Abstract
Much progress has been made in recent years toward understanding mechanisms controlling branching morphogenesis, a fundamental aspect of development in a variety of invertebrate and vertebrate organs. To gain a deeper understanding of how branching morphogenesis occurs in the mammary gland, we compare and contrast the cellular and molecular events underlying this process in both invertebrate and vertebrate organs. Thus, in this review, we focus on the common themes that have emerged from such comparative analyses and discuss how they are implemented via a battery of signaling pathways to ensure proper branching morphogenesis in diverse systems.
Keywords: Drosophila trachea, Air sacs, Lung, Kidney, Submandibular gland, Mammary gland, Prostate, Epithelial-mesenchymal crosstalk
Introduction
A major impediment to development of large and complex animals is the requirement for efficient exchange of gases, nutrients, metabolites and wastes with the environment. This problem was elegantly solved during metazoan evolution by packing a large surface area of epithelium into a relatively small volume via a process called branching morphogenesis, whereby epithelial tubules are expanded and remodeled during embryonic and postnatal development. Indeed, branching morphogenesis is a fundamental developmental process involved in formation of a variety of organs, from fly trachea and air sacs, to mammalian lungs, kidneys, vasculature, pancreas, as well as many exocrine glands, including mammary and salivary glands [1]. As a result, a deeper understanding of branching morphogenesis is not only crucial to our understanding of how these organs develop, but to understanding the molecular etiology of many human diseases, such as polycystic kidney disease, kidney and lung agenesis, and many malignancies including breast and prostate cancer [2, 3].
Given their structural simplicity and genetic accessibility, the Drosophila tracheal and air sac systems have been used to identify cellular and molecular events involved in branching. Many of the molecules and processes involved in fly branching morphogenesis are now known to be highly related to those involved in the development of complex vertebrate organs [4–6]. From these studies, it has become clear that a small number of related pathways fulfill a similar role in branching of various systems, whereas molecular differences between distinct branched structures or organs will help explain how each organ becomes unique [2, 7, 8].
Our goal in this review is to consider the cellular and molecular mechanisms governing mammary branching in the context of recent advances concerning regulation of branching morphogenesis in other organ systems, including invertebrate systems. For this reason, the hormonal control of branching morphogenesis, which is clearly important but unique to the mammary gland [9–11], is not considered here. Nor do we discuss mechanisms controlling tubulogenesis as it is beyond the scope of this review, even though branching morphogenesis is one of several ways to generate and remodel epithelial tubes [12–14]. We will first discuss various modes of branching and their underlying cellular mechanisms in diverse branched organs. In addition, we focus on the molecular basis of epithelial–mesenchymal crosstalk in branching morphogenesis and how different molecules are deployed to execute similar functions. Finally, we will discuss the versatile roles of the extracellular matrix (ECM) in regulating this process and point out interesting directions for future studies. Thus, in the current review we assemble and analyze some common themes that are shared by a variety of organs to ensure their proper branching morphogenesis in the face of molecular differences that render each of these organs unique.
Branching Patterns in the Mammary Gland
A Brief Description of Mammary Ductal Development
The “tree-like” ductal epithelium observed in the mature mammary gland is derived from a single mammary bud that forms in mice at around embryonic day 11 [15]. Unlike the primary budding that occurs in the lung, or the ureteric buds that form in the kidney, both of which arise from a hollow tube, the mammary sprout starts out as a solid cord when the mammary bud invaginates into the primary mammary mesenchyme [15–17]. The process whereby the lumen initially forms in the mammary sprout has not been carefully examined, however, it is thought to be similar to the process that occurs in the salivary gland and pancreas, where lumina form by an apoptosis-mediated cavitation process [12].
By birth, a rudimentary ductal tree consisting of ~ 10–20 branches can be seen in each of the ten mammary glands in the mouse [15]. Interestingly, with the exception of the lung, where the initial orders of branching are stereotypical, the branch patterns of other vertebrate branched organs, including the mammary gland, are stochastic. In fact, branch patterns seem to differ even between contralateral mammary glands at the same position along the anterior-posterior axis of the body [15]. Thus, an early global patterning event that is thought to occur to ‘hard-wire’ the branching pattern of the lung [5], does not seem to function in the mammary gland or in most other vertebrate branching systems. After birth, the primitive ductal network remains relatively quiescent until puberty, growing just enough to keep pace with enlargement of the mammary fat pad associated with normal body growth [18, 19]. The surging female hormones at puberty then set the “branching machinery” in motion and, for the subsequent 4–5 weeks, a rigorous epithelial invasion by ductal elongation and branching takes place until the fat pad becomes entirely filled [18, 19].
Modes of Branching
Two major modes of branch-point formation, namely primary branching and secondary branching, are thought to occur concomitantly with ductal elongation in the stromal fat pad [10, 11, 16, 18, 20]. Primary branching occurs when the tip of invading mammary epithelium, a bulbous terminal end bud (TEB), splits dichotomously at a sharp angle to give rise to two TEBs, each of which continues to invade the stroma and give rise to two subtending primary ducts. This dichotomous splitting or bifurcation of the TEB takes place at fairly regular intervals and together with ductal elongation are responsible for the formation of a simple, primary ductal system in the mature gland [20]. Secondary branching, which is sometimes called side-branching or lateral branching, also occurs during post-pubertal mammary development and denotes the budding and elongation of perpendicular secondary ducts from the already formed portion of the primary ducts behind the invading TEBs. In addition, a third mode of branching called tertiary branching takes place during recurrent estrous cycles and early pregnancy and denotes the initial formation of alveolar buds from the primary and secondary ducts [19, 20]. During late pregnancy, the surface area of the alveolar buds expands tremendously due to a significant increase of cell proliferation and differentiation to prepare for milk production for the mother’s offspring [9–11, 16, 18–20].
Similar modes of branching are thought to occur in other vertebrate branched organs, for example lung and kidney [21, 22]. However, the proposed modes of branching in these tissues are based on evidence from analyses on fixed tissues. The most serious caveat, therefore, is that important details of the highly dynamic branching process may have been missed by deducing its various aspects from a final structure. Recent technical advances in the time-lapse imaging of organ cultures in vitro have shown that this is indeed the case [23]. Using time-lapse video recording of cultured embryonic mouse kidney, in which the epithelium is labeled with green-fluorescent protein (GFP), Watanabe and Costantini [23] found that ~75% of the renal tubular branches form by bifurcation and that ~7% form by lateral branching. However, they also discovered that ~18% of branches form by a third mode of branching: trifurcation, in which the tips of the tubules (the ampulae) split in three different directions simultaneously. Remarkably, a remodeling event of the branch-point was observed, such that what was previously assumed by end-point analysis to be two separate bifurcation events could actually be the product of one trifurcation event followed by unequal elongation of the ductal epithelium [22, 23]. Similar events may also occur in other vertebrate branching systems. Thus, as a result of recent advances in long-term in vivo and cell culture-based imaging techniques as well as organotypic culture methods, we may learn surprisingly important new details of how branching actually proceeds in the mammary gland in real time.
Identity and Fate of the Functional Units of Mammary Branching
One question regarding functional units of the branched mammary epithelium (the TEBs and the ducts), is what genes specify their identities. Although we do not yet know the answer to this question, there is evidence that tips and trailing ducts of the branches are specified by different genes. In the kidney, for example, expression of Ret and its co-receptor Gfrα1, as well as expression of Wnt11, Crlf1, and CXCL14 are specifically restricted to ampulae [24–26], whereas Wnt7b and collagen XVIII are only expressed in trunks [26]. Likewise, markers that distinguish TEBs from trailing ducts have been identified in the mammary gland. For example, Wnt5a, Wnt7b, Sprr1a, MT1-MMP and numerous other genes are specifically expressed in TEBs, whereas caseins and many other genes appear to be expressed exclusively in more proximal ducts [141]. Future studies should yield important insights concerning molecular mechanisms that determine the identities of these two structures.
Another under-explored aspect of mammary gland biology regards the fates and lineages of TEBs and ducts. There are at least three models to explain the relative contribution of TEBs and ducts to the final branching network. The first model argues that TEBs and ducts are two separate lineages and that the only function of TEBs is to invade stroma and bifurcate. Thus, according to this model, it is the elongation of ducts that gives rise to the entire ductal epithelial network. The alternative model argues that ducts are passive entities, and it is the TEBs that, by giving rise to the trailing ducts, exclusively contribute to the final ductal tree. The observation that most mammary epithelial proliferation occurs in TEBs seems to support the latter hypothesis [27]. The third model takes the middle ground and proposes that TEBs and ducts are not separate lineages and they both contribute to the final branching network.
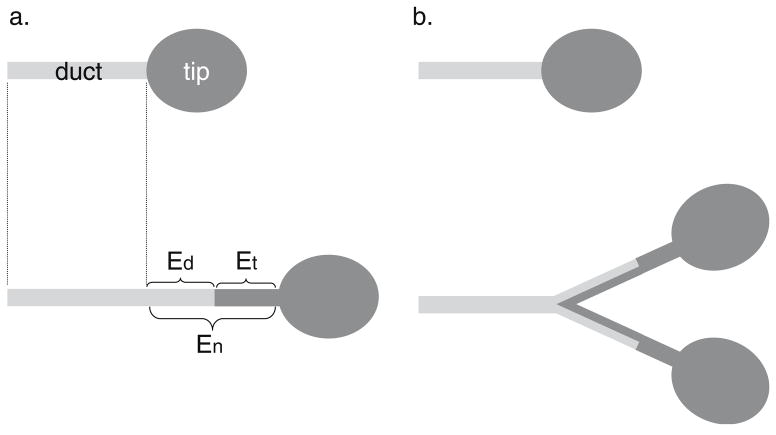
Important progress has recently been made in the field of kidney branching concerning respective fates of ampulae and ducts. Using chimeric analyses generated by injecting Ret mutant cells into wild-type blastocysts, Shakya et al. [25] created mosaic kidneys in which the ducts, but not the ampulae, were labeled with Ret mutant cells expressing GFP Mutant cells are excluded from ampulae because Ret function is required for formation of the ureteric bud (UB), the tip of which gives rise to future ampulae [25]. Consequently, mutant cells only contribute to the trunk of ureteric bud and ducts, which normally do not express Ret protein, in subsequent branching events. Because ampulae and ducts were differentially marked, their fates could be followed through live imaging. In addition to confirming that ampulae actively branch and give rise to trailing ducts, the authors found that the ducts are not passive entities, but that they, too, actively participate in ductal elongation and, most surprisingly, in remodeling of branch-points. Together, these data demonstrate that both ampulae and ducts contribute to ductal elongation and branching, and thus determine branch patterns (Fig. 1). Considering the aforementioned similarities between the branching modes seen in both kidney and mammary gland, it will be interesting to see whether mammary TEBs and ducts behave in a similar manner to their renal counterparts.
Figure 1.
Fate map of the tip and duct of branching renal epithelium. a. Relative contribution of the tip and duct to ductal elongation. The overall increase of ductal length during renal epithelial invasion is the sum of new ductal epithelium produced by extension of the existing duct (light gray) and by derivation from the tip (dark gray), b. Ductal participation in the remodeling of a branch-point. As the tip bifurcates and gives rise to two new tips. The original ductal epithelium is remodeled so that it contributes to the proximal side of each new trunk, whereas the rest of the trunk epithelium derives from each new tip. Ed, ductal contribution of elongation; Et, tip contribution of elongation; En, net increase of ductal length. Adapted from Shakya et al. [25].
Cellular Events of Branching Morphogenesis
Migration, Cell Rearrangement and Change of Cell Shape
The cellular events leading to distinct steps in branching morphogenesis are best understood in the Drosophila tracheal system [4, 6, 28]. In brief, the onset of fly tracheal development starts at 5 h after egg-laying (AEL) with formation of ten tracheal sacs on each side of the embryo by invagination of the surface ectoderm. During the next 2 h, tracheal sacs separate from the ectoderm, elongate and undergo two rounds of cell division to give rise to epithelial sacs consisting of ~80 cells. In subsequent branching events, there is no cell proliferation, apoptosis or change in cell number in the tracheal system [29]. At 7 h AEL, primary branches start to form by invaginating or budding in six different but stereotypical directions. At each bud site, two cells migrate out of the sac, followed by a small number [3–20] of cells. As they migrate, cells of the primary branch elongate and intercalate such that their initial side-by-side positioning is rearranged to an end-to-end configuration, with the lumen of resultant tubes enveloped by one single cell. At 11 h AEL, the lead cell of the primary branch extends and separates from neighboring cells to form a secondary branch. At 13 h AEL, the lead cell also elaborates fine cytoplasmic extensions, or terminal branches. Importantly, the pattern of terminal branches is variable and is determined by physiological oxygen concentration of the surrounding tissue. In essence, tracheal branching creates morphologically distinct tubes, which form apical junctions through a variety of mechanisms [28]. Together, tracheal branching is characterized by three main cellular processes: cell migration, cell rearrangement and change of cell shape [4, 6, 28].
In contrast, cellular events that occur during branching morphogenesis in vertebrates are poorly understood. At first glance, vertebrate branching is a relatively simple process, involving reiterative events of ductal elongation and branch-point formation through invagination or budding of a group of cells in an epithelial sheet, or splitting the tips of epithelial tubes. As opposed to the process that takes place in the tracheal system, cell proliferation is concomitant with all stages of branching morphogenesis in vertebrate organs. The increase in cell number, along with current limitations in organ culture and extended live animal imaging techniques have made characterization of cellular events in vertebrate systems extremely challenging. Using an in vitro lung culture model, Weaver et al. [30] showed that FGF10 beads can attract epithelial cells to migrate toward the source of ligand. Likewise, beads soaked in glial-cell-line derived neurotrophic factor (GDNF) induce and attract ectopic ureteric buds from the Wolffian duct [31–33], thus also suggesting that cell migration may contribute to vertebrate branching. However, filopodia and/or lamelliopodia have yet to be documented in vertebrate systems to argue definitively that the cells are indeed migrating, rather than undergoing directional proliferation. However, epithelial cells in some systems are very motile, constantly changing their positions in trunks and ampulae of the developing kidney [25]. Comparable observations have been made in organotypic mammary cultures and live mammary gland imaging in vivo (A. Ewald and Z. Werb, unpublished results). Such cell rearrangements may play a role in convergent extension of epithelial cells and thus participate in ductal elongation and control of tube-size in renal branching [25]. Together, cell migration, rearrangement and change of cell shape are likely to be conserved cellular events underlying vertebrate branching in general.
Epithelial–mesenchymal Transition and Cell Proliferation in Branching Morphorgenesis
The unique tubulogenic process of branching and the lack of cell proliferation in the fly trachea have also limited its usefulness as a developmental paradigm to study vertebrate branching morphogenesis. In this regard, recent characterization of fly air sac development may provide insights that help us understand vertebrate branching processes. Briefly, the thoracic air sac develops during remodeling of the tracheal system, when the increase in body-size demands higher oxygen supplies in the early third instar larva at around 70 h AEL [34]. The thoracic air sac starts out as a group of cells migrating out from an existing tracheal branch, the transverse connective of the wing imaginal disc, toward a stereotypical direction. Significantly, cell proliferation accompanies the outgrowth process of air sacs, making it similar to the budding events seen in vertebrate systems [34, 35]. In addition, as in the tracheal system, both cell rearrangement and change in cell shape occur in developing air sacs of the fly.
It has been proposed that cells at the leading edge of branching epithelium in vertebrate organ systems may undergo a transient epithelial–mesenchymal transition (EMT) [36]. Evidence for this hypothesis derives from the observation that, in MDCK cell cultures, branches form by initial depolarization and cell migration, followed by repolarization and lumen formation [37, 38]. A similar process accounts for sprouting angiogenesis [39]. In air sacs, however, migrating cells at the leading edge, as evident by their filopodial and lamellipodial extensions, seem to remain embedded within the epithelium, contact the lumen and form apical junctions with neighboring cells, thus arguing against the premise that EMT plays a role in branching morphogenesis [35]. Indeed, there is no real evidence that a transient EMT takes place during mammary branching in organotypic cultures in vitro (A. Ewald and Z. Werb, unpublished results).
A long-standing question regarding cell proliferation has been whether it plays a permissive or instructive role in branching morphogenesis in vertebrate organs. Intuitively, an increase in cell number is necessary to add more ‘building-blocks’ to branching epithelium. On the other hand, directional cell proliferation may be a powerful force to generate forward movement during ductal elongation and invasion into the mesenchyme. This question has now been addressed in fly air sacs. Using loss-of-function studies based on mitotic recombination, in which mutant cells are generated in a heterozygous environment, Cabernard and Affolter [35] examined the role of several major signaling pathways, including Dpp/BMP, Hedgehog (HH), VEGF, Frizzled, EGFR and Breathless (Btl)/FGFR signaling, in the outgrowth of fly air sacs. They found that Btl/Fgfr mutant cells are unable to take the leading edge position during migration, although they can proliferate and occupy stem and truck regions. These data suggest that Btl/Fgfr signaling is essential for cell migration but is unnecessary for cell proliferation in air sacs. On the other hand, EGFR seems to be important for cell proliferation and survival because mutant clones are much smaller than normal. Remarkably, however, mutant cells can contribute to the trunk region and to the leading edge. These data demonstrate, therefore, that cell proliferation per se is unlikely to be the driving force for branching morphogenesis, at least within fly air sacs (Fig. 2).
Figure 2.
Differential RTK signaling pathways control cell proliferation and migration in Drosophila tracheal air sac. Coordination of cell behavior in the outgrowth of a fly larval tracheal air sac. FGF signaling is required for migration of cells at the leading edge. Bnl/Fgf ligands are expressed in the cells adjacent (dark gray cloud) to the tracheal epithelium. Activation of Btl/Fgfr function in cells at the leading edge (gray) triggers changes in the cytoskeleton and cell shapes, and thus causes cells to migrate toward the source of signal. EGF signaling is required for all epithelial cells to proliferate and/or to survive and thus plays a permissive role in air sac outgrowth. Although sharing major downstream effectors, these two RTKs use different molecules to ensure distinct cellular effects. Adapted from Cabernard and Affolter [35].
That cell proliferation plays a permissive role has yet to be established in vertebrate branching systems, although there is evidence to suggest that cell proliferation and branch-point formation are differentially regulated. For example, pharmacological attenuation of Erk/MAP-kinase activities by PD98059 leads to a potent inhibition of branch-point formation in kidney organ culture [23, 40]. However, the overall length of renal tubules formed in treated cultures is barely affected. As a result, the average fragment is significantly longer than normal [23, 40]. The overall production of renal tubules is essentially a net measurement of the amount of cell proliferation that has occurred in culture. Thus, consistent with the aforementioned relationship between cell proliferation and migration in the fly air sacs, these data demonstrate that cell proliferation and branch-point formation are independent events at the molecular level in kidney branching.
Interestingly, inhibition of PI3 kinase activities blocks both ductal elongation and branch-point formation in kidney organ culture [41]. Likewise, loss of Ras function abrogates both cell proliferation and migration in the fly air sacs, a phenotype resembling that seen in the absence of both Egfr and Btl/Fgfr function [35]. Thus, cell proliferation and branch-point formation in vertebrate tissues, or cell proliferation and migration in the fly air sac, seem to be governed by similar as well as distinct molecular events. Taken together, these data highlight the complexity of molecular mechanisms responsible for branching morphogenesis and the challenges we face to fully understanding them.
Molecular Basis of Epithelial–Mesenchymal/Stromal Crosstalk in Branching Morphogenesis
Epithelial–mesenchymal crosstalk is one of the central themes in developmental biology for its essential role in specification, patterning, outgrowth and differentiation of a variety of vertebrate organs, including the limb, pharyngeal arches, and intestines to name just a few. Not surprisingly, interactions between epithelium and embryonic mesenchyme, or stroma in the case of the post-natal mammary gland, are required for branching morphogenesis to occur properly in different vertebrate systems. We discuss in the following sections molecular mechanisms governing epithelial–mesenchymal crosstalk: how the mesenchyme signals epithelium, and how the epithelium responds, and vice versa, in branching morphogenesis.
Role of Mesenchymal/Stromal Cues in Epithelial Branching Morphogenesis
Studies in the fly suggest that mesenchymal cues in vertebrate systems can serve at least two functions. The first function is to guide the direction of epithelial outgrowth and the second is to promote cell proliferation and/or survival in the epithelium. In the fly trachea, one of the most insightful observations is that Branchless (Bnl/Fgf), and its receptor Breathless (Btl/Fgfr), determine the stereotypical branch pattern [42, 43]. Bnl/Fgf, which is normally restricted to distinct cell clusters adjacent to the epithelium [4, 5], binds to and activates Btl/Fgfr located on tracheal epithelium, and thus acts as a chemoattractant to guide epithelial migration [42, 43]. Remarkably, although all tracheal epithelial cells express Btl/Fgfr and can respond to Bnl/Fgf, they compete for the ligand, and the cell with highest Bnl/Fgf signaling activities becomes the lead cell [44]. Once the lead cell has been determined, it is the only cell in the primary branch to require Bnl/Fgf signaling as a guidance cue. Remaining tracheal cells follow the lead cell in a Bnl/Fgf-independent manner [44]. As noted earlier, the cell number does not change during fly tracheal branching and specific survival cues are not required. On the other hand, in air sacs where cell proliferation accompanies epithelial out-pouching, EGF signaling is required to function as an epithelial survival factor.
Similar themes may function in vertebrate organ systems as well. Generally speaking, however, the mesenchymal environment in the vertebrate is more complicated than the fly systems and a myriad of factors, many of which are ligands of receptor tyrosine kinases (RTKs), are required for vertebrate branching morphogenesis [3, 19, 45]. Without an accurate description of cell behaviors in vertebrate branching as mentioned, one of the most challenging questions regarding mesenchymal factors is whether they function as guidance cues or survival factors for branching epithelium.
Among the mesenchymal factors, the FGFs are the most often documented factors to play a role in the epithelial branching of vertebrate organs. For example, in the lung a loss of Fgf10 expression in the mesenchyme, or of Fgfr2 in the epithelium prevents primary budding and, as a result, the organ fails to form [46–48]. Thus, FGF 10 may serve as a chemoattractant to guide migration and/or a mitogen during branching of lung epithelium [30]. In the kidney, both Fgf7 and Fgf10 are expressed in mesenchymal cells, and the loss of their receptor Fgfr2 in epithelium causes a significant reduction in branching [49]. Thus, FGF signaling also plays a role in renal branching.
In the mammary gland, FGF signaling has long been associated with normal development and with breast cancers [50]. However, its role in branching morphogenesis has not been defined due, in a large part, to an early requirement for FGF signaling in embryonic mammary placode development [51]. Using Cre-mediated gene disruption and mosaic analysis, we have found that FGFR2 function is required in TEBs for proper ductal elongation and bifurcation (P. Lu and Z. Werb, unpublished results). Likewise, down-regulation of FGF signaling by a function-blocking antibody against FGFR2 reduces branching in the submandibular gland [52]. Interestingly, the dosage of FGF 10 appears to be critical for branching morphogenesis in the lacrimal gland, since loss of only one wild-type allele is sufficient to cause defective branching in heterozygous mice [53].
In addition to FGFs, other RTK ligands are required for vertebrate branching. For example, EGF signaling regulates lung development, as a 50% reduction in branching is observed in Egfr mutant mice [54]. Similarly, mesenchymal GDNF is important both for kidney induction and for renal epithelial branching [25]. In the mammary gland, insulin growth factor (IGF) signaling appears to function downstream of female hormones to promote epithelial branching [55, 56]. Hepatocyte growth factor (HGF) has also been proposed to play a role in mammary branching based on gain-of-function studies [57], although this idea has yet to be tested through loss-of-function analysis.
Given their similar downstream signaling events, in particular their overlapping activation of the Ras–Raf–MAPK pathway, it is reasonable to assume that several mesenchymal RTK ligands may cooperate to induce epithelial migration and branching. This may indeed be the case for FGF and GDNF in the kidney. As mentioned earlier, GDNF can stimulate renal epithelial cell migration in vitro, and ectopic GDNF is sufficient to induce supernumerary ureteric buds [25, 58]. Likewise, FGF beads can cause formation of ectopic ureteric buds [143]. It is thus tempting to suggest that FGF and GNDF signaling act concordantly to promote renal epithelial branching. Alternatively, they may act sequentially in the same signaling pathway. Future studies will help distinguish between these models.
Another interesting, but challenging, question is how mesenchymal RTK ligands serve distinct functions, as they appear to do in the fly, if their downstream signaling events are similar. In air sacs, Bnl/Fgf and EGF, both of which depend on Ras function, act as guidance cues and survival factors, respectively [35]. However, at least two strategies are implemented to ensure that these two RTK ligands function independently. First, they seem to differentially impinge upon MAPK activities such that it is more strongly activated by Bnl/Fgf in cells of the leading edge. Second, they recruit distinct co-factors to initiate separate downstream events [35]. Aside from differentiating whether the mesenchymal factors act as survival or guidance cues, another major challenge will be to determine how different downstream events are elicited to control specific aspects of branching morphogenesis in the vertebrate.
Epithelial Responsiveness to Mesenchymal/Stromal Cues
Given the importance of mesenchymal/stromal factors in regulating epithelial cell behavior, it comes as no surprise that mesenchymal cell functions or signals need to be controlled. Indeed, there are at least two ways to accomplish this. First, epithelial cells can modulate their own responsiveness to mesenchymal factors. Second, the epithelium can signal the mesenchyme and proactively influence the production of cues from the latter tissue.
The Drosophila Sprouty (Spry) gene was first identified in a screen for mutations that cause defective branching in the tracheal system. In Spry mutant flies, more secondary branches are produced from the lead cell of primary branches [59]. It was discovered subsequently that Spry functions in a cell-autonomous manner to inhibit Btl/Fgfr signaling in tracheal epithelium. In its absence, the lead cells are over-sensitized to Bnl/Fgf stimulation and, as a result, excessive secondary branches form. In addition, consistent with the aforementioned role of Bnl-Btl signaling in specification of the lead cell, Spry mutant cells are more likely to adopt the lead cell fate than neighboring non-mutant cells in a mosaic environment [44]. Importantly, it is now known that Spry is not a specific antagonist to FGF signaling; rather, it also inhibits EGFR and presumably other RTKs in the fly [60–63].
Four vertebrate homologues of the fly Spry gene, Spry 1–4, have been identified in the mouse and some of them have been shown to play a role in branching morphogenesis in the mouse [60, 64, 65]. Mice lacking Spry1, for example, show polycystic kidneys due to formation of extra ureteric buds, similar to having excessive GDNF/Ret signaling during kidney induction [66]. Indeed, reducing GDNF/Ret signaling activity by removing one wild-type Gdnf allele, and thus reducing the dosage of GDNF, rescues the phenotype of Spry1 mutant mice. These results suggest that Spry1 normally functions to inhibit GDNF/Ret signaling in ureteric bud formation. Importantly, Spry1 also functions at later stages during renal branching as ectopic branching has been observed in the mutant kidney [142]. Similarly, data suggest that Spry2, which is expressed in the distal lung epithelium, negatively regulates branching morphogenesis of the lung. For example, down-regulation of Spry2 function using an antisense RNA strategy can stimulate lung branching, whereas gain of Spry2 function causes the opposite phenotype [67, 68]. However, it remains unclear which RTK signaling pathway, and which cellular behaviors are regulated by Spry2.
Sprouty-related protein (Spred) and Similar expression to Fgf (Sef) genes, have also been shown to antagonize RTK signaling [69–74]. Interestingly, Spreds are expressed in the developing lung and may play a role in vertebrate branching [75]. Given the importance of RTKs in both invertebrate and vertebrate systems, it will be important to determine how RTK signaling is regulated by these antagonists during branching morphogenesis in mammals.
Role of Epithelial Cues in Mesenchyme/Stroma
In addition to adjusting its responsiveness to mesenchymal/stromal cues, the epithelium also directly signals the mesenchyme and modulates its activities. Such mesenchymal modulation by epithelium appears to be a unique feature of vertebrate branching morphogenesis. In the fly tracheal system, for example, although the epithelium can fine-tune Bnl/Fgf signaling activities by expressing Spry, so far there has been no evidence to suggest that the epithelium can alter Bnl/Fgf expression in return. In fact, Bnl/Fgf expression is believed to be pre-determined by global patterning genes irrespective of the epithelium [4, 5]. Interestingly, in the lung, where branch pattern is also stereotyped and thought to be hard-wired, mesenchymal Fgf10 expression appears to be modulated by the epithelium [5]. In the following sections, we discuss several signaling pathways that have been proposed to modulate mesenchymal functions in vertebrate branching.
Hedgehog (HH) Signaling
The first line of evidence that lung mesenchyme is modulated by the epithelium derived from the observation that Fgf10 expression is up-regulated in mice lacking Sonic HH (Shh) function [76]. Since Patched-1 (Ptc-1), which encodes the receptor for Shh protein, is expressed in lung mesenchyme, it was suggested that Shh signaling from the epithelium restricts Fgf10 expression to discrete locations in this tissue [21, 76]. Importantly, despite up-regulation of Fgf10 expression, Shh mutant lungs fail to branch beyond primary budding [76, 77], suggesting that Shh may play additional roles in lung branching morphogenesis (see below).
Shh is also expressed in epithelium of the submandibular gland. Moreover, inhibition of Shh signaling by cyclopamine, a chemical inhibitor of this pathway, reduces branching in salivary gland organ cultures. These results suggest that HH signaling is required for salivary gland branching [78]. Surprisingly, Smoothened, a positive transducer of HH signaling, Gli3, an HH-responsive transcription activator, and Ptc-1 are expressed in the epithelium. In this context, therefore, Shh may function in an autocrine manner within the salivary epithelium compartment [78].
HH signaling may play a role in branching morphogenesis of the mammary gland. Loss of one wild-type allele of Ptc-1, which causes excess HH signaling, leads to hyperplasia of ductal epithelium [79]. Conversely, loss of stromal Gli2 causes defective ductal epithelium in pregnant animals [80]. Since both Gli2 and Ptc-1 are expressed in the stroma, HH signaling must act upon the epithelium via an unidentified stromal factor(s). However, mammary glands lacking either Shh or Indian HH (Ihh) show no defects in postnatal branching morphogenesis [81, 82]. Thus, it remains unclear at present whether Shh and Ihh play a redundant role in mammary branching. If so, removal of both genes may be required to cause discernible defects in this developmental process.
Interestingly, hedgehog-interacting protein (Hip), an inhibitor of Shh function, is expressed in the lung mesenchyme to counteract HH signaling activities. In mice lacking Hip function, Fgf10 expression is down-regulated due to excess HH signaling and, as a result, lung branching is significantly reduced [83]. Moreover, the reduction of lung branching in Hip mutants is exacerbated by additional increase of HH signaling, for example in mutants lacking one wild-type allele of Ptc-1, which is another inhibitor of HH function [83].
TGF-β Super-family
The transforming growth factor β (TGF-β) super-family contains a large number of members, including at least eight bone morphogenetic proteins (BMPs), three TGF-βs, five activins and other related proteins that function as ligands for serine-threonine kinase receptors at the cell surface [84, 85]. Binding of these ligands to their type I and type II heterodimeric receptors causes phosphorylation and kinase activation, which in a Smad-dependent or -independent manner lead to transcription of target genes [84, 85]. Available data show that TGF-β super-family ligands play an important role in a variety of developmental processes, including branching morphogenesis.
BMP
During fly tracheal development, Dpp/BMP in dorsal domains regulates Bnl/Fgf expression. In the absence of Dpp/BMP signaling in this context, Bnl/Fgf is not expressed and dorsal tracheal branches do not form [86]. The function of BMP signaling in vertebrate branching morphogenesis has been difficult to analyze. This is in part due to the fact that multiple BMPs are often expressed in rather broad and overlapping domains, where they may play redundant roles, and in part due to their dynamic nature, whose functions are spatiotemporally dependent. The complexity of BMP function is best illustrated in vertebrate limb development. In this setting, several BMPs, including Bmp2, Bmp4 and Bmp7, are expressed in a dynamic fashion in both ectoderm and mesenchyme of the developing limb [87, 88]. Using loss-of-function studies, it has been shown that BMP signaling in the ectoderm is required initially for the formation of apical ectodermal ridge (AER) [89, 90], a crucial signaling center in limb development, and, counter-intuitively, for its subsequent demise when AER function is no longer required [90].
Several BMPs are expressed in the developing lung [30, 91, 92], including Bmp4, which is expressed in distal epithelium [30]. Since removal of BMP receptor 1a in the epithelium causes cell death and reduced branching, it has been suggested that Bmp4 functions as an autocrine factor in the lung epithelium to promote cell survival [93]. In contrast, BMP signaling appears to inhibit induction, and possibly branching morphogenesis, of the kidney. For example, ectopic ureteric buds form in Bmp4 heterozygous null mice, suggesting that BMP4 normally functions to inhibit GDNF/Ret signaling during kidney induction [94]. Consistent with this notion, the opposite phenotype, a failure of ureteric bud formation, has been observed in mice lacking the BMP antagonist Gremlin. This is because loss of Gremlin causes excessive BMP signaling, which in turn prevents GDNF/Ret from inducing formation of the kidney [95]. At present it is not known whether BMP signaling plays an analogous role in branching morphogenesis of the mammary gland.
TGF-β
Generally speaking, TGF-β signaling has been found to inhibit vertebrate branching morphogenesis [2, 7, 19, 45, 91, 96]. Indeed, TGF-β family members can inhibit branching in organ cultures of lung [97, 98] and kidney [96, 99]. In addition, over-expression of TGF-β1 in epithelium causes reduced branching in the mammary gland [100]. Based on results from these gain-of-function studies, one would predict that ectopic branching should be observed in the absence of TGF-β(s). However, loss-of-function studies have not been straightforward. For example, despite the expression of TGF-β1, TGF-β2 and TGF-β3 in the developing lung [91], loss of any one of these three genes is not sufficient to cause defective branching, although TGF-β3 mutant mice show hypoplastic alveoli during late stage lung development [91, 101, 102]. From these results, one might speculate that different TGF-βs play redundant roles, and thus, simultaneous removal of two or all three may be required to cause a discernible phenotype in lung branching. It turns out, however, that this hypothesis is difficult to test, given that maternal TGF-β1 can traverse the placenta and rescue immune-deficiency and possibly other phenotypes in mutant embryos [101]. Therefore, the issue of maternal supply has so far hindered analysis of TGF-β function in the development of most branched organs in the mouse, which develop primarily before birth.
In retrospect, it comes as no surprise that the function of TGF-β signaling in branching morphogenesis is best understood in the mammary gland. This is because the mammary gland develops primarily after birth, and therefore maternal TGF-β1 does not play a major role. Indeed, in TGF-β1 heterozygous females, in which TGF-β1 protein is expressed at ~10% of normal levels, epithelial invasion is accelerated [103]. This phenotype can in part be explained by a much higher rate of cell proliferation in the epithelium. Tissue-recombination experiments and expression analysis have revealed that mammary epithelium is the source of active TGF-β1, as opposed to the stroma [103–105]. However, it appears that mammary stroma is the target for TGF-β1 since loss of TGF-β receptor II (TβRII) function in the stroma accelerates epithelial branching [106–108]. Together, these results suggest that TGF-β1 inhibits epithelial branching by targeting stromal production of a yet unidentified factor(s), which normally functions to regulate epithelial branching. Several lines of evidence suggest that stromal FGF may be regulated by TGF-β. Indeed, TGF-β1 has been shown to inhibit Fgf10 expression in fibroblasts [109], as well as in lung [110] and prostate [111] explant cultures. Future studies should address which FGF factor functions in mammary stroma and, importantly, whether its expression is indeed negatively regulated by TGF-β signaling in vivo.
Additionally, TGF-β signaling may regulate branching morphogenesis by affecting ECM remodeling, which, as discussed below, plays an essential role in vertebrate branching. For example, TGF-β1 expression has long been associated with ECM deposition. TGF-β1 regulated ECM components include collagen I, collagen III and fibronectin in developing lungs and mammary gland [112–114]. Moreover, pellets soaked with TGF-β1 alter ECM production in the mammary gland [113]. Taken together, it appears that mammary epithelial branching is highly regulated by TGF-β, likely through control of expression of stromal factors and ECM deposition in stromal cells.
Epithelium as the Source of Mesenchymal Survival Factors
Aside from modulating the production of mesenchymal cues, epithelial signals regulate cell proliferation and survival in the mesenchyme. As mentioned earlier, Shh plays dual roles in the developing lung; one by restricting sites of Fgf10 expression, the other by promoting survival of mesenchymal cells [115]. Thus, in the absence of Shh function, despite an up-regulation of Fgf10 expression, the lung fails to develop [76, 77]. Another survival factor for lung mesenchyme is Fgf9, which, curiously, is derived from both mesothelium and epithelium [115]. Similarly, in addition to its aforementioned role in epithelial branching [49], renal FGF signaling has recently been shown to play a survival function for metanephric mesenchyme [116].
In the mammary gland, some of instructive or permissive epithelial signals are provided by members of the EGF family. The first indication that EGFR signaling regulates mammary branching derives from the observation that EGF-related ligands can rescue branching defects in mice lacking ovarian hormonal stimulation or estrogen-receptor (ER) function [117–119]. Loss-of-function analysis showed that amphiregulin (AREG), but not EGF or TGF-α in the mammary epithelium is required for normal branching [120]. Interestingly, although EGFR is expressed in both epithelial and stromal compartments, its function is required only in the stroma [117]. Our laboratory has shown that ADAM17, a metalloproteinase, is required for cell surface release of AREG and other EGFR ligands. Indeed, ADAM17 mutant mice have reduced mammary [121] and lung branching [122], as do EGFR deficient mice [123]. Thus, ADAM17 plays a key role in the development of these and probably other branched tissues by releasing critical cell surface EGFR ligands, thus enabling EGFR activation and its downstream consequences to occur.
Role of ECM in Branching Morphogenesis
The extracellular matrix (ECM) is a complex, highly dynamic and critical component of all tissues in a multicellular organism [124]. Research has shown that ECM regulates cell proliferation, survival, migration, cell fate specification and other fundamental aspects of cell biology. It is now well known that regulation of ECM is required for many essential developmental processes during tissue morphogenesis, injury and repair, and that its deregulation plays a critical role in the pathogenesis of cancer and many other diseases [124, 125]. Nevertheless, our understanding of the role of ECM production and remodeling in vertebrate and invertebrate branching systems remains rudimentary. In general, functions of the ECM during branching morphogenesis can be divided into two broad categories: structural and biological.
Structural Role of ECM
The structural role of ECM in branching morphogenesis refers to its relatively inelastic and stiff properties, which act as a physical barrier to branching epithelium. For example, the site of fibronectin deposition has been correlated with branching pattern in the lung and kidney such that it is more abundant in clefts and around stalks, than in front of budding tips [126]. Similarly, sulfated glycosaminoglycans (GAGs) and type I collagen are more strongly expressed in the cleft and around ducts than in areas in front of mammary TEBs [113, 114]. Indeed, in submandibular gland organ cultures, down-regulation of fibronectin blocks branching, whereas adding fibronectin to the growth medium facilitates it [126]. It is therefore likely that regional deposition of fibronectin and other ECM proteins is a common strategy to execute branch-point decisions and guide growing epithelium in vertebrate organs.
In addition to localized synthesis, ECM is constantly being removed and/or remodeled to keep up with the dynamic nature of branching morphogenesis. For example, cells of the TEB face an ever-changing environment, where they need to remove interstitial ECM and lay down new matrix components during epithelial invasion. Moreover, the physical barrier posed by extracellular basement membrane (BM) needs to be removed for cells in the ductal epithelium to undergo side-branching. Remodeling of ECM is largely accomplished by matrix-modifying enzymes, including matrix metalloproteinases (MMPs), GAG-degrading enzymes (e.g., β-glucuronidase) and polysaccharide synthetases.
The importance of these enzymes for branching morphogenesis was first shown in cultured organs, including the kidney, lung, salivary gland and pancreas, where collagenase or hyaluronidase treatment can change branch patterns [127, 128]. In the salivary gland, collagenase appears to block epithelial bifurcation by preventing deposition of type I collagen fibrils, whereas inhibitors of collagenase cause the opposite phenotype [129]. In the kidney, selective inhibition of MMP-9 with function-perturbing antibodies causes abnormal branching, suggesting that MMP9 plays a role in renal branching morphogenesis [130]. Moreover, the kidneys of MMP9-deficient mice have 12% fewer nephrons than normal, probably reflecting a mild in vivo defect in branching morphogenesis [131]. MMP9-null mice have no overt mammary branching phenotype. However, MMP2-and MMP3-deficient mice show defective mammary ductal elongation and side-branching, respectively [132]. In addition, regulation of lung branching morphogenesis by EGFR is partly accomplished through EGFR-mediated induction of MMP14/MT1-MMP expression, and activation of MMP2 by the former enzyme [123]. Likewise, MMP14-mediated MMP2 activation and clearance of type I collagen by both enzymes appears to be required for normal mammary branching morphogenesis. This pathway probably also lies downstream of EGFR (M. Egeblad, M.D.S. and Z.W., unpublished results).
Biological Role of ECM
The outdated notion that ECM is a mere scaffold or barrier for cells has been revised to reflect the fact that it conveys important contextual information and actively regulates cell behavior [133]. Indeed, ECM components can influence cell shape, polarity, motility, growth, differentiation and survival by acting as fixed ligands for adhesion molecules that transduce ECM-derived signals to the cell interior and by sequestering other signaling molecules, such as growth factors and their binding proteins. By extension, enzymes that modify or degrade the ECM can affect cell behavior by altering the content and organization of ECM-associated information. For instance, proteolytic ECM remodeling can (1) alter or abolish existing ECM signals, (2) remove physical barriers to ductal branching and elongation, (3) unmask hidden structural cues, such as cryptic integrin binding sites on fibrillar collagen, a bio-active laminin-5 fragment that promotes epithelial motility, and anti-angiogenic fragments of collagens IV (tumstatin) and XVIII (endostatin), and (4) release ECM-sequestered signaling molecules, such as amphiregulin, FGFs, Wnts and TGF β [124].
Thus it is not surprising that the ECM, its receptors and its proteolytic remodeling influence branching. Indeed, multiple ECM receptors may participate, such that genetic ablation of any one receptor may show only subtle defects in branching. For example, deletion of the discoidin domain receptor-1 (DDR1) gene, which codes for a receptor tyrosine kinase that transmits signals upon binding fibrillar collagens, results in delayed and abnormal mammary development [134]. Thus, fibrillar collagens provide more than structural support. This fact may partly explain the delayed mammary branching phenotypes that occur in mice lacking collagenolytic enzymes MMP2 or MMP14, or that express cleavage-resistant or mutant type I collagen (M. Egeblad, M.D.S. and Z.W., unpublished results). In addition, mice that lack integrin α2β1 collagen/laminin receptors due to deletion of the α2 integrin gene show diminished but otherwise normal mammary branching [135]. Likewise, antibodies against β1 integrin cause diminished ductal elongation, as do antibodies against the laminin γ1 chain (i.e., part of a major ligand for β1 integrins) [136]. In contrast, targeted deletion of β1 integrin using MMTV-Cre is reported to have no effect on mammary development [137]. Over-expression of another laminin receptor, (β-1,4-galactosyltransferase, elicits abnormal mammary development [138], and yet another laminin receptor, dystroglycan, has been shown to regulate normal mammary epithelial cell polarity [139]. In addition, transitions between different modes of adhesion due to spatial differences in ECM deposition may play a role in ductal branch-point selection. For instance, localized fibronectin deposition is required for cleft formation in submandibular gland cultures and is associated with a switch from E-Cadherin to 0.5 α5β1 integrin-mediated adhesion, i.e., from cell-cell to cell-ECM adhesion, a transition that may stabilize bifurcation sites [126].
Concluding Remarks
It is amazing how nature uses sometimes similar, if not identical, molecular gadgets to build what clearly are non- homologous structures from the fly trachea to the human lung, kidney and mammary gland. Although a great deal has yet to be learned, several signaling pathways and common themes have emerged as conserved regulators of branching morphogenesis in diverse organs (Fig. 3 and Table 1).
Figure 3.

Common themes controlling branching morphogenesis in diverse organs. The mesenchyme produces guidance cues (Gm) and survival factors (Sm) for the epithelium during branching morphogenesis. The epithelium responses to these mesenchymal factors, via an unknown mechanism (dotted arrow and question mark), by making survival factors (Se) for the mesenchyme. In addition, the epithelium produces factors (Me) that can modulate the activities of factors produced by the mesenchyme (via an unknown mechanism, dotted arrow and question mark). The epithelium adjusts its responsiveness by expressing Re and fine-tuning its sensitivity to mesenchymal inputs. Conversely, the mesenchyme adjusts its responsiveness (Rm) to epithelial inputs. Epi, epithelium; Gm, mesenchymal guidance cues; Me, epithelial modulators; Mes, mesenchyme; Re, epithelial responsiveness; Rm, mesenchymal responsiveness; Se, epithelial survival factors; Sm, mesenchymal survival factors.
Table 1.
Molecular basis of the common themes that govern branching morphogenesis in diverse organs.
| Fly trachea | Fly air sacs | Lung | Kidney | Mammary gland | |
|---|---|---|---|---|---|
| Gm mesenchymal guidance cues | Bnl/Fgf | Bnl/Fgf | Fgf7, Fgf10 | Fgf7, Fgf10, GDNF | IGF, FGF, HGF? |
| Sm mesenchymal survival factors | - | Egf | |||
| Re epithelial responsiveness | Spry | N/A | Spry2 | Spry1 | Spry2 |
| Me epithelial modulators | - | - | Shh, TGF-β? | TGF-β? | TGF-β |
| Se epithelial survival factors | - | - | Shh, Fgf9 | Fgf | AREG |
| Rm mesenchymal responsiveness | - | - | Ptc-1, Hip, Spry4? | N/A | Ptc-1 |
An interesting question, then, is what such molecular conservation really means. Perhaps it means that there are “branching modules” consisting of a defined set of molecules and pathways that are activated whenever epithelial branching is required [140]. Alternatively, it may indicate that branching morphogenesis, irrespective of what species or organs it occurs in, is the sum of several cellular events, each of which is controlled by specific signaling pathways. Lastly, the molecular conservation may merely reflect regulatory relationships between signaling pathways that often work together in many different times and places during development. For example, directional FGF signaling, its regulation by Spry family proteins and its participation in epithelial–mesenchymal interactions are recurring themes in organogenesis, from tracheal development in flies to limb and tooth development in vertebrate organisms. So it should, after all, not be surprising that a small number of genes and pathways are used repeatedly to generate branched organs. While continuing our quest for molecules that play conserved roles in branching morphogenesis in diverse organs, we should also take note of mechanisms that determine specific differences in development of various branched structures.
One obstacle that hinders our progress in future studies on branching in vertebrate organ systems, in general, and in the mammary gland, in particular, is the lack of detailed information on cell behavior in this process. Technical improvements in organ culture and live imaging, for example, may help facilitate a better understanding of cellular events involved in mammary branching. Ultimately, such knowledge will be essential to our dissection of the effects different mesenchymal factors have on the epithelial cells and whether these factors act as guidance cues, mitogens, survival factors, or a combination thereof.
Another important area of future research will be to identify upstream regulators and downstream effectors of the signaling cascades known to influence branching morphogenesis, as well as those that have not yet been discovered, and to determine how these various pathways interact with one another in a holistic sense to direct and carry out the overall process of branching morphogenesis. What, for instance, are the critical downstream targets of FGF signaling? How do these events lead to production of epithelial signals that can affect mesenchymal activities, and vice versa? What are the downstream targets of EGF signaling in the mammary stroma? Does EGFR signaling alter production of FGFs or other stromal factors that in turn effect the epithelium; and if so, how? Lastly, we have primarily considered cellular and molecular events that occur at the tips of epithelial branches, which is where many of the branch-point decisions are made, yet side-branching also occurs [3, 7, 19, 21–23]. Although the main cellular and molecular events that regulate branching may be similar in primary branching and side-branching, deciphering their differences may be crucial to our eventual understanding of mechanisms that define mammary-specific branching and function.
Acknowledgments
We apologize to authors whose work is not cited due to space limitations. This work was supported by grants CA057621, CA058207 and ES012801 from the NIH and an institutional NRSA HL07731 (P. L.).
Abbreviations
- ADAM
a disintegrin and metalloproteinase
- AEL
after egg laying
- AER
apical ectodermal ridge
- BMP
bone morphogenetic protein
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EMT
epithelial–mesenchymal transition
- EGFR
EGF receptor
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- GDNF
glial-cell-line derived neurotrophic factor
- GFP
green fluorescent protein
- HGF
hepatic growth factor
- IGF
insulin growth factor
- MMP
matrix metalloprotease
- RTK
receptor tyrosine kinase
- SHH
sonic hedgehog
- TEB
terminal end bud
References
- 1.Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4(1):11–8. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 2.Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development. 2004;131(7):1449–62. doi: 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- 3.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296(5570):1046–9. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol. 2003;19:623–47. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- 5.Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284(5420):1635–9. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- 6.Cabernard C, Neumann M, Affolter M. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J Appl Physiol. 2004;97(6):2347–53. doi: 10.1152/japplphysiol.00435.2004. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies 10.1242/dev.02310. Development. 2006;133(9):1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Laurie GW. Organogenesis of the exocrine gland. Dev Biol. 2004;273(1):1–22. doi: 10.1016/j.ydbio.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Robinson GW. Identification of signaling pathways in early mammary gland development by mouse genetics. Breast Cancer Res. 2004;6(3):105–8. doi: 10.1186/bcr776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467–75. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 11.Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12(4):449–55. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- 12.Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3(7):513–23. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 13.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112(1):19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 14.Nelson WJ. Tube morphogenesis: closure, but many openings remain. Trends Cell Biol. 2003;13(12):615–21. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71(1):1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- 16.Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4(1):9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- 17.Hens JR, Wysolmerski JJ. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005;7(5):220–4. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–25. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 19.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8(1):201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisken C. Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia. 2002;7(1):39–48. doi: 10.1023/a:1015718406329. [DOI] [PubMed] [Google Scholar]
- 21.Chuang PT, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13(2):86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 22.Davies JA. Watching tubules glow and branch. Curr Opin Genet Dev. 2005;15(4):364–70. doi: 10.1016/j.gde.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271(1):98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122(11):3627–37. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- 25.Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8(1):65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt-Ott KM, Yang J, Chen X, Wang H, Paragas N, Mori K, et al. Novel regulators of kidney development from the tips of the ureteric bud. J Am Soc Nephrol. 2005;16(7):1993–2002. doi: 10.1681/ASN.2004121127. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, et al. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122(12):4013–22. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 28.Uv A, Cantera R, Samakovlis C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13(6):301–9. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 29.Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122(5):1395–407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 30.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127(12):2695–704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- 31.Brophy PD, Ostrom L, Lang KM, Dressier GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128(23):4747–56. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- 32.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–6. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 33.Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124(20):4077–87. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3(2):195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 35.Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9(6):831–42. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Fata IE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6(1):1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3(7):531–7. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 38.Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13(4):169–76. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 39.Dor Y, Djonov V, Keshet E. Making vascular networks in the adult: branching morphogenesis without a roadmap. Trends Cell Biol. 2003;13(3):131–6. doi: 10.1016/s0962-8924(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 40.Fisher CE, Michael L, Barnett MW, Davies JA. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development. 2001;128(21):4329–38. doi: 10.1242/dev.128.21.4329. [DOI] [PubMed] [Google Scholar]
- 41.Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressier GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243(1):128–36. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- 42.Reichman-Fried M, Dickson B, Hafen E, Shilo BZ. Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev. 1994;8(4):428–39. doi: 10.1101/gad.8.4.428. [DOI] [PubMed] [Google Scholar]
- 43.Klambt C, Glazer L, Shilo BZ. Breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992;6(9):1668–78. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- 44.Ghabrial A, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 45.Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7(6):245–51. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12(20):3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138–41. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 48.De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal—epithelial signalling during mouse organogenesis. Development. 2000;127(3):483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 49.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276(2):403–15. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickson C, Spencer-Dene B, Dillon C, Fantl V. Tyrosine kinase signalling in breast cancer: fibroblast growth factors and their receptors. Breast Cancer Res. 2000;2(3):191–6. doi: 10.1186/bcr53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, et al. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129(1):53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- 52.Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, et al. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132(6):1223–34. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 53.Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, et al. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. 2005;37(2):125–128. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- 54.Miettinen PJ, Warburton D, Bu D, Zhao JS, Berger JE, Minoo P, et al. Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev Biol. 1997;186(2):224–36. doi: 10.1006/dbio.1997.8593. [DOI] [PubMed] [Google Scholar]
- 55.Richards RG, Klotz DM, Walker MP, DiAugustine RP. Mammary gland branching morphogenesis is diminished in mice with a deficiency of insulin-like growth factor-I (IGF-I), but not in mice with a liver-specific deletion of IGF-I 10.1210/en.2003–1112. Endocrinology. 2004;145(7):3106–10. doi: 10.1210/en.2003-1112. [DOI] [PubMed] [Google Scholar]
- 56.Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142(11):4937–45. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- 57.Yant J, Buluwela L, Niranjan B, Gusterson B, Kamalati T. In vivo effects of hepatocyte growth factor/scatter factor on mouse mammary gland development. Exp Cell Res. 1998;241(2):476–81. doi: 10.1006/excr.1998.4028. [DOI] [PubMed] [Google Scholar]
- 58.Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. BioEssays. 2006;28(2):117–27. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 59.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92(2):253–63. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5(6):441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 61.Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126(11):2515–25. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- 62.Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126(18):4139–47. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- 63.Casci T, Vinos J, Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell. 1999;96(5):655–65. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- 64.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126(20):4465–75. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 65.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16(1):45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, et al. Sproutyl is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8(2):229–39. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, et al. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev. 2001;102(1–2):81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 68.Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9(4):219–22. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 69.Niehrs C, Meinhardt H. Modular feedback. Nature. 2002;417(6884):35–6. doi: 10.1038/417035a. [DOI] [PubMed] [Google Scholar]
- 70.Tsang M, Friesel R, Kudoh T, Dawid IB. Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol. 2002;4(2):165–9. doi: 10.1038/ncb749. [DOI] [PubMed] [Google Scholar]
- 71.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, et al. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412(6847):647–51. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 72.Furthauer M, Lin W, Ang SL, Thisse B, Thisse C. Sef is a feedback-induced antagonist of Ras/MAPK-mediated FGF signalling. Nat Cell Biol. 2002;4(2):170–4. doi: 10.1038/ncb750. [DOI] [PubMed] [Google Scholar]
- 73.Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell. 2005;8(5):689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 74.Torii S, Kusakabe M, Yamamoto T, Maekawa M, Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7(1):33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Hashimoto S, Nakano H, Singh G, Katyal S. Expression of Spred and Sprouty in developing rat lung. Gene Expr Patterns. 2002;2(3–4):347–53. doi: 10.1016/s1567-133x(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 76.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8(19):1083–6. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 77.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20(1):58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 78.Jaskoll T, Leo T, Witcher D, Ormestad M, Astorga J, Bringas P, Jr, et al. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn. 2004;229(4):722–32. doi: 10.1002/dvdy.10472. [DOI] [PubMed] [Google Scholar]
- 79.Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Scott MP, et al. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126(22):5181–93. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- 80.Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Hui C, et al. The GH2 transcription factor is required for normal mouse mammary gland development. Dev Biol. 2001;238(1):133–44. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- 81.Michno K, Boras-Granic K, Mill P, Hui CC, Hamel PA. Shh expression is required for embryonic hair follicle but not mammary gland development. Dev Biol. 2003;264(1):153–65. doi: 10.1016/s0012-1606(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 82.Gallego MI, Beachy PA, Hennighausen L, Robinson GW. Differential requirements for shh in mammary tissue and hair follicle morphogenesis. Dev Biol. 2002;249(1):131–9. doi: 10.1006/dbio.2002.0761. [DOI] [PubMed] [Google Scholar]
- 83.Chuang P-T, Kawcak TN, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17(3):342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 85.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14(6):627–44. [PubMed] [Google Scholar]
- 86.Vincent S, Ruberte E, Grieder NC, Chen CK, Haerry T, Schuh R, et al. DPP controls tracheal cell migration along the dorsoventral body axis of the Drosophila embryo. Development. 1997;124(14):2741–50. doi: 10.1242/dev.124.14.2741. [DOI] [PubMed] [Google Scholar]
- 87.Lu P, Minowada G, Martin GR. Increasing Fgf4 expression in the mouse limb bud causes polysyndactyly and rescues the skeletal defects that result from loss of Fgf8 function. Development. 2006;133(1):33–42. doi: 10.1242/dev.02172. [DOI] [PubMed] [Google Scholar]
- 88.Pizette S, Abate-Shen C, Niswander L. BMP controls proximo-distal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development. 2001;128(22):4463–74. doi: 10.1242/dev.128.22.4463. [DOI] [PubMed] [Google Scholar]
- 89.Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128(22):4449–61. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- 90.Pizette S, Niswander L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development. 1999;126(5):883–94. doi: 10.1242/dev.126.5.883. [DOI] [PubMed] [Google Scholar]
- 91.Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57(5 Pt 2):26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi H, Ikeda T. Transcripts for two members of the transforming growth factor-beta superfamily BMP-3 and BMP-7 are expressed in developing rat embryos. Dev Dyn. 1996:207. doi: 10.1002/(SICI)1097-0177(199612)207:4<439::AID-AJA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 93.Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291(1):67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 94.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105(7):863–73. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–10. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 96.Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, et al. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266(2):285–98. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 97.Serra R, Pelton RW, Moses HL. TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development. 1994;120(8):2153–61. doi: 10.1242/dev.120.8.2153. [DOI] [PubMed] [Google Scholar]
- 98.Zhao J, Bu D, Lee M, Slavkin HC, Hall FL, Warburton D. Abrogation of transforming growth factor-beta type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev Biol. 1996;180(1):242–57. doi: 10.1006/dbio.1996.0298. [DOI] [PubMed] [Google Scholar]
- 99.Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol. 1993;160(2):293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- 100.Pierce DF, Jr, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7(12A):2308–17. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- 101.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science. 1994;264(5167):1936–8. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- 102.Kaartinen V, Voncken JW, Staler C, Warburton D, Bu D, Heisterkamp N, et al. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11(4):415–21. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 103.Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, et al. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160(6):2081–93. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pollard JW. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001;3(4):230–7. doi: 10.1186/bcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serra R, Crowley MR. Mouse models of transforming growth factor beta impact in breast development and cancer. Endocr-Relat Cancer. 2005;12(4):749–60. doi: 10.1677/erc.1.00936. [DOI] [PubMed] [Google Scholar]
- 106.Joseph H, Gorska AE, Sohn P, Moses HL, Serra R. Over-expression of a kinase-deficient transforming growth factor-beta type II receptor in mouse mammary stroma results in increased epithelial branching. Mol Biol Cell. 1999;10(4):1221–34. doi: 10.1091/mbc.10.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crowley MR, Bowtell D, Serra R. TGF-beta, c-Cbl, and PDGFR-alpha the in mammary stroma. Dev Biol. 2005;279(1):58–72. doi: 10.1016/j.ydbio.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 108.Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, et al. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24(32):5053–68. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15(18):2211–8. doi: 10.1038/sj.onc.1201383. [DOI] [PubMed] [Google Scholar]
- 110.Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech Dev. 1999;86(1–2):125–36. doi: 10.1016/s0925-4773(99)00124-0. [DOI] [PubMed] [Google Scholar]
- 111.Tomlinson DC, Grindley JC, Thomson AA. Regulation of Fgf10 gene expression in the prostate: identification of transforming growth factor-beta1 and promoter elements. Endocrinology. 2004;145(4):1988–95. doi: 10.1210/en.2003-0842. [DOI] [PubMed] [Google Scholar]
- 112.Heine UI, Munoz EF, Flanders KC, Roberts AB, Sporn MB. Colocalization of TGF-beta1 and collagen I and III, fibronectin and glycosaminoglycans during lung branching morphogenesis. Development. 1990;109(1):29–36. doi: 10.1242/dev.109.1.29. [DOI] [PubMed] [Google Scholar]
- 113.Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-beta1 -growth-inhibited mouse mammary gland. J Cell Biol. 1990;110(6):2209–19. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silberstein GB. Postnatal mammary gland morphogenesis. Microsc Res Tech. 2001;52(2):155–62. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 115.White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133(8):1507–17. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- 116.Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, et al. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291(2):325–39. doi: 10.1016/j.ydbio.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 117.Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, et al. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9(9):777–85. [PubMed] [Google Scholar]
- 118.Kenney NJ, Bowman A, Korach KS, Barrett JC, Salomon DS. Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouse. Breast Cancer Res Treat. 2003;79(2):161–73. doi: 10.1023/a:1023938510508. [DOI] [PubMed] [Google Scholar]
- 119.Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127(2):304–15. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- 120.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126(12):2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 121.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132(17):3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J, Chen H, Peschon JJ, Shi W, Zhang Y, Frank SJ, et al. Pulmonary hypoplasia in mice lacking tumor necrosis factor-alpha converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev Biol. 2001;232(1):204–18. doi: 10.1006/dbio.2001.0176. [DOI] [PubMed] [Google Scholar]
- 123.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115(Pt 4):839–48. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16(5):558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 127.Wessells NK, Cohen JH. Effects of collagenase on developing epithelia in vitro: lung, ureteric bud, and pancreas. Dev Biol. 1968;18(3):294–309. doi: 10.1016/0012-1606(68)90037-7. [DOI] [PubMed] [Google Scholar]
- 128.Banerjee SD, Cohn RH, Bernfield MR. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977;73(2):445–63. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakanishi Y, Sugiura F, Kishi J, Hayakawa T. Collagenase inhibitor stimulates cleft formation during early morphogenesis of mouse salivary gland. Dev Biol. 1986;113(1):201–6. doi: 10.1016/0012-1606(86)90122-3. [DOI] [PubMed] [Google Scholar]
- 130.Lelongt B, Trugnan G, Murphy G, Ronco PM. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J. Cell Biol. 1997;136(6):1363–1373. doi: 10.1083/jcb.136.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lelongt B, Ronco P. Role of matrix metalloproteinases in kidney development and glomerulopathy: lessons from transgenic mice. Nephrol Dial Transplant. 2002;17(Suppl 9):28–31. doi: 10.1093/ndt/17.suppl_9.28. [DOI] [PubMed] [Google Scholar]
- 132.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162(6):1123–33. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8(11):437–41. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 134.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–17. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161(1):337–44. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, et al. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215(1):13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- 137.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6(2):159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 138.Hathaway HJ, Shur BD. Mammary gland morphogenesis is inhibited in transgenic mice that overexpress cell surface beta1, 4-galactosyltransferase. Development. 1996;122(9):2859–72. doi: 10.1242/dev.122.9.2859. [DOI] [PubMed] [Google Scholar]
- 139.Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62(23):7102–9. [PubMed] [Google Scholar]
- 140.Davies JA. Do different branching epithelia use a conserved developmental mechanism? BioEssays. 2002;24(10):937–48. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- 141.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006 Oct 12; doi: 10.1002/dvdy.20978. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Constantini FD, et al. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006 Nov 15;299(2):466–77. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 143.Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, et al. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wntl1, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131(14):3345–56. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]