Abstract
Human melanoma tumors cells are known to express the enzyme, inducible nitric oxide synthase (iNOS), which is responsible for cytokine induced nitric oxide (NO) production during immune responses. This constitutive expression of iNOS in many patients’ tumor cells, as well as its strong association with poor patient survival, have led to the consideration of iNOS as a molecular marker of poor prognosis, as well as a possible target for therapy. The expression of iNOS in patient tumors was found to associate with nitrotyrosine, COX2, pSTAT3, and arginase. Using human melanoma patients’ samples as well as cell lines, we have further evidence supporting intracellular NO production by detection of nitrotyrosine and also by use of DAF-2 DA staining. Experiments were performed to scavange the endogenous NO (with c-PTIO) resulting in melanoma cell growth inhibition; this was restored with SIN-1 (NO and O2- donor) providing data to support a functional role of this gas. Our goal is to understand the aberrant biology leading to this curious phenomenon, and to regulate it in favor of patient treatments.
Keywords: Melanoma, nitrotyrosine, nitric oxide, biochemotherapy
Introduction
Melanoma is the most malignant of skin cancers for which early detection and surgical excision are the only reliable means of control. Patients often metastasize and progress rapidly, with only 20% of patients with visceral metastasis living beyond 5 years. The incidence and death rates in the US from melanoma continue to increase, with over a 600x increase in incidence, and 15x increase in deaths since 1950 documented by the American Cancer Society (SEER database, 2007). Unfortunately, our poor understanding of the biology of melanoma is reflected by the lack of effective therapies. No systemic therapies currently exist that increase survival of patients with metastatic disease beyond a 10 – 15% produced by biotherapies such as IFNs, IL-2, or experimental adoptive immunotherapy efforts. Our findings of iNOS expression in melanoma tumor cell cytoplasm provides a novel marker and possible target for therapy [1]; [2].
In normal melanocytes, which are the precursor cell of melanoma, the pigment molecule eumelanin provides a redox function supporting an antioxidant intracellular environment; however in melanoma tumor cells, a pro-oxidant status has been reported [3]. Both reactive oxygen species (ROX) and reactive nitrogen oxidants (RNS) can be identified in melanoma. Our published studies provide strong circumstantial evidence that endogenous Nitric Oxide (NO) is produced by some human melanomas and that in these cases, melanoma cell survival appears dependent on NO [4]. Molecular analysis supports the hypothesis that iNOS-produced NO can be responsible for driving proliferation as well as regulating resistance to apoptosis; this is indicated by results of experiments in which chemical quenching of NO resulted in G2 growth arrest followed by a gain of cisplatin-induced apoptosis [4]. An increasing series of publications support the hypothesis that that NO is the product of aberrant constitutive expression of the cytokine-inducible nitric oxide synthase (iNOS) as detected by immunohistochemistry (IHC) [1], [2]. Antibodies to iNOS and to nitrityrosine are both used in these reports. Our data indicate that the presence of inducible nitric oxide synthase (iNOS) protein, which we find in the melanoma tumor cytoplasm of ~60% of advanced patients, provides independent prognostic value by predicting decreased survival, so that the Hazard Ratio of iNOS positive patients is 4.63 in our most recent report [2].
The independent validation of iNOS in melanoma as a prognostic marker is now being pursued outside of M.D. Anderson. The mechanism of iNOS expression and of the molecular pathways affected by NO are currently active areas of research. Interesting suggestions of key molecules in melanoma growth promotion and apoptosis resistance being regulated by NO-mediated post-translational modifications (eg., p53 and N-ras) are further being pursued. In addition, efforts to identify specific nitrated and S-nitrosated proteins in melanoma as a hypothesis-generating approach for future targeting of growth and survival pathways are in progress.
While specific quenchers and inhibitors of NO are being used for in vitro cell line assays, a long-term goal of this project is to test any drugs with anti iNOS activity in vitro in melanoma lines and then in a melanoma xenograft mouse models, and finally in human cancer patients. Our ultimate goal is to understand and regulate the production of intracellular NO in melanoma, which is considered a previously overlooked yet highly significant bioactive mediator for which data and literature suggests a critical role in neovascularization, growth support, and apoptosis resistance in melanoma and some other cancers.
MATERIAL and METHODS
Tumor Samples
The melanoma tumor samples used in this study consist of metastases surgically removed from patients with stage III disease who were enrolled in an institutionally approved neoadjuvant biochemotherapy trial. The biochemotherapy regimen consisted of cisplatin; vinblastine; dacarbazine; IL-2, and IFN-alfa-2a administered weekly for cycle of 4 weeks. All patients received a minimum of two cycles of therapy prior to surgery. Surgery was usually performed within 2–4 weeks after the last biochemotherapy cycle and tumor processed by formalin fixation and paraffin embedding, followed by sectioning onto slides for analysis in this report. Survival of patients was updated annually for this data. Several human melanoma cell lines (A375, Mewo) are also employed as detailed previously [4].
Reagents
Anti-iNOS mouse monoclonal antibody (Becton Dickinson Transduction Laboratories,) was used for iNOS immunohistochemistry, and anti-NT mouse monoclonal antibody (Upstate Biotechnology, Lake Placid, NY) was used for NT staining; both antibodies were confirmed as being cross-reactive between species. Preimmune normal mouse IgG (Vector Laboratories, Burlingame, CA) was used as a negative control. Anti-vimentin antibody (BioGenex Laboratories, San Ramon, CA) was used as a positive control for all melanoma staining. Other reagents including sulfanilamide, N-1-naphthyl-ethylenediamine, ammonium formate, zinc sulfate, sodium nitrate and sodium nitrite was purchased from Sigma Chemical Company, Inc. (St. Louis, MO).
Donors (3-morpholino-sydnonimin (SIN-1)), and quenchers ((2-(4-carboxyphenyl)-4,4,5,5-tetramethylimida-zoline-1-oxyl-3-oxide (cPTIO)) for NO were obtained from Alexis Corp. (San Diego) and DAF-2 diacetate was purchased from Calbiochem, and used according to manufacturers instructions.
Immunohistochemical Staining
Immunohistochemical staining was performed with 10% formalin-fixed paraffin-embedded melanoma tissue, cut 4–6 µm thick. Sections were placed on silanized slides (Histology Control Systems, Glen Head, NY), deparaffinized in xylene, and rehydrated in descending grades (from 100 to 85%) of ethanol. To enhance the immunostaining and restore the maximal antigenicity of cytokines, sections then were placed in antigen unmasking solution (Vector Laboratories, Burlingame, CA) and microwaved intermittently for up to 10 min to maintain a boiling temperature. After the slides were cooled at room temperature for 30 min, they were washed in distilled water and PBS. After this initial preparation, the slides were removed from PBS and covered with 3% H2O2 (Sigma Chemical Co.) in methanol to block endogenous peroxidase activity. All incubations were carried out at room temperature in a humidified covered slide chamber. The slides were washed in PBS before incubation in TRIS-buffered saline (TBS) containing 0.05% Triton X-100 (Sigma Chemical Co.) for 15 min to permeabilize the cells. An avidin-biotin-peroxidase complex (ABC) kit (Vectastain; Vector Lab.) was then used to detect the primary antibody staining. These kits are specific for the species of primary antibody used and contain a blocking serum, a secondary biotinylated antibody, and the ABC reagent. After the slides were incubated for 30 min with the blocking serum, the primary antibody at various dilutions (1:100 to 1:200 for iNOS and 1:50 for NT) was added and the slides incubated for 60 min at room temperature. The slides were then washed, incubated for 30 min with secondary biotinylated antibody, washed again, and then incubated for 30 min with the ABC reagent. After the slides were washed in PBS, the immunostaining was developed with the use of 3-amino-9-ethylcarbazole as a chromogen for 15 min. Slides were counterstained with hematoxylin (Vector Lab) and mounted with Aqua-Mount (Lerner Laboratories, Pittsburgh, PA). Control tissues employed were the samples of formalin fixed and paraffin-embedded normal human nevi obtained as incidental material from our Pathology Department. Immunolabeling was scored for number of positive melanoma cells defined as greater than 5% positive cells for this particular study, while more recent groups of tumors have analyzed based on percentage of cells positive as well as intensity of staining. The slides were independently interpreted by two readers without knowledge of the clinical data. Any discrepancies in scores were subsequently reconciled.
Results
Detection of iNOS and NT in human tumor specimens
In melanoma patient tumor specimens, expression of iNOS was variable, and ranged from completely negative (~ 40% of all patients) to almost 100% of cells positive (in less that 10% of patients), with most tumor samples being intermediate. Figure 1 displays positive and negative melanoma tumors specimens. IHC is currently the preferred method for patient sample analysis so that any stromal cells positive for iNOS can be excluded from the tumor analysis. Both macrophages and endothelial cells have been noted to be positive in some samples, but this feature appeared to be independent of tumor iNOS.
Figure 1.
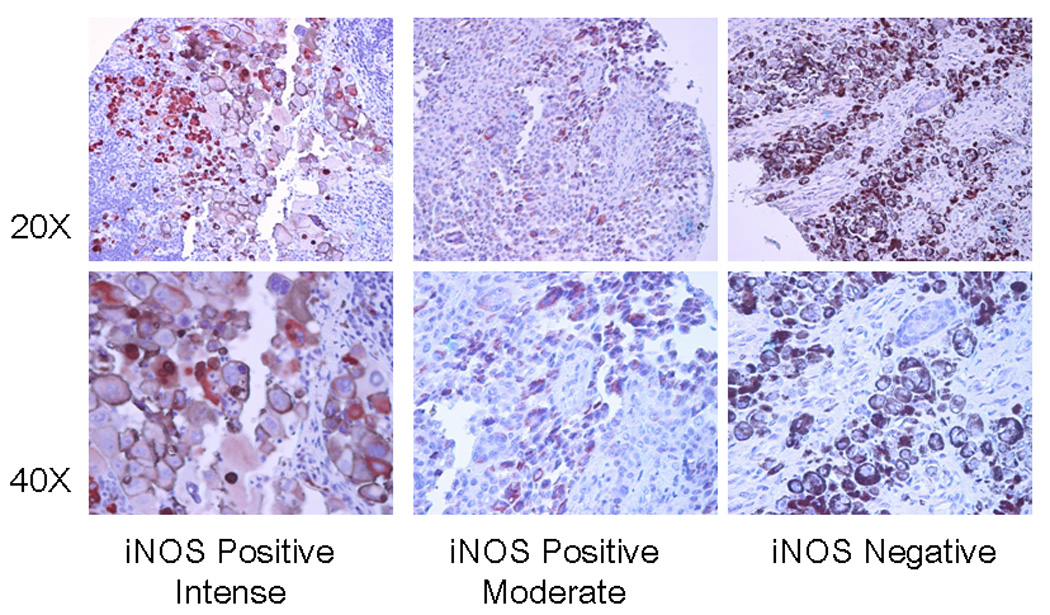
IHC of metastatic melanoma for iNOS is shown at low and higher powers to display the cytoplasmic staining in three human surgical specimen melanoma tumors, one very intense and one moderate, and a third one which is negative.
IHC was also employed for further evaluation of nitrotyrosine (NT) as well as other inflammatory markers such as COX2, pSTAT3, and the arginase also so that tumor expression of these markers could be distinguished from that of any inflammatory or stromal cells. NT is formed when NO chemically combines with superoxide to form ONOO-, one possible molecule responsible for protein nitration and nitrosation, as well as DNA adduct and lipid peroxidation reactions. Statistically significant association was identified between NT intensity and iNOS intensity (p < 0.0001).
Association of iNOS and NT in human melanoma tumors with poor survival
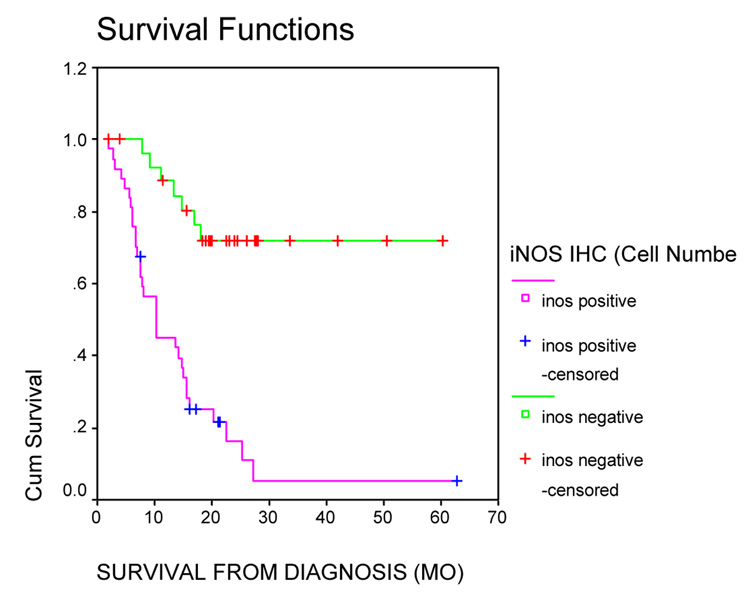
Our first observation of iNOS in 20 melanoma patient tumor cells, was from tumors excised from patients in a neoadjuvant trial in which the patients were receiving a combination of chemotherapy and the approved IL-2 and IFN-alfa biotherapy, hence called “biochemotherapy.” [5] We hypothesized that the chemotherapy would inhibit the concomitant biotherapy, and as part of studying immune cell activation status a monoclonal antibody to iNOS was employed to identify activated macrophages. Although iNOS was observed as positive in occasional macrophages, there was no association with patient progression or survival. The novel data is that iNOS was positive in some melanoma tumor cell cytoplasm. More recent data has attempted to quantify both the percentage of melanoma tumor cells positive and intensity of staining, although in the original study we used analysis of positive or negative [2]. Our new data is from testing tumors from 65 patients enrolled on this same trial, and for which we have over 5 years of follow-up (Figure 3).
Figure 3.
Melanoma Tumors were excised after at least two cycles of biochemotherapy. Tumors from these 65 patients were immunostained for iNOS. iNOS staining status was plotted as a function of survival. There were 28 iNOS negative tumors, with 21 censored observations (Mean survival 47.04 mo; 95% CI: 38. – 55.4 mo) and 37 iNOS positive tumors (Mean survival 14.9 mo; 95% CI: 9.8–19.9 mo; and median survival 10.15 mo; 95% CI: 3.8 – 16.6).
Melanoma cell line proliferation can be regulated by NO
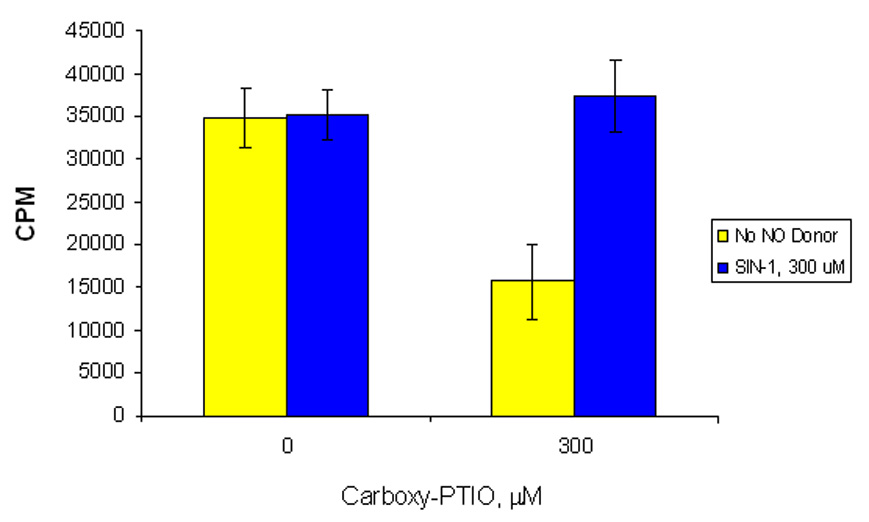
Treatment of the melanoma cell line A375 with the nitric oxide scavenger c-PTIO at a concentration of 0.3mM resulted in decreased cell growth (Figure 4), suggesting that low to moderate levels of NO are required for growth by this line. Addition of the stochiometric nitric oxide and superoxide anion donor SIN-1 at a concentration of 0.3mM restored growth in both experiments, which controls for toxicity as not responsible for the c-PTIO inhibition. In parallel, propidium iodide staining of the treated and untreated cells showed that the c-PTIO-treated cells showed a large population of sub-GO cells, while the SIN-1 alone resulted in only 2% in sub-GO (data not shown). While these data are preliminary, they support the hypothesis that endogenous nitric oxide serves as a mutable survival factor in A375 melanoma cells and that inhibition of NO production results in an increase in apoptosis.
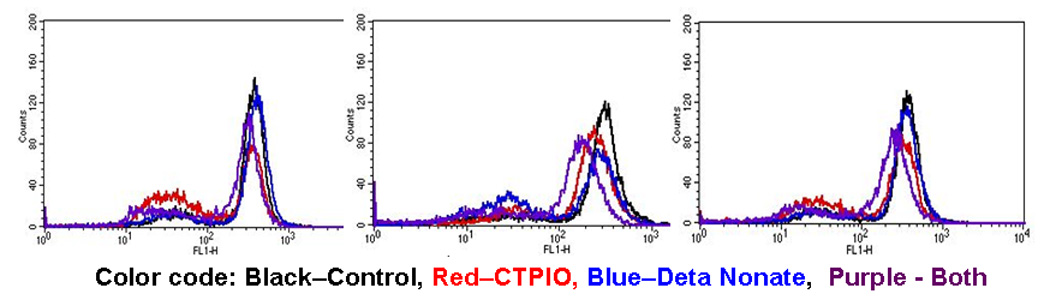
Figure 4. Effects of NO scavenger and donor on DAF-2 DA staining in three melanoma cell lines.
Cells were cultured in the indicated NO scavenger (CTPIO) or donor (Deta Nonate) at 300uM overnight. The cells were trypsinized, washed and DAF-2DA was added in modified HBSS with treatment for 30 min. Cells were then washed resuspended inmodifed HBSS with treatment and counted by flow cytometry.
These results further support our contention that constitutive iNOS leading to intracellular NO production is directly responsible for supporting melanoma growth and that reduction in the levels of NO can inhibit melanoma growth [4]. Therefore, we have initiated preliminary studies into possible mechanisms controlling iNOS expression, as well as methods to measure immediate changes in the intracellular NO content.
Application of DAF-2 DA to observe relative intracellular NO levels
Currently there is no reliable nor quantitative means to measure intracellular NO. Use of the DAF-2DA dye has been popularized lately as providing a relative measure of intricallular nitric oxide species, and our testing of it in melanoma cell lines has proven useful, as the peak fluorescence falls with known inhibitors and rises with donors. Means to measure intracellular NO changes in cancer cells is needed for a more complete understanding of this biology.
Discussion
Our unexpected finding of melanoma expressing iNOS, which is an ”activated macrophage marker,” has not only been consistently observed by us but is also consistently observed in other sets of melanoma, such as in tumors from a prospective analysis of Stage III patients who had not received CVD-Bio or any other systemic therapy [2] and others employing varying sources of tumor [6]. In our additional prospective study, 132 sequential patients tumors diagnosed with Stage III cutaneous melanoma at MD Anderson Cancer Center in Houston, Texas were collected and iNOS expression was examined by IHC in the metastatic (not cutaneous primary) tumors of melanoma patients. Expression was measured by the number of positive cells as well as intensity of staining [2]. The median follow up time from Stage III diagnosis was 49.5 months, and over the period of observation 72 had died and 60 remained alive. Overall, iNOS positive patients Hazard Ratio was 4.63 compared to iNOS negative patients. Therefore, it appears that the treatment had little or no impact on the iNOS status of the tumors, and that from both trials iNOS negative patients had not reached median survival. Therefore, consistent reports of melanoma tumor iNOS associated with poor patient prognosis suggests that an identifiable set of patients may be selected by this means.
Our use of IHC methodology for these analysis is considered necessary at this time, as iNOS in the tumor cells must be distinguished from iNOS in stromal cells. Although the readers are blinded as to the patient outcome, only the positive and negative criteria were considered optimal for this study. It is hoped that automated technologies using cellular masks and tumor specific markers for melanoma will become widely available soon, an objective quantitation of numbers of cells positive intensity of staining can be normalized and a continuous score for iNOS measurement will be possible.
Our finding that iNOS expression predicted shortened survival in both treated as well as untreated Stage III melanoma patients supports the value of this enzyme as a marker and further begs the investigation of whether NO was actually produced and if so whether it was functional in maintaining the more aggressive phenotype of cancer found in these patients. To date, there is no method that specifically measures NO in tissues, although Iron-dithiocarbamate complexes are widely used as one relative measure, and efforts to improve their specificity are anticipated [7]. Our use of the DAF method for cell lines provides a rapid and relative measure of total NO forms using standard flow cytometry instrumentation, and its application to human tumors remains distant. The Greiss reaction has been used by us [8] and many others for years to measure NO oxidation products in fluids, but this technology has neither the sensitivity nor technological basis for use in tissues.
Means to quantify intracellular NO is likely to be useful to determine whether there is a direct quantitative relationship of NO and prognosis, such that more NO leads to earlier death. Currently, NO products can be measured in the circulation, but using the Griess reaction for total NO oxidation product generation as we have performed previously [8] is viewed as nonspecific. Therefore, methods are needed to quantify NO in tumor, and the DAF system is a start, but provides only relative information. Specific means to quantify intracellular NO are needed, as it is likely that some very low levels are required for all cells to survive, but an intermediate level leads to altered signaling as we hypothesize is occurring in these tumors. The induction of higher levels of NO or iNOS has been associated with direct stimulation of necrotic or apoptotic pathways. This is a reasonable alternative approach to pursue in melanoma therapy, as perhaps stimulation of the endogenous iNOS to the very high levels needed to induce apoptosis. One such example is exogenous NO regulating Fas-induced apoptosis via chemical modification on YY1 transcription factors [9]. NO effects are likely to be influenced by not only effective concentration but also the intracellular microenvironment, such as the pro-oxidant status of melanoma. The complexity and heterogeneity of cancers and within cancers, such as shown by our own studies that some melanoma are negative for iNOS and the patients with this type of tumor have a much less aggressive disease, argue strongly in favor of detailed research continuing in this field. We have previously reported that the iNOS enzyme is active in melanoma producing up to 8 pM/min/mg using the classic arginine to citrulline enzymatic assay [10], thus not only confirming that NO is produced by iNOS, but suggesting that the DAF is likely to be measuring NO as well.
In conclusion, melanoma tumor expression of iNOS provides an independent predictor of disease-specific survival for patients with Stage III melanoma. Not only do we propose this as useful information for patient prognosis, we further propose regulation of iNOS or quenching of NO in melanoma patients’ tumors as an adjunct approach to future therapeutic regimens in patients with iNOS positive tumors.
Figure 2.
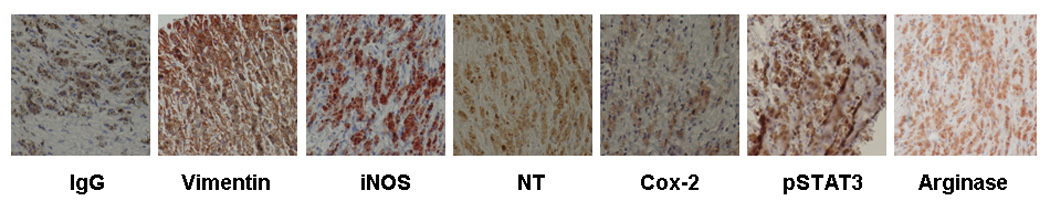
IHC of iNOS, and associated inflammatory markers, using isotype specific IgG as the negative control and anti-Vimentin as the positive control.
Figure 5. Growth inhibition of melanoma by NO scavenger.
3H uptake assay. A375 treated with c-PTIO (NO scavenger) followed by SIN-1 (NO donor to control for possible c-PTIO toxicity) overnight. Error bars represent +/− SD of triplicates.
Acknowledgements
The authors are grateful for the assistance of Engene T. Walch, Ph.D. in application of the DAF dye to our melanoma system. We also acknowledge the help of Omar Eton, M.D., and Antonio Buzaid, M.D, who directed the clinical trials in biochemotherapy on which the patients were enrolled. We are most grateful to Jeffrey E. Gershenwald and Victor Prieto for their support in tumor tissue acquisition and patient bioinformatics support. This work was supported by NIH SPORE in Melanoma, P50 CA093459 (EAG, JAE, SE) and by R21 CA111369 (JAE) and K22 CA097983 (JAE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, Grimm EA. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6:4768–4775. [PubMed] [Google Scholar]
- 2.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int. J. Cancer. 2006;119:861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 3.Meyskens FL, Jr, McNulty SE, Buckmeier JA, Tohidian NB, Spillane TJ, Kahlon RS, Gonzalez RI. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic. Biol Med. 2001;31:799–808. doi: 10.1016/s0891-5849(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 4.Tang CH, Grimm EA. Depletion of endogenous nitric oxide enhances cisplatin-induced apoptosis in a p53-dependent manner in melanoma cell lines. J Biol. Chem. 2004;279:288–298. doi: 10.1074/jbc.M310821200. [DOI] [PubMed] [Google Scholar]
- 5.Eton O, Legha SS, Bedikian AY, Lee JJ, Buzaid AC, Hodges C, Ring SE, Papadopoulos NE, Plager C, East MJ, Zhan F, Benjamin RS. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J. Clin. Oncol. 2002;20:2045–2052. doi: 10.1200/JCO.2002.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Salvucci O, Carsana M, Bersani I, Tragni G, Anichini A. Antiapoptotic role of endogenous nitric oxide in human melanoma cells. Cancer Res. 2001;61:318–326. [PubMed] [Google Scholar]
- 7.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol. Life Sci. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson CM, Buzaid AC, Sussman J, Lee JJ, Ali-Osman F, Braunschweiger PG, Plager C, Bedikian A, Papadopoulos N, Eton O, Legha SS, Grimm EA. Nitric oxide and neopterin levels and clinical response in stage III melanoma patients receiving concurrent biochemotherapy. Melanoma Res. 1998;8:149–155. doi: 10.1097/00008390-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Garban HJ, Bonavida B. Nitric oxide inhibits the transcription repressor Yin-Yang 1 binding activity at the silencer region of the Fas promoter: a pivotal role for nitric oxide in the up-regulation of Fas gene expression in human tumor cells. J. Immunol. 2001;167:75–81. doi: 10.4049/jimmunol.167.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Zheng M, Ekmekcioglu S, Walch ET, Tang CH, Grimm EA. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004;(3):165–171. doi: 10.1097/01.cmr.0000129374.76399.19. [DOI] [PubMed] [Google Scholar]