Summary
Germline mutations of BRCA1 predispose women to breast and ovarian cancers. However, the downstream mediators of BRCA1 function in tumor suppression remain elusive. We found that human BRCA1-associated breast cancers have lower levels of SIRT1 than their normal controls. We further demonstrated that mammary tumors from BRCA1 mutant mice have low levels of SIRT1 and high levels of Survivin, which is reversed by induced expression of BRCA1. BRCA1 binds to the SIRT1 promoter and increases SIRT1 expression, which in turn inhibits Survivin by changing the epigenetic modification of histone H3. Absence of SIRT1 blocks the regulation of Survivin by BRCA1. Furthermore, we demonstrated that activation of SIRT1 and inhibition of Survivin expression by resveratrol elicit a more profound inhibitory effect on BRCA1-mutant cancer cells than on BRCA1-wild type cancer cells both in vitro and in vivo. These findings suggest that resveratrol treatment serves as an excellent strategy for targeted therapy for BRCA1-associated breast cancer.
Keywords: BRCA1, Resveratrol, SIRT1, Survivin, Cancer treatment
Introduction
Breast cancer is one of the most prevalent cancers and affects 1/8 of the women during their lifetime. About 8% of breast cancer cases are inheritable, caused by mutations of tumor suppressor genes, such as breast cancer associated gene-1 and -2 (BRCA1 and BRCA2) and other unidentified tumor suppressor genes (Alberg et al., 1999; Eccles and Pichert, 2005; Zhang and Powell, 2005). BRCA1 is the most frequently mutated tumor suppressor gene found in familial breast cancers. It is estimated that BRCA1 mutation carriers have a 50–80% risk of developing breast cancer by age 70 (Easton et al., 1995; Ford et al., 1998; Struewing et al., 1997).
BRCA1 null mice died at early embryonic developmental stages due to cellular defects, developmental retardation and genetic instability (reviewed in (Deng, 2002)). Two strains carrying targeted truncation or deletion of BRCA1 exon 11 survived to adulthood in a specific genetic background (Ludwig et al., 2001) or a p53+/− mutation background (Brca1Δ11/Δ11;p53+/−) (Xu et al., 2001). Analysis of these mutant mice and mutant cells revealed that BRCA1 plays essential roles in numerous important biological processes, including protein degradation, cell cycle progression, apoptosis, DNA damage repair, centrosome duplication, transcription regulation, and animal aging (reviewed in (Deng, 2006)). BRCA1 mutant mice carrying a Cre-LoxP mediated deletion of full-length BRCA1 in mammary tissues (Brca1Co/Co;MMTV-Cre) developed mammary tumors in about a year and a half (Brodie et al., 2001; Xu et al., 1999). Of note, after deletion of one p53 wild type allele, about 95% of the mutant mice (Brca1Co/Co;MMTV-Cre;p53+/−) developed mammary tumors before 12 months of age (Brodie et al., 2001; Xu et al., 1999). Mammary tumor cells derived from mammary specific knockout mouse models have also been used to study chemoprevention and therapeutic potential of various drugs (Simeone et al., 2005; Tominaga et al., 2007).
Resveratrol, a phytoalexin found in grapes, fruits, and root extracts of the weed polygonum cuspidatum, is an important constituent of traditional Japanese and Chinese medicine. Recent publications have demonstrated that resveratrol has chemopreventive activity against various cancers including leukemia (Estrov et al., 2003), DMBA induced mammary tumors in rat (Whitsett et al., 2006), skin cancer (Aziz et al., 2005), and prostate cancer (Hsieh and Wu, 2000). It was shown that resveratrol inhibits tumor progression by inducing cell cycle arrest and apoptosis while presenting very low cytotoxicity. Moreover, by binding to plasma proteins, resveratrol in the blood exhibits a prolonged effect and is sufficient for anti-invasive activity. Interestingly, low doses of resveratrol can sensitize cells to low doses of cytotoxic drugs, therefore facilitating innovative strategies to enhance the efficacy of anticancer therapy in various human cancers (reviewed in (Aggarwal et al., 2004; Delmas et al., 2006)). While the underlying mechanism remains elusive, it has been shown that resveratrol has the ability to activate SIRT1 deacetylase activity (Howitz et al., 2003) and to inhibit intracellular Survivin level (Aziz et al., 2005).
SIRT1 is the mammalian homolog of yeast Sir2 and belongs to the 7 member family of NAD+ dependent type III histone and protein deacetylases. At telomeres, yeast Sir2 is required to establish and maintain telomeric heterochromatin (Denu, 2003; Gasser and Cockell, 2001). Sir2 also plays important roles in DNA damage repair and in suppressing the recombination between rDNA repeats (Guarente, 2000). The mammalian SIRT1 deacetylates many non-histone proteins, including p53 and translational elongation factor II. It has been shown that SIRT1 plays a role in many important biological processes including apoptosis, neuronal protection, adaptation to calorie restriction, organ metabolism and function, cellular senescence, and aging (Baur et al., 2006; Haigis and Guarente, 2006). The role of SIRT1 in tumorigenesis, however, remains elusive due to some conflicting data obtained in different experimental systems.
Survivin is one of the apoptosis inhibitors that has been implicated in controlling cell division and inhibiting apoptosis (Zaffaroni et al., 2005). Survivin is also required for a sustained spindle checkpoint arrest in response to the lack of tension at the kinetochores of sister-chromatids (Lens et al., 2003). Because expression level of Survivin is dramatically elevated in many types of tumors, it is believed that Survivin maintains cell viability and promotes tumor growth (Kennedy et al., 2003; Ma et al., 2003). In support of this theory, down regulation of Survivin triggers apoptosis and arrests growth of many types of tumor cells both in vitro and in vivo (reviewed in (Zaffaroni et al., 2005)). A change in Survivin expression level also provides an accessible biomarker of target validation for patients treated with inhibitors of HSP90, the EGFR family, or CDK1 (reviewed in (Altieri, 2008)).
In this study, we have investigated relationship among BRCA1, SIRT1, Survivin, and resveratrol. We found that SIRT1 and Survivin serve as downstream mediators of BRCA1 function in tumor suppression, and that resveratrol inhibited growth of BRCA1 mutant tumor cells in culture and in allografted nude mice. We demonstrated that the ability of resveratrol to inhibit BRCA1-mutant tumor growth is associated with upregulation of SIRT1 activity followed by reduction of Survivin, triggering apoptosis of the tumor cells.
Results
BRCA1 positively regulates SIRT1 expression
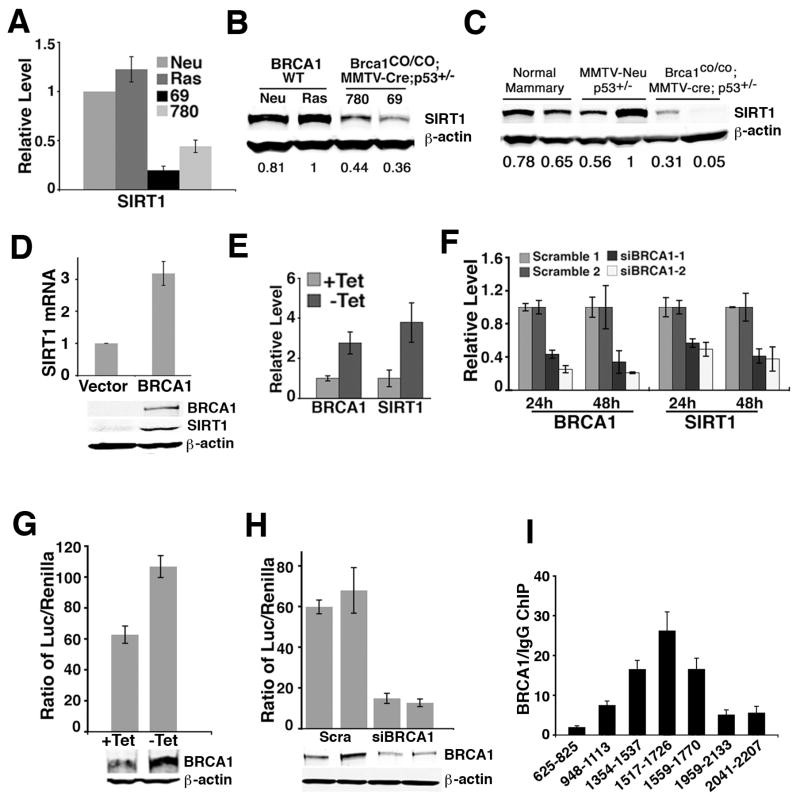
To study the role of SIRT1 in BRCA1-associated tumorigenesis, we examined the expression of SIRT1. We found that SIRT1 mRNA and protein levels were significantly lower in cell lines derived from mammary tumors lacking full-length BRCA1 as compared to cell lines derived from tumors without BRCA1 mutation (Fig. 1A, B). Primary mammary tumors harvested from Brca1Co/Co;MMTV-Cre;p53+/− mice also exhibited lower levels of SIRT1 than tumors isolated from MMTV-cNeu;p53+/− transgenic mice that were wild type for BRCA1, and wild type mammary glands (Fig. 1C). We also detected a reduction of SIRT1 levels in two human BRCA1 mutant breast cancer cell lines, HCC1937 and L56Br-C1 than in two cell lines derived from sporadic breast cancers, BT20 and MCF-7 (Supplementary Fig. 1A). Analyzing a public database, we found significantly reduced SIRT1 mRNA levels in 31 breast cancer samples with a BRCA1 mutation compared with 128 breast cancer samples without BRCA1 mutation (Supplementary Fig. 1B), and in 18 breast cancers with a BRCA1 mutation compared with 97 breast cancers with no BRCA1 mutation (Supplementary Fig. 1C). Positive correlation between BRCA1 and SIRT1 expression was also observed from immunohistochemical staining of 45 human breast cancer samples (Supplementary Fig. 1D). These observations suggest that BRCA1 plays a role in maintaining SIRT1 expression.
Figure 1.
BRCA1 positively regulates SIRT1 expression. (A, B) Levels of SIRT1 in mouse mammary tumor cells lines, Neu and Ras, derived from BRCA1 wild type (MMTV-cNeu and MMTV-ras transgenic mice, respectively), and 69 and 780, derived from BRCA1 mutant (Brca1Co/Co;MMTV-Cre;p53+/−) mice measured using 3 pairs of SIRT1 primers by Reatime RT-PCR (A) and Western blot (B). The intensity of bands was measured by Quantity One Software (Bio-Rad) and normalized using intensity of β-actin. The quantified numbers are shown at the bottom of the picture. (C) Western blot showing SIRT1 levels in normal mammary glands of wild type mouse, and primary mammary tumors derived in MMTV-cNeu;p53+/− and Brca1Co/Co;MMTV-Cre;p53+/− mice. (D) Expression of SIRT1 in vector and wild type BRCA1 reconstituted HCC-1937 cell lines by Western blot and Realtime RT-PCR. (E) Increased BRCA1 in UBR60 cells (by tetracycline withdrawal, −Tet) up-regulates endogenous SIRT1 as revealed by Realtime RT-PCR. (F) Acute knockdown of BRCA1 in UBR60 cells by BRCA1 specific, but not scrambled, siRNA decreased SIRT1 expression, revealed by Realtime RT-PCR. (G,H) BRCA1 positively regulates the SIRT1 promoter. UBR60 cells were transfected with a SIRT1 luciferase reporter (S-2852) and SIRT1 promoter activity was measured 24 hours post transfection in the presence (+Tet), absence (−Tet) of over-expressed BRCA1 (G), or acute knockdown of endogenous BRCA1 (H). Two sets of scrambled siRNA and BRCA1 siRNA were used, and BRCA1 protein levels were shown below the panels. (I) ChIP assay by Realtime PCR showing that BRCA1 binds to SIRT1 promoter from position 1354-1770 bp. Data in D–I are presented as average±SD.
To provide further evidence that BRCA1 is indeed required for SIRT1 expression, we compared expression levels of SIRT1 between HCC1937, a human BRCA1 mutant breast cancer cell line, before and after BRCA1 reconstitution, and found that expression of BRCA1 upregulated SIRT1 at both protein and mRNA levels (Fig. 1D). To investigate this further, we used UBR60 cells, in which BRCA1 cDNA was stably transfected under the control of a tet-off promoter (Harkin et al., 1999). In the absence of tetracycline (−Tet), the expression of SIRT1 was increased upon induction of BRCA1 expression (Fig. 1E, Supplementary Fig. 2A). Conversely, acute knockdown of BRCA1 using BRCA1 specific siRNA, but not a control siRNA, reduced the expression level of SIRT1 (Fig. 1F, Supplementary Fig. 2B). To investigate whether BRCA1 regulates the SIRT1 promoter, we performed a luciferase reporter activity assay controlled by a 2.8 kb fragment of the SIRT1 promoter that was reported previously (Nemoto et al., 2004) in UBR60 cells. We showed that increasing BRCA1 by withdrawing tetracycline raised the Luc activity about 2 fold (Fig. 1G). UBR60 cells also express endogenous BRCA1 (+Tet). We showed that RNAi-mediated knockdown of BRCA1 decreased the luciferase activity about 5 fold (Fig. 1H).
To further study the regulation of SIRT1 promoter by BRCA1, chromatin immunoprecipitation assay (ChIP) with three individual BRCA1 antibodies was performed. PCR assays using the primers that cover 5 fragments in the 5′ regulatory region of the SIRT1 promoter in UBR60 cells under inducible condition (without tetracycline) showed that BRCA1 bound to 1354–1902 but not the other regions in the SIRT1 promoter, as revealed by regular PCR (Supplementary Fig. 2C) and real-time PCR (Fig. 1I). This data indicates that BRCA1 binds to the promoter of SIRT1.
Ectopic expression of SIRT1 inhibits BRCA1-associated cancer formation
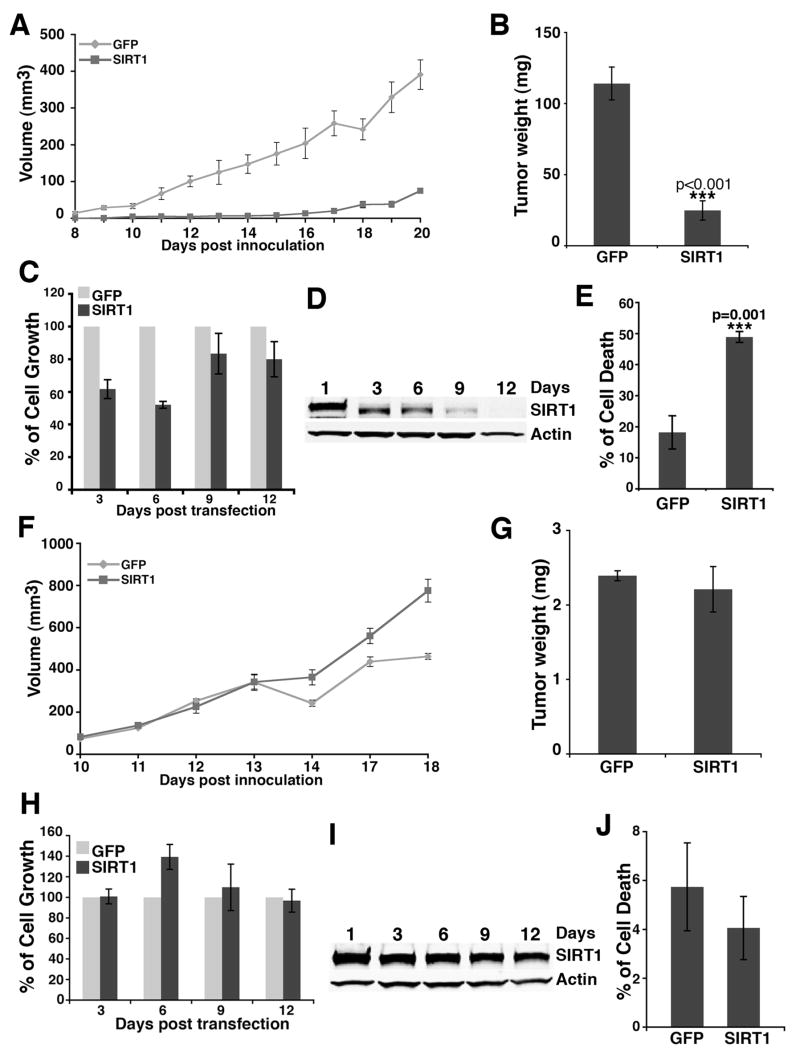
Reduced SIRT1 expression in BRCA1 mutant cancer prompted us to test if over expression of SIRT1 in these cells could inhibit tumor formation. However, we found that stable expression of SIRT1 in BRCA1 mutant cells inhibited cell growth (data not shown), which prevented the establishment of a stable SIRT1-expressing cell line. To overcome this difficulty, Brca1Co/Co;MMTV-Cre;p53+/− tumor cells were transfected with either SIRT1 or GFP, and 24 hours later, the transfected cells were sorted to 90% purity by AutoMACS and transplanted into nude mice. The results revealed that transfection of SIRT1 significantly inhibited the ability of Brca1Co/Co;MMTV-Cre;p53+/− cells to form tumors in nude mice (Fig. 2A). During the time course, SIRT1 transfected tumor cells had much more difficulty to initiate tumor formation. At harvest, tumor weight from the GFP transfected group (228 mg) was, on average, 4.56 fold of the weight from the SIRT1 over-expressed group (49.8 mg) (Fig. 2B). When the same batch of cells was followed in vitro during the same time course, we found that SIRT1 transfected cells grew poorly during the first 6 days, displaying a proliferation rate that was about 50–60% of the control (Fig. 2C). Corresponding to the reduced cell number during this period, the SIRT1 transfected cells, which expressed high levels of transfected SIRT1 (Fig. 2D), also exhibited dramatically increased cell death compared to control after day 5 (Fig. 2E). In contrast, ectopic overexpression of SIRT1 in a BRCA1 wild type cell line, Neu, did not inhibit tumor formation, nor cell proliferation (Fig. 2F–J). These data indicate that over expression of SIRT1 could inhibit BRCA1 mutant cell growth and tumor formation.
Figure 2.
Over-expression of SIRT1 inhibits growth of Brca1Co/Co; MMTV-Cre;p53+/− tumor cells. (A, B) Over-expression of SIRT1 inhibits the allografted tumor growth of Brca1Co/Co;MMTV-Cre;p53+/− tumor cells (cell line: 69) compared with GFP transfected cells as reflected by average tumor volume (mm3) ±SE (A) and average weight (mg) ±SE (B). The tumor size was measured daily starting one week post transplantation, and the tumor weight was recorded when animals were sacrificed. P is a student t-test value and represents the comparison between the GFP and SIRT1 tranfected tumors. (C–E) Over-expression of SIRT1 reduced growth of 69 cells (C) and induced cell death at the same time. Cell death at day 6 is shown. (E). (D) Western blot to measure the expression level of SIRT1 in SIRT1 transfected 69 cells at 1 to 12 days after sorting. (F, G) Over-expression of SIRT1 did not inhibit the allografted tumor growth of MMTV-Neu;p53+/−tumor cells (cell line: Neu) compared with GFP transfected cells by volume (F) and weight (G). (H–J) Over-expression of SIRT1 did not affect the growth (H) and cell death of Neu cells. Cell death at day 6 is shown. (J). (I) Western blot analysis to monitor the expression of SIRT1 in SIRT1 transfected Neu tumor cells at 1 to 12 days after sorting. For the nude mice study, each group of data was collected from 10 individual mice, bearing 2 inoculated tumors each. Data is presented as average±SD if it is not specifically mentioned.
Resveratrol inhibits BRCA1 mutant tumor growth
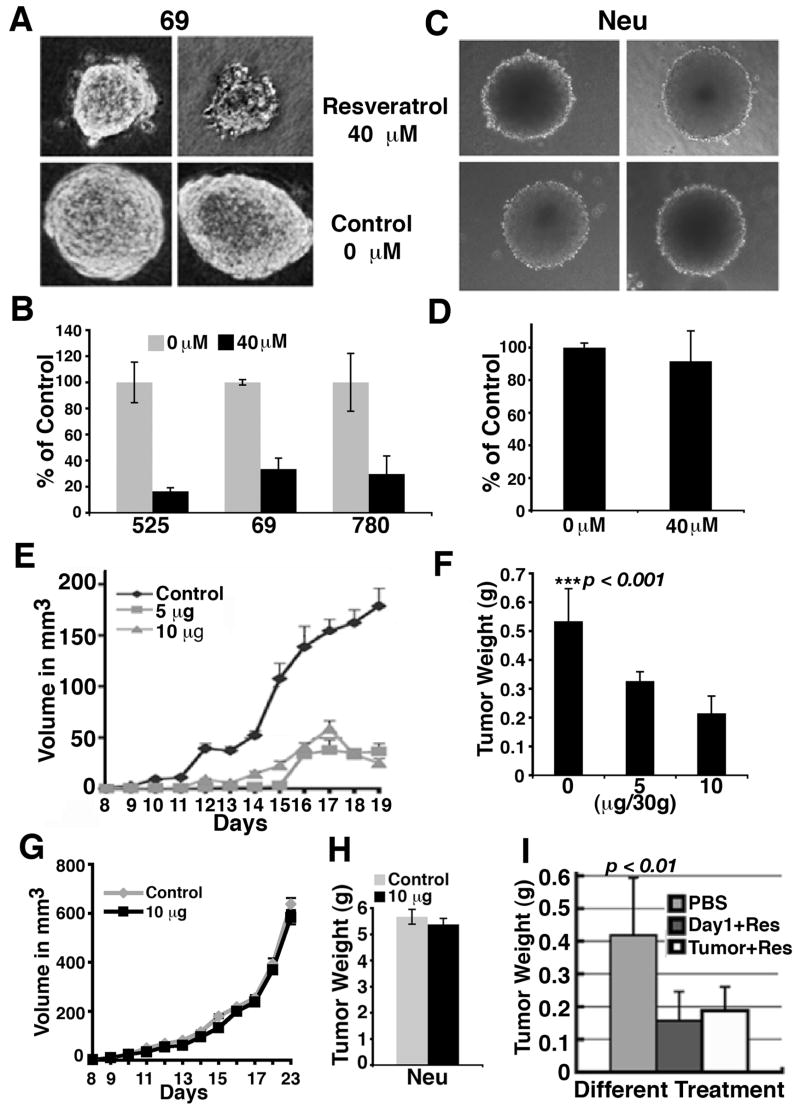
Resveratrol is a well-known agonist of SIRT1 (Aziz et al., 2005; Howitz et al., 2003). Since over-expression of SIRT1 was able to inhibit BRCA1 mutant tumor growth both in vitro and in vivo, we hypothesized that resveratrol could serve as a therapeutic agent that inhibits growth of BRCA1 mutant cancer cells. Consistently, we found resveratrol dramatically reduced the capability of BRCA1 mutant tumor cells to form colonies in soft agar, causing about a 5 fold decrease in colony size (Fig. 3A) and number (Fig. 3B) compared with untreated controls. Such an inhibitory effect was not obvious for a BRCA1 wild type cell line tested under identical conditions (Fig. 3C, D). Our data further indicated that resveratrol (40 μM) induced a much higher incidence of apoptosis in BRCA1 mutant tumor cells (69) than the BRCA1 wild type cells (Neu) (data not shown).
Figure 3.
Resveratrol inhibits Brca1Co/Co;MMTV-Cre;p53+/− tumor cell growth in vitro and in allograft tumors. (A, B) Resveratrol reduces Brca1Co/Co;MMTV-Cre;p53+/− tumor cell colony formation in soft agar in both size (A) and number (B). (C, D) Resveratrol does not affect MMTV-Neu;p53+/− tumor cell colony formation in soft agar in both size (C) and number (D). In (B) and (D), data are average ± SD. (E) Nude mice were pretreated with carrier (control), 5μg, or 10μg revesveratrol per 30g body weight daily by IP injection for 10 days. The Brca1Co/Co;MMTV-Cre;p53+/− tumor cells were implanted subcutaneously and resveratrol treatment was provided daily. The tumor size was measured daily starting 1-week post transplantation (E). Data shown is mean ±SE. The tumor weight was recorded when animals were sacrificed (F). Data is Mean±SE. P is a Student t-test value and represents the comparison between the control and two treated groups. (G, H) Resveratrol treatment did not inhibit allografted MMTV-Neu;p53+/− tumor cells in volume (G) and weight (H). In (G) and (H), data are Mean±SE. (I) Brca1Co/Co;MMTV-Cre;p53+/− tumor cells were innoculated subcutaneously into nude mice and resveratrol treatment started at 5μg/30g either next day (Day1+Res) or until the tumor was visible (Tumor+Res). For all in vivo resveratrol treatment, data for each group of allografted tumors was collected from 10 individual nude mice, bearing 2 tumors each. The data is presented as Mean±SE.
To study the effect of resveratrol on tumor growth in vivo, we designed 3 sets of experiments. In the first set, we pretreated the nude mice with resveratrol for ten days before mouse BRCA1mutant tumor cells were implanted. After the implantation, the treatment continued daily until the tumors were collected. We demonstrated that the treatment with either 5 μg or 10 μg of resveratrol significantly delayed tumor initiation and growth (Fig. 3E, F), whereas treatment of BRCA1 wild type tumor with 10 μg reveratrol did not inhibit tumor growth (Fig. 3G, H). In two other sets of experiments, the BRCA1 tumor cells were implanted into nude mice and resveratrol was applied either the next day for one set or right after the tumors became visible for the second set to test its therapeutic effects. Our data indicated that both treatment protocols were capable of significantly reducing tumor formation in the recipient mice (Fig. 3I). In addition, we found that the resveratrol treated tumors became less proliferative as revealed by BrdU incorporation than controls (Supplementary Fig. 3). TUNEL assay on these tumor sections demonstrated that resveratrol treatment induced apoptosis (Supplementary Fig. 3). During the treatment, all recipient mice showed no sign of drug toxicity, weight loss and growth abnormalities. These data suggest that resveratrol has chemoprevention potential for tumors induced by BRCA1 mutation.
Resveratrol inhibits BRCA1 mutant tumor growth through activating SIRT1
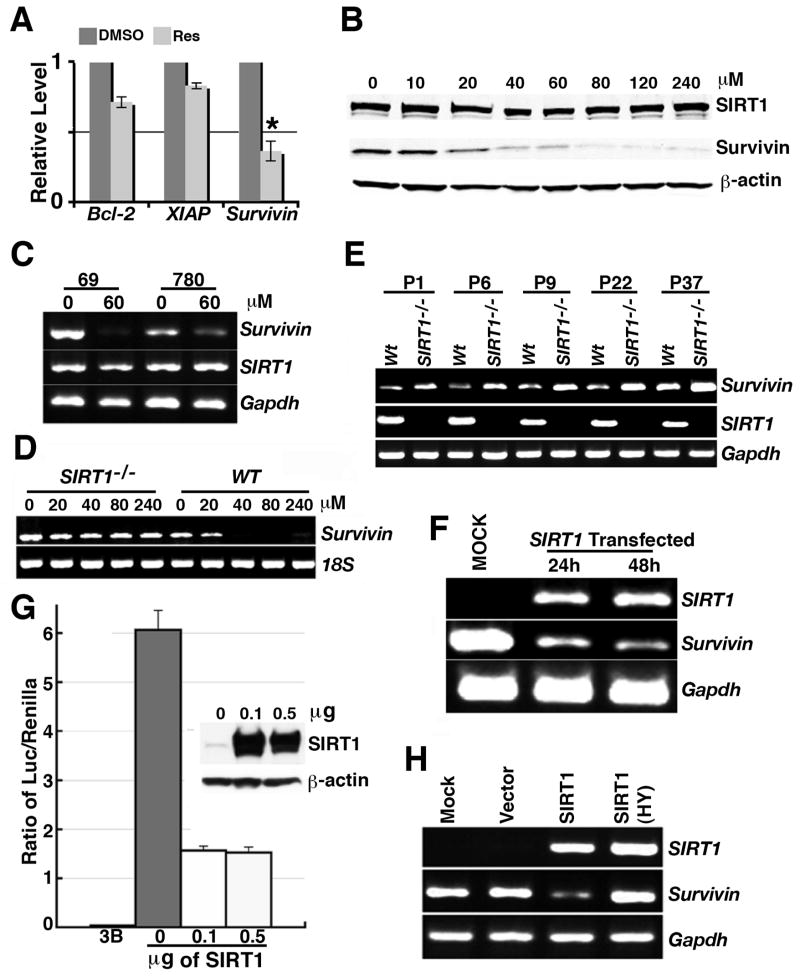
Because resveratrol induces apoptosis in cultured cells and allografted tumors, we checked expression of a number of genes that are involved in apoptosis. Our analysis by real-time RT-PCR revealed a significant reduction of Survivin expression upon resveratrol treatment while expression of Bcl2 and XIAP was also reduced but not significantly (Fig. 4A). Because Survivin is a well-known anti-apoptotic protein, we decided to investigate further how resveratrol treatment reduced expression of Survivin. Our data revealed that resveratrol treatment reduced Survivin, an anti-apoptotic gene, at both protein (Fig. 4B) and mRNA (Fig. 4C) levels. In contrast, there was no obvious effect on the expression levels of SIRT1 upon the treatment of resveratrol, which is consistent with the notion that resveratrol affects SIRT1 primarily through increasing its enzymatic activity rather than its expression. To provide evidence that the effect of resveratrol on Survivin is indeed mediated by SIRT1, we studied Survivin expression in resveratrol treated SIRT1+/+ and SIRT1−/− mouse embryonic fibroblast cells (MEFs). Absence of SIRT1 completely blocked the ability of resveratrol to inhibit Survivin transcription (Fig. 4D) providing compelling evidence that resveratrol inhibits Survivin expression through SIRT1. We next followed expression of endogenous Survivin in MEFs from passage 1 up to 33 passages, and found that the absence of SIRT1 resulted in up-regulation of Survivin transcription in all passages tested (Fig. 4E), suggesting that SIRT1 inhibits Survivin expression.
Figure 4.
Resveratrol down-regulates Survivin through SIRT1 at the transcriptional level. (A) Resveratrol treatment significantly inhibited expression of Survivin and also reduced expression of Bcl2 and XIAP in 69 cells. “*” represents p < 0.05 by Student T-test. Data is average±SD.(B) Resveratrol treatment reduced Survivin protein level but did not change SIRT1 level in Brca1Co/Co;MMTV-Cre;p53+/− tumor cells three days after the treatment. (C) Resveratrol inhibits Survivin expression from mRNA level in Brca1Co/Co;MMTV-Cre;p53+/− tumor cells (69 and 780) as assessed by RT-PCR. (D) In Sirt1−/− cells, resveratrol cannot reduce Survivin mRNA level. (E) In Sirt1−/− MEFs, Survivin expression was increased. Data from MEFs of different passages (P) are shown. (F) Reconstituting SIRT1 in Sirt1−/− cells was able to reduce Survivin mRNA expression. (G) SIRT1 down-regulates Survivin promoter activity. Insert shows the levels of SIRT1 after transfection with increasing amount of DNA. Data is average±SD. (H) Deacetylase activity of SIRT1 is required for inhibition of Survivin mRNA expression by SIRT1. SIRT1 knockout MEFs were transfected with plasmids either carrying wildtype SIRT1 (SIRT1) or catalytic site mutated SIRT1 (SIRT1-HY).
To provide evidence that SIRT1 regulates Survivin expression, we performed two further experiments. First, a SIRT1 expression vector was reconstituted into Sirt1−/− MEFs. RT-PCR analysis revealed that reconstitution of SIRT1 in Sirt1−/− cells decreased Survivin expression (Fig. 4F). We then analyzed Survivin promoter activity using a luciferase reporter assay (Chen et al., 2004) after transfection of SIRT1 (Fig. 4G). We showed that over-expression of SIRT1 decreased Survivin expression by about 4 fold, indicating that SIRT1 is capable of efficiently inhibiting Survivin promoter activity.
SIRT1 negatively regulates Survivin expression through epigenetic chromatin modification
Since SIRT1 is a well-known deacetylase (Blander and Guarente, 2004; Guarente, 2000), we were interested in determining whether SIRT1 regulates Survivin expression through its deacetylase activity. To address this, we transfected wild type SIRT1 and its mutant form, SIRT1 (HY) that lacks the deacetylase activity, into Sirt1−/− MEFs, respectively. RT-PCR analysis revealed that while the wild type SIRT1 construct sufficiently repressed Survivin transcription, the deacetylase defective form of SIRT1 failed to reduce Survivin expression (Fig. 4H). This observation provides compelling evidence that the deacetylase activity of SIRT1 is required for suppressing Survivin transcription.
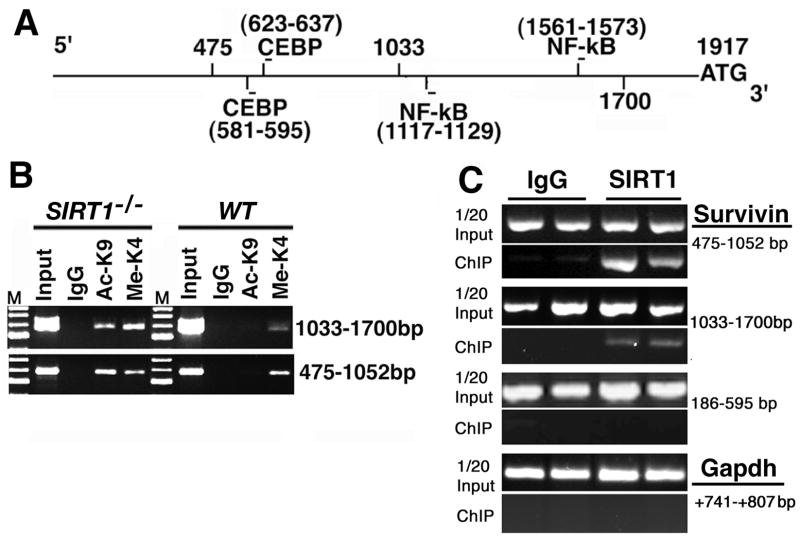
Next, we investigated whether SIRT1 affects the Survivin promoter through modification of histone H3 lysine 9 (K9), a well-known SIRT1 deacetylase target (Shankaranarayana et al., 2003). To explore this possibility, we carried out a ChIP assay in both wild type and SIRT1 knockout MEFs on a 2kb region of the Survivin promoter upstream of the start codon (Fig. 5A). Within this 2kb fragment, there are 2 regions (475–1052bp and 1033–1700bp) in which the acetylation of lysine 9 was dramatically increased in Sirt1−/− cells (Fig. 5B). To follow this up, we performed ChIP analysis with a SIRT1 antibody. Our data indicated that SIRT1 bound to both fragments, with a stronger binding to the fragment from 475-1052 bp than the fragment from 1033-1700bp in the Survivin promoter (Fig. 5C). In contrast, SIRT1 did not bind to the fragment from 186–595 and the promoter of the Gapdh gene, both of which served as negative controls (Fig. 5C).
Figure 5.
SIRT1 displays important epigenetic regulation of the Surivivn promoter. (A) Diagram of the 2kb region of the mouse Survivin promoter, showing the NF-κB and CEBP binding sites. (B) Loss of SIRT1 increases the acetylation level of histone H3 lysine 9 on two fragments of the Survivin promoter, 1033-1700 bp and 475-1052 bp, revealed by ChIP assay. (C) SIRT1 binds to the Survivin promoter on fragment 475-1052bp strongly, and on 1033-1700 weakly as revealed by ChIP. SIRT1 does not bind to the fragment 186–595 in Survivin promoter, and the promoter of the Gapdh gene, which was used as negative controls.
Survivin plays an important role in BRCA1 associated mammary tumor formation
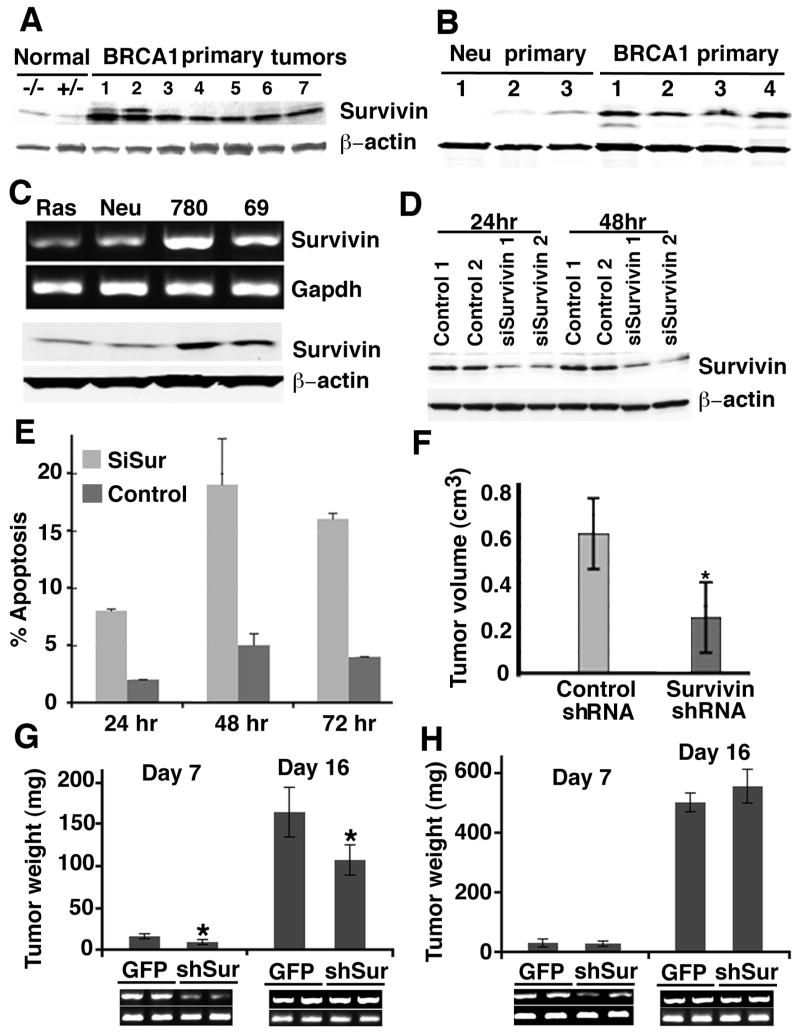
Survivin has been reported to be involved in tumorigenesis in many types of cancer. Since both SIRT1 and Survivin expression level changed when BRCA1 level was manipulated, we wanted to study the role of Survivin in BRCA1-associated tumorigenesis. Survivin expression level was examined in primary tumors isolated from Brca1Co/Co;MMTV-Cre;p53+/− mice. As all of these tumors had lost the remaining wild-type allele of p53 (Brodie et al., 2001; Xu et al., 1999), we used normal mammary tissues isolated from pregnant p53−/− and p53+/− mice at day 14.5 as controls. Western blot analysis revealed a slight increase of Survivin in p53−/− mammary tissues in comparison with p53+/− mammary tissues (Fig. 6A). This observation suggests that p53 represses Survivin expression in mammary tissue, which is consistent with a previous finding observed in cultured cells (Hoffman et al., 2002). The tumors had an average of 10 fold more Survivin than normal mammary tissues (Fig. 6A). Similarly increased Survivin was also observed in primary mammary tumors derived from Brca1Co/Co; MMTV-Cre;p53+/− mice compare with MMTV-Neu transgenic mice (Fig. 6B) and in cultured BRCA1 mutant cancer cells (Fig. 6C). Significantly, increased Survivin mRNA levels were also found in human breast cancer samples with a BRCA1 mutation compared with breast cancer samples without BRCA1 mutation (Supplementary Fig. 4). These data suggest that Survivin may play an important role during tumorigenesis associated with BRCA1 deficiency.
Figure 6.
Brca1Co/Co;MMTV-Cre;p53+/− tumors show increased Survivin expression. (A–C) Brca1Co/Co;MMTV-Cre;p53+/− primary tumors (A,B) and tumor cell lines (C) express higher Survivin as revealed by Western blot and mRNA level. (D) Western blot analysis showing knockdown efficiency of two mouse Survivin specific siRNA oligos. (E) Transfection with mouse Survivin siRNA oligos induced apoptosis in Brca1Co/Co;MMTV-Cre;p53+/− tumor cells. (F–H) Survivin shRNA significantly inhibited tumor formation of Brca1Co/Co;MMTV-Cre;p53+/− tumor cells in nude mice allograft as reflected by volume (F) and weight (G), but did not reduce tumor formation of MMTV-cNeu;p53+/− tumor cells (H) in nude mice. The RT-PCR on the bottom shows Survivin expression (upper panel) from 2 individual tumors of each treatment. Lower panel is Gapdh level as internal control. From (E) to (H), data are presented as average ± SD.
Since Survivin inhibits apoptosis, we hypothesized that over expression of Survivin could protect mammary cells from cell death associated with BRCA1 deficiency. To test this, we performed RNA interference (RNAi) in BRCA1 mutant mammary tumor cells using two specific siRNA oligos to mouse Survivin and two control oligos (Fig. 6D). Transfection of Survivin RNAi triggered apoptosis in about 15–20% cells at 48h post-transfection (Fig. 6E). Next, we transfected BRCA1 mutant tumor cells with a vector-based shRNA that was shown to specifically knockdown Survivin mRNA (Coumoul et al., 2004). The transfected cells were sorted to 90% purity by AutoMACS and then transplanted into nude mice. ShRNA specific for Survivin significantly slowed the growth of BRCA1 mutant tumors in volume (Fig. 6F) and weight (Fig. 6G), but it did not have significant effects on MMTV-Neu mediated tumor growth (Fig. 6H). These observations suggest that Survivin plays an important role in BRCA1 associated mammary tumor formation but it is not essential for mammary tumors driven by activation of MMTV-Neu signaling in these model systems.
Interplay among BRCA1, SIRT1, and Survivin
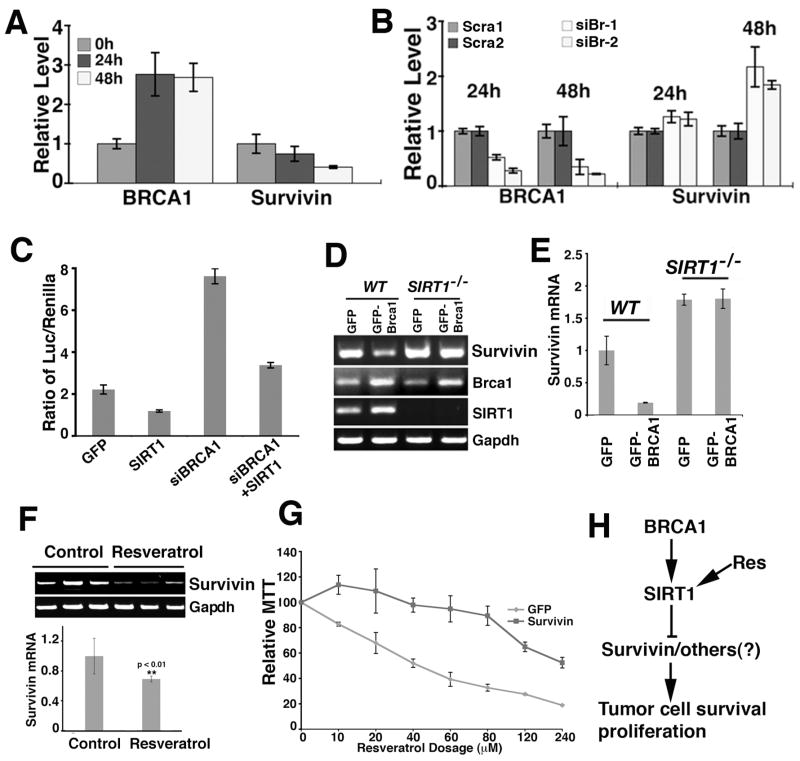
Since both SIRT1 and Survivin expression levels changed in BRCA1 mutant cells, next we studied the potential relationship among BRCA1, SIRT1 and Survivin. First, we investigated whether BRCA1 can affect Survivin expression. Using UBR60 cells, we found that induction of BRCA1 inhibited Survivin expression (Fig. 7A) and, conversely, acute suppression of BRCA1 using BRCA1 specific RNAi increased Survivin levels (Fig. 7B). To investigate whether BRCA1 regulates the Survivin promoter, luciferase activity assays were carried out in UBR60 cells. We showed that reducing BRCA1 by an RNAi approach elevated Luc activity more than 5 fold. Of note, transfection of a SIRT1 expression vector significantly inhibited the effect of BRCA1 RNAi (Fig. 7C). These data suggest that BRCA1 plays a very important role in maintaining the correct level of both SIRT1 and Survivin.
Figure 7.
BRCA1 regulates Survivin through SIRT1. (A–C) BRCA1 negatively regulates Survivin expression. Over-expression of BRCA1 decreases the level of Survivin (A), while down-regulation of BRCA1 by siRNA up-regulates Survivin as revealed by Realtime PCR (B). (C) Down-regulation of BRCA1 results in about a 5 fold increase in Survivin promoter activity, while over-expression of SIRT1 on top of siBRCA1 significantly reduced the activity. Two BRCA1 siRNA exhibited similar results and data from one siRNA was shown. (D, E) Over-expression of BRCA1 only reduces Survivin in SIRT1 wild type cells, but not in SIRT1−/− cells as revealed by RT-PCR (D), and by Realtime RT-PCR (E). (F) Resveratrol treatment reduced Survivin expression in Brca1Co/Co;MMTV-Cre;p53+/− allografted tumors in nude mice. Tumors from 3 pairs of individual nude mice were analyzed. (G) Ectopic overexpression of Survivin significantly reversed the lethality caused by resveratrol. (H) A model showing a BRCA1-SIRT1-Survivin axis signaling pathway involved in tumor cell survival and proliferation. Our data indicates that resveratrol treatment induces deacetylase activity of SIRT1, which inhibits Survivin and potentially other factors leading to reduced cell proliferation and increased apoptosis. Data in (A–B), (C), (E–G) are average ± SD.
Next, we investigated whether the inhibition of BRCA1 on Survivin is mediated by SIRT1. To address this, we compared Survivin expression levels in Sirt1−/− and Sirt1+/+ MEFs after ectopically expressing BRCA1 and a GFP control vector. Our data indicated that expression of BRCA1 could only inhibit Survivin expression in Sirt1+/+ cells but not in Sirt1−/− cells (Fig. 7D, E). This observation supports the notion that Survivin mediates the inhibitory effect of resveratrol on BRCA1 mutant cells. Consistently, tumors from resveratrol treated nude mice contained much less Survivin than the tumors from mock treated mice (Fig. 7F). To provide evidence that reduced Survivin is responsible for the decreased tumor growth, we transfected cells with a vector that expresses Survivin from the SV40 promoter that cannot be regulated by SIRT1. The data indicated that ectopic expression of Survivin reversed the lethal effect of resveratrol treatment up to 80 μM, while reduced proliferation was observed after treatment with higher concentrations of resveratrol (Fig. 7G). Because higher concentrations of resveratrol could also inhibit BRCA1 wild type tumor cells (data not shown), this data indicate that ectopical over expression of Survivin renders the BRCA1 mutant cells resistant to resveratrol to a certain level, comparable with BRCA1 wild type cells.
Discussion
We have studied the therapeutic effect of resveratrol on BRCA1 mutant breast cancer cells. There are a number of findings that can be concluded from this study. First, we demonstrated that BRCA1 is involved in modulating expression of SIRT1 and Survivin. The absence of BRCA1 results in low levels of SIRT1 and high levels of Survivin. Second, we found that resveratrol treatment decreases expression of Survivin through increasing SIRT1 activity. We further demonstrated that SIRT1 negatively regulates Survivin expression through its deacetylase activity, which epigenetically modifies the Survivin promoter and turns the promoter into a transcription silent configuration. Third, we demonstrated that resveratrol is a potent inhibitor for the initiation and progression of BRCA1 mutant cancer both in vitro and in vivo. These findings suggest that resveratrol may serve as an excellent reagent for targeted therapy for BRCA1 associated breast cancers.
It has been shown that BRCA1 acts as a transcription factor that positively or negatively regulates expression of many important genes, including p21, Gadd45, Mad2, and IGF axis members (Harkin et al., 1999; Shukla et al., 2006; Wang et al., 2004). Here we found that BRCA1 is involved in maintaining SIRT1 expression as reflected by reduced expression of SIRT1 in BRCA1 mutant MEFs, cultured cancer cells, and primary tumors. We further revealed that BRCA1 positively regulates SIRT1 promoter activity. Currently, several factors have been reported to affect SIRT1 expression, including p53, HIC1 and Foxo3a (Chen et al., 2005b; Nemoto et al., 2004). The potential relationship between BRCA1 and these factors in relation to SIRT1 expression is unclear, and remains to be addressed in future studies.
In yeast, Sir2 is involved in aging and longevity (Guarante, 2005); in mice, SIRT1 is responsible for an increased physical activity caused by caloric restriction (Chen et al., 2005a). Our previous study reveals that BRCA1 mutant mice undergo premature aging and suffer from high risk of spontaneous tumor formation (Cao et al., 2006; Cao et al., 2003; Cao et al., 2007). Thus, reduced SIRT1 expression upon BRCA1 deficiency is consistent with the premature aging phenotype exhibited in the BRCA1 mutant mice. On the other hand, the role of SIRT1 in cancer formation is currently under active discussion. It was shown that activation of SIRT1 down-regulates stress-induced p53 and Foxo pathways and protects cells from apoptosis (anti-apoptosis) (Brunet et al., 2004; Motta et al., 2004). In contrast, it was shown that activation of SIRT1 by resveratrol treatment inhibits NF-kappaB activity and sensitized cells to TNFα-induced apoptosis (pro-apoptosis) (Yeung et al., 2004). Based on the fact that SIRT1 deacetylates p53 to decrease its activity, it was hypothesized that increased SIRT1 activity may elevate the risk of cancer in mammals (Chen et al., 2005b). However, recent studies revealed that SIRT1 might serve as a tumor suppressor for certain tumor types in mice (Firestein et al., 2008, Wang et al. 2008).
These observations, which are contradictory on the surface, suggest that SIRT1 may play different or opposite roles in different populations of cells. This perhaps is because SIRT1 can affect multiple pathways with different biological functions, whereas the availability of these pathways in different cells may cause different phenotypes upon activation of SIRT1. For example, the previous finding that activation of SIRT1 might reduce p53 activity and therefore, elevate the risk of cancer in mammals (Chen et al., 2005b) should not be a concern in our study as virtually all BRCA1 mutant cancer cells are p53 deficient (Brodie et al., 2001; Xu et al., 1999). This may account for the reason that activation of SIRT1 does not cause proliferation, but rather induces apoptosis through inhibition of Survivin expression. More importantly, we showed that ectopically over-expression of SIRT1 in BRCA1 mutant cells inhibits tumor formation in nude mice and reduces cell growth in cultured cells. Thus, our data indicate that SIRT1 serves as a tumor suppressor in the context of BRCA1 deficiency, although it remains elusive whether SIRT1 has a general tumor suppressor role of SIRT1 in other types of tumors.
Survivin is a well-known anti-apoptotic protein (Zaffaroni et al., 2005). We demonstrated that all BRCA1 mutant primary tumors and cultured tumor cell lines express Survivin at levels significantly higher than controls. Thus, we concluded that escaping apoptosis is one of the major events that BRCA1 tumor cells adapt to survive and proliferate. This is primarily because loss of BRCA1 triggers apoptosis due to extensive DNA damage and abnormal cell cycle progression (reviewed in (Deng, 2006)). Apparently, an increase in Survivin raises the apoptotic threshold, providing a suitable environment for cells with genetic instability to proliferate. This may explain why inhibition of Survivin kills BRCA1 mutant cells more profoundly than cells driven by activated ErbB2/Neu signaling tested in this study. We further demonstrated that resveratrol treatment could significantly decrease levels of Survivin, reduce cell growth and increase apoptosis. Our data indicates that SIRT1 binds to the Survivin promoter and inhibits Survivin expression. Furthermore, it was recently reported that NF-kB also plays a positive role in Survivin expression (Kawakami et al., 2005). Therefore, the decreased levels of Survivin upon resveratrol treatment could be due to a direct inhibitory role of SIRT1 on Survivin and/or an indirect role of SIRT1 through decreased NF-kB activity. In all three protocols tested, the resveratrol treatment clearly delays the onset of tumor initiation and retards their progression. We also noticed that the resveratrol treatment alone does not completely block tumor development. This may be due to the fact that BRCA1 associated cancers have undergone many additional molecular/genetic alterations (Brodie et al., 2001). Furthermore, some other factors, besides SIRT1, may also regulate Survivin expression (Sato et al., 2006; Xia et al., 2006). These observations suggest that targeting Survivin specifically in addition to a combination of traditional chemotherapeutic drugs is needed to eliminate the cells with genetic mutations for tumorigenesis and minimize the frequency of tumor re-occurrence.
In conclusion, our data indicates that BRCA1 plays an important tumor suppressor function through maintenance of SIRT1 expression, which in turn inhibits expression of Survivin directly and/or indirectly (Fig. 7H). The absence of BRCA1 causes reduced levels of SIRT1 and increased expression of Survivin, allowing BRCA1 deficient cells to overcome growth defects and apoptosis and finally undergo malignant transformation. Our analysis reveals that resveratrol strongly inhibits the growth of BRCA1 mutant tumors both in vitro and in vivo. This is accomplished through up-regulating SIRT1 activity followed by reduction of Survivin expression, leading to growth arrest and apoptosis of BRCA1 deficient cancer cells (Fig. 7H). This provides a basis for the use of resveratrol on chemoprevention and therapeutic treatment of BRCA1 associated cancer patients.
Experimental procedures
Cell culture and in vitro resveratrol treatment
Mouse BRCA1 mutant cell lines (69, 525 and 780, derived from mammary tumors of Brca1Co/Co;MMTV-Cre;p53+/− mice containing a targeted deletion of full-length BRCA1) and BRCA1 wild type cell lines (Neu and Ras, derived from mammary tumors of MMTV-cNeu and MMTV-Ras mice, respectively) (Brodie et al., 2001) were cultured in DMEM with 10% FBS and supplemented with glutamine, non-essential amino acids, and antibiotics (Invitrogen, CA). L56Br-C1 (Johannsson et al., 2003), HCC1937, and HCC1937 reconstituted with wild type BRCA1 (Yu et al., 2003) were cultured following description. SIRT1 wild-type and mutant MEFs were derived from E12.5 embryos. UBR60 cells (Harkin et al., 1999) are maintained in 2 μg/ml tetracycline medium. For the MTT assay, cells were seeded into 24-well plates at 2×104 cells/well for overnight before resveratrol (Calbiochem) was added. Then the cells were either incubated with different concentrations of resveratrol for 3 days or with a fixed resveratrol concentration at the IC50 for 7 days. Cells were stained with MTT (Sigma) and read at OD560. For soft agar assay, 35-mm dishes were first layered with 1% agarose solution with 10% FBS. The agarose was allowed to solidify. Cells were trypsinzed and resuspended at 1000 cells/ml. Top agarose layer contains 0.6% agarose, 10% FBS, and 500 cells with different concentrations of Resveratrol. Two sets of triplicates were done for each cell line and concentration. The colony number was counted under microscope.
In vivo study
69, 780, Neu and Ras cells were implanted sub-cutaneously into nude mice at 1×106 cells/100μl/spot. Each group contained ten female nude mice, each mouse carried two implanted spots. In total, each group contained 20 implanted spots. The day of implantation was designated as day 0. The mice were injected with either resveratrol (dissolved with DMSO, diluted with PBS) or carrier (DMSO+PBS) through i.p daily at dosage of 5 or 10 μg/30g body weight. The entire experiments lasted about 3 weeks when the tumors in control group reached 2 cm that required harvesting or there were tumor ulcerations.
To investigate functions of SIRT1 in tumor formation in vivo, pUSE-SIRT1 (Upstate) or GFP was eletroporated into BRCA1 mutant tumor cells (Nucleofector, amaxa, MD) together with pMACS Kk.II (Miltenyl Biotec). Twenty-four hours post transfection, the cells were collected and sorted with AutoMACS (Miltenyl Biotec) to over 90% purity. Then the sorted cells were implanted into nude mice and tumor growth was monitored daily. The same batch of cells was also followed up in tissue culture for cell growth and cell death during the same time course.
All animal experiments were approved by the Animal Care and Use Committee of National Institute of Diabetes, Digestive and Kidney Diseases (ACUC, NIDDK).
Supplementary Material
Acknowledgments
We thank Dr. Zhi-Ming Zheng for critical reading of the manuscript, Dr. Stina Oredsson for L56Br-C1 cell line, and Dr. Junjie Chen for the HCC1937 and HCC1937-BRCA1-WT cell lines. This work was supported by the intramural Research Program of National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- Alberg AJ, Lam AP, Helzlsouer KJ. Epidemiology, prevention, and early detection of breast cancer. Curr Opin Oncol. 1999;11:435–441. doi: 10.1097/00001622-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Afaq F, Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–7523. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Cao L, Kim S, Xiao C, Wang RH, Coumoul X, Wang X, Li W, Xu X, De Soto J, Takai H, et al. ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J. 2006;25:2167–2177. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Li W, Kim S, Brodie SG, Deng CX. Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev. 2003;17:201–213. doi: 10.1101/gad.1050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Xu X, Cao LL, Wang RH, Coumoul X, Kim SS, Deng CX. Absence of full-length Brca1 sensitizes mice to oxidative stress and carcinogen-induced tumorigenesis in the esophagus and forestomach. Carcinogenesis. 2007;28:1401–1407. doi: 10.1093/carcin/bgm060. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005a;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chen JS, Liu JC, Shen L, Rau KM, Kuo HP, Li YM, Shi D, Lee YC, Chang KJ, Hung MC. Cancer-specific activation of the survivin promoter and its potential use in gene therapy. Cancer Gene Ther. 2004;11:740–747. doi: 10.1038/sj.cgt.7700752. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005b;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Coumoul X, Li W, Wang RH, Deng C. Inducible suppression of Fgfr2 and Survivin in ES cells using a combination of the RNA interference (RNAi) and the Cre-LoxP system. Nucleic Acids Res. 2004;32:e85. doi: 10.1093/nar/gnh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- Deng CX. Roles of BRCA1 in centrosome duplication. Oncogene. 2002;21:6222–6227. doi: 10.1038/sj.onc.1205713. [DOI] [PubMed] [Google Scholar]
- Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response, and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci. 2003;28:41–48. doi: 10.1016/s0968-0004(02)00005-1. [DOI] [PubMed] [Google Scholar]
- Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- Eccles DM, Pichert G. Familial non-BRCA1/BRCA2-associated breast cancer. Lancet Oncol. 2005;6:705–711. doi: 10.1016/S1470-2045(05)70318-1. [DOI] [PubMed] [Google Scholar]
- Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser SM, Cockell MM. The molecular biology of the SIR proteins. Gene. 2001;279:1–16. doi: 10.1016/s0378-1119(01)00741-7. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14:1021–1026. [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wu JM. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer Res. 2000;20:225–228. [PubMed] [Google Scholar]
- Johannsson OT, Staff S, Vallon-Christersson J, Kytola S, Gudjonsson T, Rennstam K, Hedenfalk IA, Adeyinka A, Kjellen E, Wennerberg J, et al. Characterization of a novel breast carcinoma xenograft and cell line derived from a BRCA1 germ-line mutation carrier. Lab Invest. 2003;83:387–396. doi: 10.1097/01.lab.0000060030.10652.8c. [DOI] [PubMed] [Google Scholar]
- Kawakami H, Tomita M, Matsuda T, Ohta T, Tanaka Y, Fujii M, Hatano M, Tokuhisa T, Mori N. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. Int J Cancer. 2005;115:967–974. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- Kennedy SM, O’Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, O’Higgins NJ, Parkinson M, Linehan R, Clynes M. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. Embo J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Fisher P, Ganesan S, Efstratiadis A. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 2001;15:1188–1193. doi: 10.1101/gad.879201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Sato A, Oya M, Ito K, Mizuno R, Horiguchi Y, Umezawa K, Hayakawa M, Murai M. Survivin associates with cell proliferation in renal cancer cells: regulation of survivin expression by insulin-like growth factor-1, interferon-gamma and a novel NF-kappaB inhibitor. Int J Oncol. 2006;28:841–846. [PubMed] [Google Scholar]
- Shankaranarayana GD, Motamedi MR, Moazed D, Grewal SI. Sir2 regulates histone H3 lysine 9 methylation and heterochromatin assembly in fission yeast. Curr Biol. 2003;13:1240–1246. doi: 10.1016/s0960-9822(03)00489-5. [DOI] [PubMed] [Google Scholar]
- Shukla V, Coumoul X, Cao L, Wang R, Xiao C, Xu X, Ando S, Yakar S, LeRoith D, Deng D. Absence of the full-length BRCA1 leads to increased expression of IGF signaling axis members. Cancer Research. 2006;66:7151–7157. doi: 10.1158/0008-5472.CAN-05-4570. [DOI] [PubMed] [Google Scholar]
- Simeone AM, Deng CX, Kelloff GJ, Steele VE, Johnson MM, Tari AM. N-(4-Hydroxyphenyl)retinamide is more potent than other phenylretinamides in inhibiting the growth of BRCA1-mutated breast cancer cells. Carcinogenesis. 2005;26:1000–1007. doi: 10.1093/carcin/bgi038. [DOI] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- Tominaga Y, Wang A, Wang RH, Wang X, Cao L, Deng CX. Genistein inhibits Brca1 mutant tumor growth through activation of DNA damage checkpoints, cell cycle arrest, and mitotic catastrophe. Cell Death Differ. 2007;14:472–479. doi: 10.1038/sj.cdd.4402037. [DOI] [PubMed] [Google Scholar]
- Wang RH, Yu H, Deng CX. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc Natl Acad Sci U S A. 2004;101:17108–17113. doi: 10.1073/pnas.0407585101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008 doi: 10.1016/j.ccr.2008.09.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett TG, Jr, Carpenter DM, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Bisi J, Strum J, Liu L, Carrick K, Graham KM, Treece AL, Hardwicke MA, Dush M, Liao Q, et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66:1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, Harris CC, Deng CX. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet. 2001;28:266–271. doi: 10.1038/90108. [DOI] [PubMed] [Google Scholar]
- Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. Epub 2004 May 2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chini CC, He M, Mer G, Chen J. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med. 2005;9:360–372. doi: 10.1111/j.1582-4934.2005.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res. 2005;3:531–539. doi: 10.1158/1541-7786.MCR-05-0192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.