IR and SOM-IR Cells | Total # TH-IR Cells | % double-labeled |
|---|---|---|---|---|
| P18 | 2 | 8 | 316 | 3 |
| P20 | 2 | 9 | 141 | 6 |
| P24 | 5 | 1 | 195 | 0.5 |
| P34 | 3 | 3 | 155 | 2 |
| P40 | 3 | 0 | 59 | 0 |
Table 5.
Many cortical TH-IR neurons were double-labeled for calretinin (CAL).
| Age | Brain Examined | # TH-IR and CAL-IR Cells | Total # TH-IR Cells | % double-labeled |
|---|---|---|---|---|
| P16 | 3 | 238 | 617 | 39 |
| P18 | 5 | 920 | 2387 | 39 |
| P20 | 3 | 318 | 892 | 36 |
| P24 | 4 | 447 | 972 | 46 |
| P30 | 4 | 301 | 677 | 45 |
| P34 | 5 | 436 | 910 | 48 |
| P40 | 3 | 117 | 219 | 53 |
3. Discussion
Here, we describe a subpopulation of cortical nonpyramidal neurons that produce TH outside the classical catecholaminergic cell groups (Hokfelt et al., 1984). The number of immunohistochemically-detectable cortical TH cell profiles peaks then declines during postnatal development, with no evidence for cell death in these cells. The majority of these TH-IR neurons contain phosphorylated TH, some of these cells coexpress GAD, and this subpopulation displays partial overlap with the calretinin-containing subset of interneurons.
One potential explanation for the decline in the number of cortical TH-IR neurons observed developmentally is that some of these cells undergo programmed cell death. Although overlap existed between the ages at which we observed the maximal number of cortical TH-IR cell profiles and ages previously reported to contain TUNEL-positive cells (Ferrer et al., 1994; Spreafico et al., 1995; Thomaidou et al, 1997), we found no evidence for the death of these TH-IR cells. In a previous study using chromatin condensation and electrophoretic laddering of DNA as criteria to identify cell death, apoptosis was concluded to be the probable explanation for transient expression of neuropeptide Y in the developing hamster paraventricular area (Botchkina et al., 1996). In other reports that utilized TUNEL, cell death was rejected as the cause of transient histamine immunoreactivity in the developing rat raphe nucleus (Kinnunen et al., 1998) and as an explanation for transient TH immunostaining in developing rat primary auditory neurons (Trigueiros-Cunha et al., 2003). We observed neither DNA fragmentation detected with TUNEL nor active caspase 3 immunostaining in cortical TH-IR neurons at any age, making cell death an unlikely explanation for the reduction in TH-IR cell profile number seen during postnatal development.
Previous studies presented a conflicting picture regarding the transient nature of TH production in cortical neurons of the rat. Some reports described cortical TH-IR neurons as developmentally transient and rarely observed in adults (Berger et al., 1985), whereas others reported a higher number of TH-IR cells in the adult cortex (Kosaka et al., 1987a; 1987b). The greater number of TH-IR neurons observed by Kosaka and coworkers (1987a; 1987b) may be explained by protocol differences, including the use of glutaraldehyde in the fixative, colchicine administration in some rats, and the use of amplification detection systems such as the avidin-biotin complex. However, we found no differences in the number of TH-IR cell profiles observed in the adult cortex using biotinylated secondary antibodies detected with fluorochrome-conjugated streptavidin for amplification compared to visualization with fluorochromes directly conjugated to secondary antibodies. In contrast to detection sensitivity, strain differences provide another plausible explanation for the different findings. Kosaka et al. (1987a; 1987b) used Wistar-Imamichi rats, whereas Sprague-Dawley rats were used here. Physiologically-relevant differences between these two rat strains have been reported (Shimada et al., 2002; Imamura and Shimada, 2005). The developmental increase in brain volume and maturation-induced changes in antibody penetration of tissue sections could, at least in part, potentially explain the observed decrease in TH-IR cell profile number.
Our observation that the number of cortical TH-IR cell profiles varied over time is not unprecedented in light of reports showing that TH production may change dramatically in some developing cells. In the developing rat autonomic nervous system, for example, sympathetic neurons that innervate sweat glands (Landis and Keefe, 1983; Francis and Landis, 1999) and periosteum (Asmus et al., 2000) down-regulate catecholaminergic traits, including TH production, in response to cholinergic-inducing signals derived from the target tissue. In rat primary auditory neurons, transient TH production is concurrent with the development of hearing (Trigueiros-Cunha et al., 2003). When grown in culture, embryonic rat and human cerebral cortical cells, which do not produce TH in vivo, are capable of TH expression (Iacovitti et al., 1987; Theofilopoulos et al., 2001). Cells transiently immunoreactive for TH are present in the developing rodent CNS in a variety of areas in addition to the cerebral cortex, including the inferior colliculus (Jaeger and Joh, 1983), medial geniculate nucleus (Nagatsu et al., 1996), cerebellar Purkinje cells (Hess and Wilson, 1991), limbic forebrain (Verney et al., 1988), and anterior olfactory nucleus (Nagatsu et al., 1990). Thus, developmental plasticity in TH production is not uncommon and may be the explanation for the transitory increase in cell number that we note here. Moreover, developing cortical interneurons display plasticity in their peptide and calcium-binding protein content (Del Rio et al., 1991; Wahle, 1993; Alcantara et al., 1996; Schierle et al., 1997). Recent work has begun to elucidate the factors involved in the development of the diverse array of cortical interneuron phenotypes (Carl and Wonders, 2006).
The end-product of TH in cortical interneurons is not known. No catecholamine-synthesizing enzymes subsequent to TH, including AADC, have been observed in TH-IR neurons in the developing or adult rodent or primate cortex, calling into question the catecholaminergic status of these cells (Berger et al., 1985; Gaspar et al., 1987; Satoh and Suzuki, 1990; Ikemoto et al., 1999; Weihe et al., 2006). Additionally, in the developing rat, labeling of the cortical TH-IR neurons was insensitive to 6-hydroxydopamine toxicity, and no endogenous catecholamines were detected in cortical somata (Berger et al., 1985). To address whether or not TH in cortical neurons is active, we examined the phosphorylation of TH at Ser19 because a variety of physiological stimuli induce TH phosphorylation at this residue and at Ser31 and/or Ser40 (Lew et al., 1999; Bobrovskaya et al., 2004; Witkovsky et al., 2004). The majority of cortical interneurons identified by the phosphorylation-independent TH antibody also contained TH phosphorylated at Ser19 in the postnatal and adult rat. Some reports suggest that Ser40 phosphorylation has a greater direct effect on enzyme activity compared to phosphorylation at Ser19 or Ser31 (Haycock et al., 1998; Bobrovskaya et al., 2007). Phosphorylation at Ser40 activates TH by decreasing dopamine feedback inhibition (Daubner et al., 1992), but cortical TH neurons likely do not produce dopamine. Phosphorylation of TH at Ser19 in cortical neurons may increase enzyme stability (Royo et al., 2005).
If cortical TH-IR neurons contain active TH but lack AADC, as previous reports indicate, then these cells may be classified as “postclassical” catecholaminergic cells that are dopaergic based on the description proposed by Weihe and coworkers (2006). Dopaergic cells appear to be capable of synthesizing L-DOPA as an end-product but lack any known transmitter secretory mechanism. Dopaergic cells are seen in the adult primate and rat cortex. As postulated by Weihe et al. (2006), cortical TH-IR (dopaergic) neurons may supply L-DOPA to neighboring AADC-containing, TH-negative neurons, which then may be capable of synthesizing dopamine. Dopamine production by monoenzymatic cells was reported in the hypothalamic arcuate nucleus of developing rats (Ugrumov et al., 2004). On the other hand, L-DOPA may function as a transmitter, as posited by Misu and colleagues (2003). Cortical dopaergic cells may be more common in the developing nervous system because they play a role in establishing cortical circuitry and/or because they represent immature cells en route to their mature phenotype.
The small size and mainly bipolar, nonpyramidal morphology suggest that many, if not all, cortical TH-IR cells are interneurons. Furthermore, some cortical TH-IR neurons colocalized with GAD67, the isoform of the GABA-synthesizing enzyme most common in cortical neuron somata (Erlander et al., 1991; Feldblum et al., 1993; Esclapez et al., 1994). The intensity of GAD67 immunoreactivity in many double-labeled cell profiles was faint, suggesting that the number of cell profiles scored for TH and GAD colocalization was an underestimate. Previous studies showed that some developing GABAergic interneurons lack detectable GAD67 immunoreactivity (Dayer et al., 2005). The lack of GAD67 immunostaining in some cortical TH-IR neurons may also be due to the loss of detectable TH prior to the onset of GAD67 production. Finally, it is possible that a subset of these TH-IR neurons does not acquire a GABAergic phenotype.
The current report is the first to classify cortical TH-IR neurons into one of the three nonoverlapping subsets of cortical interneurons that are defined by their production of parvalbumin, somatostatin, or calretinin (Kubota et al., 1994; Gonchar and Burkhalter, 1997; Xu et al., 2004). The finding that approximately one-third to one-half of cortical TH-IR cell profiles were colocalized with calretinin, whereas very few TH cell profiles were colabeled with parvalbumin or somatostatin, suggests that many of these TH-IR neurons belong to the calretinin-containing subset. Colocalization with calretinin provides indirect evidence that at least some cortical TH-IR cells migrate from the CGE during development (Wichterle et al., 2001; Nery et al., 2002; Xu et al., 2004; Flames and Marin, 2005; Wonders and Anderson, 2006).
Although the number of calretinin-IR neurons in the rat cortex increases then decreases during development (Schierle et al., 1997), the transient calretinin-IR neurons do not appear to overlap with the TH-IR neurons described in the present report. The number of cortical calretinin-IR neurons changes most dramatically between P0 and P10, but TH-IR neurons are not seen in the cortex prior to P12. Furthermore, the laminar distribution and morphology of some of the neurons transiently immunostained for calretinin do not match those characteristics of cortical TH-IR neurons. By P20, the age of peak TH immunoreactivity, the number and distribution of calretinin-IR cells are similar to those in the adult cortex. Most of the calretinin-IR neurons in layer II/III are bipolar (Gonchar and Burkhalter, 1999), similar to the morphology of TH-IR neurons. Interestingly, the majority of the calretinin-IR neurons in this layer make synaptic connections with other GABAergic interneurons (Gonchar and Burkhalter, 1999). One possible function of the TH- and calretinin-copositive interneurons may be to inhibit other inhibitory neurons, thereby disinhibiting pyramidal cells (Gonchar and Burkhalter, 1999).
Our results suggest that the subset of cortical interneurons producing TH does not die but may undergo a change in neurochemical phenotype in which TH production is diminished below detectable levels as the mature phenotype is acquired. Cortical TH-IR neurons, at least some of which belong to the calretinin-containing subset of interneurons, may play a dopaergic role in cortical circuitry. Interneuron-related disorders may arise when cortical development goes awry (Levitt et al., 2004; Di Cristo, 2007). An aberrant number and/or distribution of specific subsets of cortical interneurons have been linked to several major neurological disorders, including epilepsy (Powell et al., 2003; Garbelli et al., 2006), schizophrenia (Reynolds et al., 2002; Lewis et al., 2005; Daskalakis et al., 2007), and depression (Rajkowska et al., 2007). In a rat model of fetal alcohol syndrome, one outcome of ethanol exposure during the first postnatal week is an increase in the number of cortical calretinin-IR neurons in the adult (Granato, 2006). Furthermore, loss of TH-IR neurons in the human cortex was noted in dementia (Marui et al., 2003) and Parkinson’s disease (Fukuda et al., 1999). Thus, characterization of cortical TH-IR neurons may provide insight into the role that these neurons play in cortical circuitry.
4. Experimental Procedure
4.1 Animals and Tissue Processing
Timed-pregnant Sprague-Dawley rats and pups (Zivic Laboratories, New Castle, PA) were maintained and handled according to guidelines set by the authors’ institution and the National Institutes of Health. Each litter was housed with its mother until weaning at P21. The day of birth was considered P0. Male and female rats were used at all ages sampled; no sex-related differences were observed in the properties of cortical neurons examined here. Rats from at least two different litters were included at all ages studied.
Based on preliminary studies and previous reports (Berger et al., 1985), our analysis focused on ages P16 through P40, during which maximal cortical TH immunoreactivity and its subsequent decline were seen. Rats at ages younger than and between those listed below (e.g. P12, P17 and P19) were analyzed in pilot studies. Three adult rats greater than six months old also were examined. Rats at P40 were used as adults for quantification studies to control for possible differences in perfusion efficacy that could result from large body size differences between two week old rats and rats greater than six months old.
Rats at P16 (n=5), P18 (n=5), P20 (n=5), P24 (n=6), P30 (n=4), P34 (n=5), and P40 (n=4) were deeply anesthetized and transcardially perfused with 325–450 ml (increasing volume with age) of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed, post-fixed at room temperature for one hour, then cryoprotected in 20% sucrose in 0.1 M phosphate buffer containing 0.1% sodium azide at 4°C for at least 24 hours. Brains were cryosectioned in the coronal plane at 14 µm, and non-consecutive sections at 70–112 µm intervals (increasing interval length with age) were mounted onto gelatin-coated slides.
4.2 Immunohistochemistry and DNA Fragmentation (TUNEL) Assay
Sections were double-labeled by preparing cocktails of primary antibodies raised in different species and diluted in 0.01 M phosphate buffer, 2.9% NaCl, 0.3% Triton, 0.1% sodium azide, and 2% bovine serum albumin. Overnight incubation with primary antibodies was followed by incubation for 1–2 hours with a cocktail of species-specific secondary antibodies conjugated to distinct fluorochromes. Each incubation step was followed by rinses in potassium phosphate-buffered saline (KPBS), and all incubations were conducted at room temperature. Finally, sections were coverslipped with glycerol/KPBS (1:1).
One of two TH antibodies (rabbit polyclonal anti-TH, 1:200, Pel-Freez Biologicals, Rogers, AR, or mouse monoclonal anti-TH, 1:200, ImmunoStar, Hudson, WI) was used in combination with one of the following antibodies: rabbit polyclonal anti-active caspase 3 (1:1,000, R&D Systems, Minneapolis, MN), mouse monoclonal anti-calretinin (1:1,000, Chemicon/Millipore, Burlington, MA), mouse monoclonal anti-GAD67 (1:2,000, Chemicon), rabbit polyclonal anti-somatostatin (1:200, ImmunoStar), mouse monoclonal anti-neuronal nuclei (NeuN, 1:1,000, Chemicon), mouse monoclonal anti-parvalbumin (1:1,000, Chemicon), rabbit polyclonal anti-TH phosphoserine 19 (1:750, Chemicon). The secondary antibodies used were Cy2- or Rhodamine Red-X-conjugated donkey anti-rabbit and Rhodamine Red-X-conjugated donkey anti-mouse (all at 1:50, Jackson ImmunoResearch, West Grove, PA). Control experiments confirmed that secondary antibodies bound only to the appropriate primary antibodies.
To identify TH-IR neurons undergoing DNA fragmentation, some sections were sequentially double-labeled by immunostaining with rabbit anti-TH visualized with Rhodamine Red-X-conjugated secondary antibody followed by TUNEL using an in situ apoptosis detection kit according to the manufacturer’s protocol (TACS TdT Kit, R&D Systems). Immediately following the final immunostaining rinse, sections were equilibrated in labeling buffer for 10 minutes at room temperature and then incubated in the TUNEL staining solution containing biotinylated deoxynucleotides, terminal transferase, and Mn++ in labeling buffer for one hour at 37°C. The reaction was terminated in stop buffer. To visualize the biotinylated nucleotides, sections were incubated in streptavidin conjugated to either FITC (1:100, Jackson ImmunoResearch) or Alexa Fluor 488 (1:200, Molecular Probes, Eugene, OR).
To confirm that both the TUNEL assay and the active caspase 3 antibody labeled cells undergoing programmed cell death in vitro and in situ, respectively, control experiments were performed using CHO cells (National Cell Culture Center, Minneapolis, MN) and embryonic rat paw (Zivic Laboratories). Cultured CHO cells were grown in a 1:1 mixture of Dulbecco’s Modified Eagle Medium (DMEM) and F12 (Invitrogen Gibco, Carlsbad, CA) supplemented with 5% newborn calf serum and antibiotics. Some cells were exposed to ultraviolet (UV) light for 20 minutes to induce apoptosis. After two to four hours, UV-treated and untreated cells were fixed in 4% paraformaldehyde then sequentially labeled for DNA fragmentation using TUNEL and for active caspase-3 by immunofluorescence staining as described above. Paraformaldehyde-fixed rat paws at E16.5 were cryosectioned and stained using TUNEL or anti-caspase 3 immunostaining as above.
4.3 Data collection and analysis
Sections were analyzed and images captured and annotated using a Zeiss Axioskop 2 Plus fluorescence microscope equipped with an AxioCam MRc5 CCD digital camera operated with AxioVision software (Carl Zeiss, Thornwood, NY). Photomicrographic images were cropped and lettered using Adobe Photoshop CS software (Adobe Systems, Seattle, WA). The images were not otherwise altered.
Cortical neurons were scored for TH immunoreactivity if they were above background staining compared to labeling of classical catecholaminergic cell and fiber groups in the same section and if at least one extension off a clearly defined cell body could be seen. Colocalization of TH with other markers was deemed by the overlap of cell body immunofluorescence in the same focal plane. Cell profiles immunoreactive for TH alone or double-labeled were counted at 400X magnification in nonadjacent sections through the neocortex. The analysis included the cingulate cortex medially to the rhinal fissure laterally. Data from both hemispheres were pooled in each section. An average of 768 µm (approximately 55 sections) per brain were analyzed to compare the average relative number of profiles of TH-IR neurons per section in brains of different ages. A non-stereological approach was taken because averages of relative, not absolute, numbers of cell profiles were compared at different ages. For each brain, the number of profiles of single- and double-labeled cortical TH-IR neurons per section was averaged. Statistical analysis (1-way ANOVA and post-hoc comparisons) was conducted using R version 2.4.1 The average number of profiles of cortical TH-IR neurons at different ages was analyzed by 1-way ANOVA, and confidence intervals for the difference between means were constructed using Tukey's Honest Significant Difference method. P-values <0.05 were considered significant.
Acknowledgements
The authors thank Dr. Kevin Wood for preliminary work on the project, Drs. Anthony Kincaid and Peggy Richey for comments on the manuscript, and Dr. Rob Ziemba for assistance with statistical analysis. The National Cell Culture Center kindly provided CHO cells. This work was funded primarily by NIH AREA grant 1 R15 NS046330-01A1 to SEA. Funding was also provided by a Kentucky Academy of Science Special Research Award (SEA) and from an institutional Kresge Foundation grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E. Transient colocalization of parvalbumin and calbindin D28k in the postnatal cerebral cortex: evidence for a phenotypic shift in developing nonpyramidal neurons. Eur J Neurosci. 1996;8:1329–1339. doi: 10.1111/j.1460-9568.1996.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Asmus SE, Parsons S, Landis SC. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J Neurosci. 2000;20:1495–1504. doi: 10.1523/JNEUROSCI.20-04-01495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Distribution of neurons expressing tyrosine hydroxylase in the human cerebral cortex. J Anat. 2007;211:212–222. doi: 10.1111/j.1469-7580.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Verney C, Gaspar P, Febvret A. Transient expression of tyrosine hydroxylase immunoreactivity in some neurons of the rat neocortex during postnatal development. Brain Res. 1985;355:141–144. doi: 10.1016/0165-3806(85)90013-6. [DOI] [PubMed] [Google Scholar]
- Bobrovskaya L, Dunkley PR, Dickson PW. Phosphorylation of Ser19 increases both Ser40 phosphorylation and enzyme activity of tyrosine hydroxylase in intact cells. J Neurochem. 2004;90:857–864. doi: 10.1111/j.1471-4159.2004.02550.x. [DOI] [PubMed] [Google Scholar]
- Bobrovskaya L, Gelain DP, Gilligan C, Dickson PW, Dunkley PR. PACAP stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell Signal. 2007;19:1141–1149. doi: 10.1016/j.cellsig.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Lyubsky S, Hagag NG. Transient expression of neuropeptide Y (NPY) immunoreactivity in the developing hamster paraventricular thalamic area is due to apoptosis. Cell Mol Neurobiol. 1996;16:649–659. doi: 10.1007/BF02151902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Res Rev. 2007;56:427–442. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem. 1992;267:12639–12646. [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Cortical interneurons: from Cajal to 2001. Prog Brain Res. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Soriano E, Ferrer I. A transitory population of substance P-like immunoreactive neurones in the developing cerebral cortex of the mouse. Brain Res Dev Brain Res. 1991;64:205–211. doi: 10.1016/0165-3806(91)90227-a. [DOI] [PubMed] [Google Scholar]
- Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Tortosa A, Blanco R, Martin F, Serrano T, Planas A, Macaya A. Naturally occurring cell death in the developing cerebral cortex of the rat. Evidence of apoptosis-associated internucleosomal DNA fragmentation. Neurosci Lett. 1994;182:77–79. doi: 10.1016/0304-3940(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Flames N, Marin O. Developmental mechanisms underlying the generation of cortical interneuron diversity. Neuron. 2005;46:377–381. doi: 10.1016/j.neuron.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Takahashi J, Tanaka J. Tyrosine hydroxylase-immunoreactive neurons are decreased in number in the cerebral cortex of Parkinson's disease. Neuropathology. 1999;19:10–13. doi: 10.1046/j.1440-1789.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- Garbelli R, Meroni A, Magnaghi G, Beolchi MS, Ferrario A, Tassi L, Bramerio M, Spreafico R. Architectural (Type IA) focal cortical dysplasia and parvalbumin immunostaining in temporal lobe epilepsy. Epilepsia. 2006;47:1074–1078. doi: 10.1111/j.1528-1167.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Voltattorni C. Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci Lett. 1987;80:257–262. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cereb Cortex. 1999;9:683–96. doi: 10.1093/cercor/9.7.683. [DOI] [PubMed] [Google Scholar]
- Granato A. Altered organization of cortical interneurons in rats exposed to ethanol during neonatal life. Brain Res. 2006;1069:23–30. doi: 10.1016/j.brainres.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Lew JY, Garcia-Espana A, Lee KY, Harada K, Meller E, Goldstein M. Role of serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site-and phosphospecific antibodies and site-directed mutagenesis. J Neurochem. 1998;71:1670–1675. doi: 10.1046/j.1471-4159.1998.71041670.x. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Wilson MC. Tottering and leaner mutations perturb transient developmental expression of tyrosine hydroxylase in embryologically distinct Purkinje cells. Neuron. 1991;6:123–132. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Tork I, De Tribolet N. Morphology of tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex. Exp Brain Res. 1989;76:12–20. doi: 10.1007/BF00253618. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Lee J, Joh TH, Reis DJ. Expression of tyrosine hydroxylase in neurons of cultured cerebral cortex: evidence for phenotypic plasticity in neurons of the CNS. J Neurosci. 1987;7:1264–1270. doi: 10.1523/JNEUROSCI.07-04-01264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto K, Kitahama K, Nishimura A, Jouvet A, Nishi K, Arai R, Jouvet M, Nagatsu I. Tyrosine hydroxylase and aromatic L-amino acid decarboxylase do not coexist in neurons in the human anterior cingulate cortex. Neurosci Lett. 1999;269:37–40. doi: 10.1016/s0304-3940(99)00409-7. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Shimada H. Differential pharmacokinetics of acetohexamide in male Wistar-Imamichi and Sprague-Dawley rats: role of microsomal carbonyl reductase. Biol Pharm Bull. 2005;28:185–187. doi: 10.1248/bpb.28.185. [DOI] [PubMed] [Google Scholar]
- Jaeger CB, Joh TH. Transient expression of tyrosine hydroxylase in some neurons of the developing inferior colliculus of the rat. Brain Res. 1983;313:128–132. doi: 10.1016/0165-3806(83)90208-0. [DOI] [PubMed] [Google Scholar]
- Kinnunen A, Lintunen M, Karlstedt K, Fukui H, Panula P. In situ detection of H1-receptor mRNA and absence of apoptosis in the transient histamine system of the embryonic rat brain. J Comp Neurol. 1998;394:127–137. [PubMed] [Google Scholar]
- Kosaka T, Hama K, Nagatsu I. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exp Brain Res. 1987a;68:393–405. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Storm-Mathisen J, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1987b;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Martin-Vasallo P, Peress NS. Lewy bodies in tyrosine hydroxylase-synthesizing neurons of the human cerebral cortex. Neurosci Lett. 1989;106:49–54. doi: 10.1016/0304-3940(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Landis SC, Keefe D. Evidence for neurotransmitter plasticity in vivo: developmental changes in properties of cholinergic sympathetic neurons. Dev Biol. 1983;98:349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- Lee KK, Chan WY, Sze LY. Histogenetic potential of rat hind-limb interdigital tissues prior to and during the onset of programmed cell death. Anat Rec. 1993;236:568–572. doi: 10.1002/ar.1092360317. [DOI] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lew JY, Garcia-Espana A, Lee KY, Carr KD, Goldstein M, Haycock JW, Meller E. Increased site-specific phosphorylation of tyrosine hydroxylase accompanies stimulation of enzymatic activity induced by cessation of dopamine neuronal activity. Mol Pharmacol. 1999;55:202–209. doi: 10.1124/mol.55.2.202. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Marui W, Iseki E, Kato M, Kosaka K. Degeneration of tyrosine hydroxylase-immunoreactive neurons in the cerebral cortex and hippocampus of patients with dementia with Lewy bodies. Neurosci Lett. 2003;340:185–188. doi: 10.1016/s0304-3940(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Misu Y, Kitahama K, Goshima Y. L-3,4-Dihydroxyphenylalanine as a neurotransmitter candidate in the central nervous system. Pharmacol Ther. 2003;97:117–137. doi: 10.1016/s0163-7258(02)00325-x. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nagatsu I, Komori K, Takeuchi T, Sakai M, Yamada K, Karasawa N. Transient tyrosine hydroxylase-immunoreactive neurons in the region of the anterior olfactory nucleus of pre- and postnatal mice do not contain dopamine. Brain Res. 1990;511:55–62. doi: 10.1016/0006-8993(90)90224-y. [DOI] [PubMed] [Google Scholar]
- Nagatsu I, Takeuchi T, Sakai M, Karasawa N, Yamawaki Y, Arai R, Nagatsu T. Transient appearance of tyrosine hydroxylase-immunoreactive non-catecholaminergic neurons in the medial geniculate nucleus of postnatal mice. Neurosci Lett. 1996;211:183–186. doi: 10.1016/0304-3940(96)12753-1. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL, Zhang ZJ. Understanding the neurotransmitter pathology of schizophrenia: selective deficits of subtypes of cortical GABAergic neurons. J Neural Transm. 2002;109:881–889. doi: 10.1007/s007020200072. [DOI] [PubMed] [Google Scholar]
- Royo M, Fitzpatrick PF, Daubner SC. Mutation of regulatory serines of rat tyrosine hydroxylase to glutamate: effects on enzyme stability and activity. Arch Biochem Biophys. 2005;434:266–274. doi: 10.1016/j.abb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Satoh J, Suzuki K. Tyrosine hydroxylase-immunoreactive neurons in the mouse cerebral cortex during the postnatal period. Brain Res Dev Brain Res. 1990;53:1–5. doi: 10.1016/0165-3806(90)90119-j. [DOI] [PubMed] [Google Scholar]
- Schierle GS, Gander JC, D'Orlando C, Ceilo MR, Vogt Weisenhorn DM. Calretinin-immunoreactivity during postnatal development of the rat isocortex: a qualitative and quantitative study. Cereb Cortex. 1997;7:130–142. doi: 10.1093/cercor/7.2.130. [DOI] [PubMed] [Google Scholar]
- Shimada H, Nagano M, Yasutake A, Imamura Y. Wistar-Imamichi rats exhibit a strong resistance to cadmium toxicity. Journal of Health Science. 2002;48:201–203. [Google Scholar]
- Spreafico R, Frassoni C, Arcelli P, Selvaggio M, De Biasi S. In situ labeling of apoptotic cell death in the cerebral cortex and thalamus of rats during development. J Comp Neurol. 1995;363:281–295. doi: 10.1002/cne.903630209. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos S, Goggi J, Riaz SS, Jauniaux E, Stern GM, Bradford HF. Parallel induction of the formation of dopamine and its metabolites with induction of tyrosine hydroxylase expression in foetal rat and human cerebral cortical cells by brain-derived neurotrophic factor and glial-cell derived neurotrophic factor. Brain Res Dev Brain Res. 2001;127:111–122. doi: 10.1016/s0165-3806(01)00125-0. [DOI] [PubMed] [Google Scholar]
- Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigueiros-Cunha N, Renard N, Humbert G, Tavares MA, Eybalin M. Catecholamine-independent transient expression of tyrosine hydroxylase in primary auditory neurons is coincident with the onset of hearing in the rat cochlea. Eur J Neurosci. 2003;18:2653–2662. doi: 10.1046/j.1460-9568.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- Trottier S, Geffard M, Evrard B. Co-localization of tyrosine hydroxylase and GABA immunoreactivities in human cortical neurons. Neurosci Lett. 1989;106:76–82. doi: 10.1016/0304-3940(89)90205-x. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV, Melnikova VI, Lavrentyeva AV, Kudrin VS, Rayevsky KS. Dopamine synthesis by non-dopaminergic neurons expressing individual complementary enzymes of the dopamine synthetic pathway in the arcuate nucleus of fetal rats. Neuroscience. 2004;124:629–635. doi: 10.1016/j.neuroscience.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Verney C, Gaspar P, Febvret A, Berger B. Transient tyrosine hydroxylase-like immunoreactive neurons contain somatostatin and substance P in the developing amygdala and bed nucleus of the stria terminalis of the rat. Brain Res. 1988;470:45–58. doi: 10.1016/0165-3806(88)90200-3. [DOI] [PubMed] [Google Scholar]
- Wahle P. Differential regulation of substance P and somatostatin in Martinotti cells of the developing cat visual cortex. J Comp Neurol. 1993;329:519–538. doi: 10.1002/cne.903290408. [DOI] [PubMed] [Google Scholar]
- Weihe E, Depboylu C, Schutz B, Schafer MK, Eiden LE. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol. 2006;26:659–678. doi: 10.1007/s10571-006-9053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–4249. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, Lew JY, Harada K, Aman K, Goldstein M, Deutch A, Haycock JW, Hokfelt T. Immunohistochemical studies on phosphorylation of tyrosine hydroxylase in central catecholamine neurons using site- and phosphorylation state-specific antibodies. Neuroscience. 1998;82:727–738. doi: 10.1016/s0306-4522(97)00189-9. [DOI] [PubMed] [Google Scholar]