Abstract
Aedes albopictus (Skuse) (Diptera: Culicidae) is a major nuisance mosquito and a potential arbovirus vector. The host-feeding patterns of Ae. albopictus were investigated during the 2002 and 2003 mosquito seasons in suburban neighborhoods in Wake County, Raleigh, NC. Hosts of blood-fed Ae. albopictus (n = 1,094) were identified with an indirect enzyme-linked immunosorbent assay, by using antisera made in New Zealand White rabbits to the sera of animals that would commonly occur in peridomestic habitats. Ae. albopictus fed predominantly on mammalian hosts (83%). Common mammalian hosts included humans (24%), cats (21%), and dogs (14%). However, a notable proportion (7%) of bloodmeals also was taken from avian hosts. Some bloodmeals taken from birds were identified to species by a polymerase chain reaction-heteroduplex assay (PCR-HDA). Ae. albopictus fed predominantly on chickens and a northern cardinal. PCR-HDA failed to produce detectable products for 29 (58%) of 50 bloodmeals for which DNA had been amplified, indicating that these mosquitoes took mixed bloodmeals from avian and nonavian hosts. Ae. albopictus preference for humans, dogs, and cats was determined by calculating host-feeding indices for the three host pairs based on the proportion of host specific blood-fed mosquitoes collected in relation to the number of specific hosts per residence as established by a door-to-door survey conducted in 2003. Estimates of the average amount of time that residents and their pets (cats and dogs) spent out of doors were obtained. Host-feeding indices based only on host abundance indicated that Ae. albopictus was more likely to feed on domestic animals. However, when feeding indices were time-weighted, Ae. albopictus fed preferentially upon humans. Ae. albopictus blood feeding on humans was investigated using a STR/PCR-DNA profiling technique that involved amplification of three short tandem repeats loci. Of 40 human bloodmeals, 32 (80%) were from a single human, whereas eight (20%) were multiple bloodmeals taken from more than one human host. We conclude that the blood-feeding preference of Ae. albopictus for mammals will limit acquisition of arboviruses by this species from infected avian amplification hosts. This feeding preference likely limits the vector potential of Ae. albopictus for North American arboviruses.
Keywords: Aedes albopictus, blood feeding, host availability, feeding indices, microsatellite analyses
Aedes albopictus (Skuse) (Diptera: Culicidae), a container-inhabiting mosquito indigenous to South East Asia, has spread to areas of Africa, the Middle East, Europe, the Caribbean, and South and North America (Gratz 2004). Ae. albopictus is now extensively distributed along the east coast and throughout the southeastern and midwestern United States (Moore 1999). Ae. albopictus is a major nuisance mosquito throughout its geographic range. Although Ae. albopictus has been found to be naturally infected with some indigenous arboviruses, this mosquito species has not been associated with epidemics of any arbovirus in the United States with the exception of dengue in Hawaii (Gratz 2004).
Knowledge of the blood-feeding habits of a mosquito species provides insight into its vector potential. Blood-feeding behavior can influence vector potential depending on the vertebrate host groups with which the mosquito makes contact. If reservoir and amplification hosts are the primary focus of vector blood feeding, the likelihood of pathogen acquisition by the vector increases. In addition, the blood-feeding behavior of a vector may influence the spatial distribution of a disease (Dye and Hasibeder 1986). Results of Ae. albopictus host-feeding studies (Savage et al. 1993, Niebylski et al. 1994) indicate that the limited vector potential of this species in the United States may result from its opportunistic feeding habits. However, these studies have been conducted in rural areas where the abundance of humans and domestic animals was unknown but would be expected to be lower than in suburban habitats.
In this report, we present results of a host-feeding study of Ae. albopictus conducted in a suburban area of North Carolina. The objectives of our investigation were to 1) describe the host-feeding patterns of Ae. albopictus and some sympatric mosquitoes inhabiting peridomestic areas of suburban landscapes, 2) determine effects of the availability of humans and domestic animals on host selection, 3) determine whether avian species were used as hosts, and 4) describe the feeding behavior of Ae. albopictus on humans.
Materials and Methods
StudyAreas
Our research was conducted in Raleigh (76.6° W, 35.8° N) in Wake County, NC, where ≈300,000 people presently reside. The suburban neighborhoods in our study were composed primarily of single-family dwellings, but three neighborhoods also had an apartment complex. From May to the end of October of the 2002 and 2003 mosquito seasons, four and eight neighborhoods, respectively, were included in our investigation. There were from 20 to 42 houses in the neighborhoods with an average of ≈50 m between dwellings and a property size ranging between 0.09 and 0.33 ha. Domestic animals, primarily dogs and cats, were present in most residences and a few residences in one neighborhood were in proximity to some farm animals (rabbits, horses, sheep, and chickens). The landscape of a typical residence consisted of a grass lawn in the front and back yards with shrubbery planted immediately adjacent to the dwelling. Along the edge of the property, there were woodlands composed of deciduous trees with undergrowth of grasses and low shrubs.
Host Survey
During the 2003 mosquito season, the abundance and spatial distribution of potential human and domestic animal hosts in each neighborhood were determined through a door-to-door census. Information collected in the survey included: 1) number of residents per house and their estimated weekly time spent outdoors during the summer, 2) number and kind of domestic animals and their approximate weekly time spent outdoors during the summer, and 3) time of day (morning, afternoon, and evening) residents and domestic animals were outdoors.
Collection and Processing of Mosquitoes
In each neighborhood, landscape vegetation on the grounds of each of 10 residences was aspirated for 10 min by using a large-bore aspirator (Nasci 1981). Sampling was weekly in 2002 and bimonthly in 2003. Mosquitoes were collected from shaded areas containing tall grass, herbaceous plants, or other knee-high vegetation. The aspirator was moved from side to side through the vegetation while the collector walked at a pace of ≈ 0.5 m/s. Immediately after collection, each sample bag was placed on wet ice in a cooler, and samples were transported to the laboratory for processing within 3 h of collection. The same personnel were used to make all aspirator collections. Residences within each neighborhood were not selected randomly for sampling because we wanted to maximize the numbers of mosquitoes collected. Consequently, collections were made at the same residences from peridomestic vegetation that provided a shaded environment for resting mosquitoes.
In the laboratory, each collection bag was placed in a freezer to kill the insects collected from foliage. Subsequently, mosquitoes were sorted under 10× magnification against a white background from debris, transferred to labeled vials, and stored in the freezer. Later, mosquitoes in each sample were identified to species (Slaff and Apperson 1989) and sex and counted. In addition, females were examined to determine their gonotrophic status (unfed, blood fed, or gravid). Blood-fed mosquitoes were placed singly into labeled tubes and stored in a freezer for subsequent bloodmeal host identification, whereas unfed and gravid mosquitoes were discarded after they were counted. The quality of each bloodmeal was scored based on the stage of blood digestion described by Sella stage (Detinova 1962). In 2002, all blood-fed mosquitoes were analyzed for host source, regardless of the Sella stage; however, in 2003, a more selective approach was used with only “freshly” blood-fed mosquitoes classified as Sella stage ≤3 tested. In this way, we could conclude that bloodmeals that were unidentified were likely taken from hosts that were unidentifiable with the antisera included in our tests.
Bloodmeal Analyses
Hosts of blood-fed mosquitoes were identified using an indirect enzyme-linked immunosorbent assay (ELISA) described by Irby and Apperson (1988). Briefly, blood-fed mosquitoes were homogenized in 0.01 M phosphate-buffered saline, pH 7.4. Homogenates were clarified through centrifugation (5000 × g for 10 min), and the supernatant was used for bloodmeal analyses. Assays used host-specific antisera made in New Zealand White rabbits against vertebrate serum proteins (Irby and Apperson 1988). Antisera used included anti-human, -dog, -cat, -white-tailed deer, -horse, -raccoon, -squirrel, -cotton tail rabbit, -opossum, -frog, -turtle, and -bird. Immediately after serologic testing, 0.125 M EDTA, pH 8.0, was added (10 μl) to each remaining bloodmeal extract. All extracts were stored at −80°C and subsequently subjected to molecular analyses as described below.
The feeding behavior of Ae. albopictus on humans was investigated using a subset of bloodmeals that tested positive for human blood by ELISA. The propensity of Ae. albopictus to take multiple bloodmeals from more than one human was evaluated using a polymnerase chain reaction (PCR)-DNA profiling technique (Chow-Shaffer et al. 2000) that amplified three short tandem repeats (STR) loci (CSF1PO, TPOX, and TH01). Briefly, DNA was extracted from saline extracts of mosquito bloodmeals as described previously (Wetton et al. 1987). The final DNA pellets were resuspended in 20 μl of DNA-grade water. Alleles were amplified with the GenePrint STR Multiplex system for CTT (Promega, Madison, WI). PCR reactions were completed using 5–15 ng of template DNA according to directions provided by the manufacturer (Promega 2001). Allelic DNA was separated on 6% acrylamide gels by electrophoresis. After silver staining, gels were digitally photographed. The allelic banding pattern for each mosquito was visually examined and scored relative to a reference allelic ladder. The number of identical allelic profiles was determined with MatchID software (version 2.0) (Chow-Shaffer et al. 2000). The probability of amplifying matching allelic profiles from different humans with the CTT STR triplex is 1 in 1,590 for African-Americans, 1 in 435 for Caucasian-Americans, and 1 in 549 for Hispanic-Americans (Promega 2001). All three races were present in our study neighborhoods, but the racial structure of the resident population of each neighborhood was unknown.
Ae. albopictus (n = 83) and Aedes vexans (Meigen) (n = 59) bloodmeals testing positive by ELISA against bird antisera were processed further to identify the species of avian hosts. A PCR-heteroduplex assay (HDA) was used as described previously (Lee et al. 2002) to identify avian hosts. When DNA was amplified but the PCR-HDA failed to produce a product, the amplified DNA was classified as nonavian, indicating that a mixed bloodmeal was taken. Subsequently, some of these amplicons were arbitrarily selected and subjected to DNA sequence analysis. Hosts were identified by searching for matching cytochrome b sequences in the GenBank sequence database (http://www.ncbi.nlm.nih.gov/BLAST). GenBank sequence matches of <98%, but ≥90% were classified as a species-like bird for the closest matching avian species.
Geographic Information System
A geographic information system (GIS) was created for each neighborhood so that spatial patterns of blood-feeding activity in relation to the abundance of humans and domestic animals could be visually examined. Shapefiles for property boundaries, buildings, and street centerlines for all study sites were downloaded from the Wake County government GIS Web site (http://www.wakegov.com/tax/propertyandmapping/gisdigitaldata.htm) and imported into ArcMap (ESRI, Redlands, CA). Residential lot sizes were determined from data in attribute tables of property boundary shapefiles.
Data Analyses
Variables related to host surveys and mosquito host preferences were analyzed separately for humans, dogs, and cats for all households in each neighborhood and at households where host-specific bloodmeals were collected over the 2003 mosquito season. With Kolmogorov–Smirnov tests, we determined that the numbers of hosts per residence and numbers of blood-fed mosquitoes collected per sample were not normally distributed (PROC UNIVARIATE, SAS Institute 2000). Consequently, before calculating feeding indices, the numbers of hosts (humans, dogs, and cats) per residence and per hectare as well as numbers of host-specific blood-fed mosquitoes per aspiration sample were log transformed [log (x + 1)] to achieve approximate normality.
Host-Feeding Patterns
The percentage of the total numbers of bloodmeals identified for different host groups (mammalian, avian, turtle, and frog) was calculated for both mosquito seasons for each mosquito species separately for which we had successfully tested ≥50 specimens. Species-specific mammalian and some avian bloodmeals were further enumerated for these mosquito species.
Host-Feeding Indices
We examined the relationship between host abundance and the blood-feeding frequency of Ae. albopictus through a host-feeding index (HFI) modified from Kay et al. (1979). The index was calculated as follows:
where Nx and Ny are the mean numbers of bloodmeals taken from hosts x and y per residence or hectare, respectively; and Ax and Ay are the mean number per residence or hectare of hosts x and y, respectively.
We evaluated the host preference of Ae. albopictus for dogs, cats, and humans by calculating separate HFIs for the three pairs of hosts for each neighborhood. Additionally, we recalculated HFIs incorporating a temporal component of availability, to derive a time-weighted feeding index, HFIT, as follows:
where Tx and Ty are estimated time in hours (T) spent outdoors per week for host x and host y, respectively.
An HFI or HFIT > 1 indicated that host x was preferentially fed upon, whereas a value <1 indicated that host y was preferentially fed upon. In applying the concept of a feeding index to characterize host preference, we made the following assumptions: 1) the amount of time spent out of doors during mosquito season did not change for hosts, 2) the time of day that hosts were available out of doors did not change during mosquito season; 3) the abundances of humans and pet animals were constant throughout the mosquito season; and 4) host-defensive behavior did not alter mosquito-feeding success.
A one-way analysis of variance (PROC GLM, SAS Institute 2000) was used to determine whether there were significant differences in the mean HFI and HFIT values for the three pairs of hosts across all eight neighborhoods. Tukey’s honestly significant difference (HSD) tests were used to separate significantly different means.
Results
Bloodmeal Analysis
In the 2002 and 2003 mosquito seasons, blood-fed mosquitoes (n = 3,065) of 15 species from six genera were tested. The three most commonly collected blood-fed mosquito species were Ae. albopictus (n = 1,172; 38% of total), Ae. vexans (n = 920; 30% of total), and Aedes triseriatus Say (n = 601; 20% of total) (Table 1). A small percentage (1–5%) of bloodmeals were taken by these three species from frogs and turtles, but the majority (>70%) of mosquitoes fed on mammals. Mammalian hosts fed upon most frequently were humans, deer, and squirrels, respectively (Table 2); however, bloodmeals were taken frequently from other mammalian hosts, including dogs, cats, raccoons, horses, rabbits, and opossums. Ae. albopictus fed most frequently on humans (24%), cats (21%), and dogs (14%) of the mammalian hosts for which we tested bloodmeals. Serological analyses indicated that ≈6% of Ae. albopictus bloodmeals were taken from more than one host. Culex pipiens L. complex species Culex restuans Theobald and Psorophora ferox (Humboldt) were the less commonly collected blood-fed mosquitoes (Table 1). Culex mosquitoes mostly fed on birds, whereas Ps. ferox mainly took bloodmeals from mammals.
Table 1.
Host-feeding frequency of different mosquito species during the mosquito seasons of 2002–2003
| Mosquito species | Total no. tested | No. identified (% of total tested) | No. feeding on (% of total identified)
|
||||
|---|---|---|---|---|---|---|---|
| >1 host | Mammal | Bird | Frog | Turtle | |||
| Ae. albopictus | 1,172 | 1,094 (93) | 56 (6) | 909 (83) | 82 (7) | 20 (2) | 17 (2) |
| Ae. triseriatus | 601 | 574 (96) | 72 (13) | 407 (71) | 53 (9) | 14 (2) | 28 (5) |
| Ae. vexans | 920 | 896 (97) | 44 (5) | 782 (87) | 51 (6) | 9 (1) | 10 (1) |
| Cx. pipiens | 72 | 71 (99) | 2 (4) | 11 (15) | 52 (73) | 6 (8) | |
| Cx. restuans | 185 | 183 (99) | 9 (5) | 19 (10) | 150 (83) | 3 (1) | 2 (1) |
| Ps. ferox | 67 | 62 (93) | 5 (8) | 50 (81) | 7 (11) | ||
Table 2.
Mammalian hosts fed upon by different mosquito species during the mosquito seasons of 2002–2003
| Mosquito species | No. tested | Mammalian hosts (% of total mammalian hosts identified)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Dog | Cat | Deer | Horse | Rabbit | Squirrel | Raccoon | Opossum | ||
| Ae. albopictus | 909 | 219 (24) | 124 (14) | 193 (21) | 25 (3) | 40 (4) | 88 (10) | 104 (11) | 53 (6) | 63 (7) |
| Ae. triseriatus | 407 | 28 (8) | 14 (3) | 38 (9) | 9 (2) | 13 (3) | 47 (12) | 221 (54) | 29 (7) | 8 (2) |
| Ae. vexans | 782 | 23 (3) | 14 (2) | 45 (6) | 494 (64) | 62 (8) | 77 (10) | 36 (5) | 24 (3) | 7 (1) |
| Cx. pipiens | 11 | 1 (9) | 2 (18) | 1 (9) | 1 (9) | 6 (55) | ||||
| Cx. restuans | 19 | 1 (5) | 4 (21) | 1 (5) | 4 (21) | 3 (16) | 1 (5) | 1 (5) | 3 (16) | 1 (5) |
| Ps. ferox | 50 | 1 (2) | 9 (18) | 18 (36) | 9 (18) | 7 (14) | 2 (4) | 1 (2) | 3 (6) | |
Only 21 (26%) of 82 Ae. albopictus that were ELISA-positive were identified by PCR-HDA to bird species or an avian like-species. Of these 21 specimens, 17 (81%) mosquitoes fed on domestic chickens, three (14%) females fed on a white pelican-like bird, and one (5%) mosquito had taken a bloodmeal from a northern cardinal. For Ae. vexans, 17 (29%) of 59 ELISA-positive mosquitoes were identified to avian species or avian-like species. Seven (41%) mosquitoes had fed on domestic chickens, six (35%) females had taken bloodmeals from white pelican-like birds, and four (25%) mosquitoes had fed on northern cardinals. Mixed feedings on different avian species were not detected in bloodmeals analyzed for either mosquito species.
DNA was amplified from 50 (61%) of the 82 bird bloodmeals identified by ELISA. However, PCR-HDA failed to produce detectable products for 28 (56%) of the 50 Ae. albopictus avian bloodmeal extracts for which DNA was amplified. These 28 samples were classified as containing mixed bloodmeals taken from avian and nonavian hosts. Some (n = 18) of the nonavian PCR products were subjected to DNA sequence analysis so that the host source of the bloodmeals could be identified. In addition to having fed on birds, 17 mosquitoes were determined to have fed on humans, and one mosquito fed on a white-tailed deer. For Ae. vexans, DNA was amplified from 36 (61%) of 59 avian bloodmeal extracts. PCR-HDA failed to produce detectable products for 18 (50%) of the 36 avian bloodmeal extracts from which DNA was amplified. These 18 samples represented mixed feedings on avian and nonavian hosts. Through sequencing of 13 of the 18 nonavian PCR products, humans (n = 6), cows (n = 4), and white-tailed deer (n = 3) were identified as additional hosts of mosquitoes that had also fed on birds.
Human microsatellite primers were used in PCR analyses to fingerprint Ae. albopictus bloodmeals. Allelic DNA profiles were amplified from 40 (36%) of 112 bloodmeals that were identified by ELISA as having been taken from humans. A bloodmeal containing more than one allelic pattern was interpreted to result from the mosquito feeding on more than one person (Chow-Shaffer et al. 2000). The allelic profiles indicated that 32 (80%) of 40 bloodmeals were taken from a single human host, seven (17.5%) mosquitoes had fed on more than one person, and one (2.5%) mosquito had fed on more than two different people (Fig. 1). A different allelic profile was found in each of six pairs of mosquitoes (= 12 mosquitoes), suggesting that six humans had each been fed upon by two different mosquitoes.
Fig. 1.
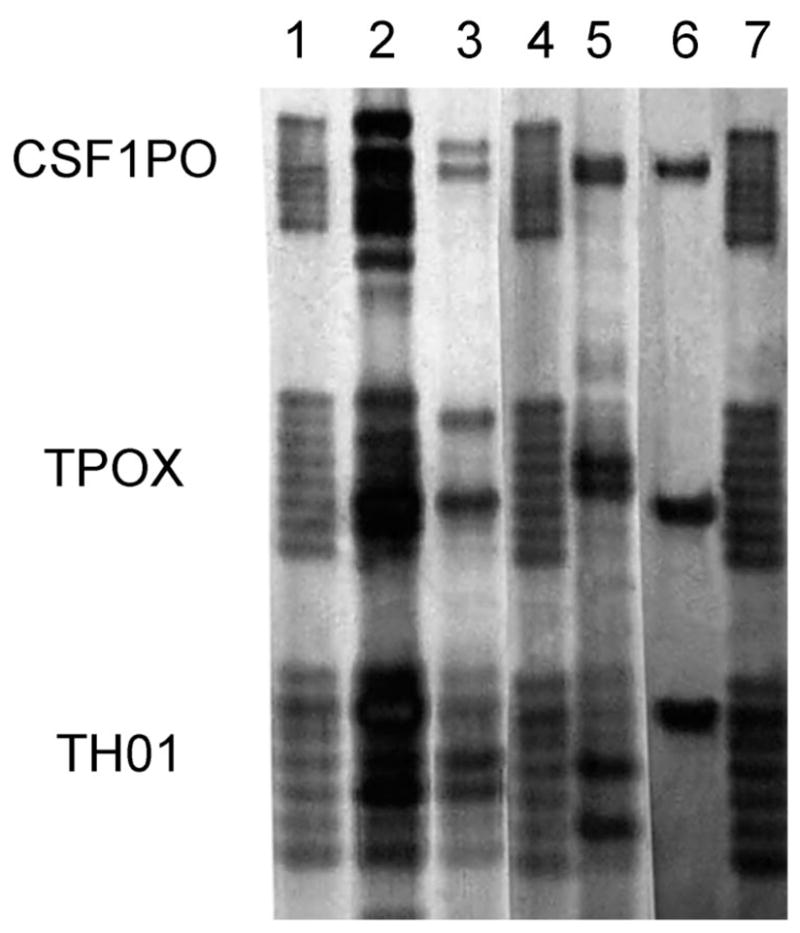
Analysis for CTT (CSF1PO, TPOX, TH01) STR loci amplified from DNA extracted from blood-fed Ae. albopictus collected from suburban neighborhoods in Raleigh, NC, during the 2002 and 2003 mosquito seasons. Alleles were separated by electrophoresis on 6% acrylamide gels and visualized by silver staining. Lanes 3, 5, and 6 illustrate mosquito bloodmeals each taken from a different person; lane 2 shows a multiple feeding taken from more than two people. Lanes 1, 4, and 7 present allelic markers.
Effects of Host Abundance and Availability on Blood Feeding
In our study neighborhoods, humans were more abundant than pets on a per residence and per hectare basis (Table 3). The mean number of dogs and humans per residence was higher at residences where dog- and human-specific blood-fed Ae. albopictus were collected. Conversely, the mean number of cats per residence was lower at residences where cat-specific blood-fed Ae. albopictus were collected. However, on a per hectare basis, humans, dogs, and cats were more abundant at residences where human-, dog-, and cat-specific blood-fed Ae. albopictus were collected.
Table 3.
Abundance of humans, dogs, and cats in suburban neighborhoods (n = 8) in Raleigh, NC, during the 2003 mosquito season
| Mean no. (± SE)
|
||
|---|---|---|
| Host | per residence | per hectare |
| At all residences where bloodmeals were collected | ||
| Humans | 2.2 (0.1) | 11.6 (1.1) |
| Dogs | 0.4 (0.1) | 2.5 (0.4) |
| Cats | 0.4 (0.1) | 3.9 (1.4) |
| At residences only where host-specific bloodmeals were collected | ||
| Humans | 2.2 (0.1) | 18.8 (1.5) |
| Dogs | 1.2 (0.1) | 12.6 (1.3) |
| Cats | 2.5 (0.5) | 41.4 (11.6) |
When the total number of hosts in each neighborhood was included in an analysis of Ae. albopictus feeding on humans compared with domestic animals, we found a preference for feeding on dogs and cats relative to humans and equivalent feeding on cats and dogs (Table 4). We found significant differences between mean HFI values for the three pairs of hosts (F = 7.94; df = 2, 21; P = 0.003). The mean HFIcats versus humans and mean HFIdogs versus humans were significantly higher (P <0.05) compared with other HFIs when all residences were considered. In subsequent analyses that solely included residences in all neighborhoods where host-specific blood-fed Ae. albopictus were collected, we again found significant differences between the mean HFIs for the three pairs of hosts (F =11.48; df = 2, 21; P = 0.0004). Ae. albopictus preferred to feed on dogs and cats relative to humans, and the mean HFIdogs versus humans was significantly higher (P <0.05) compared with other HFIs (Table 4).
Table 4.
Host preference of Ae. albopictus for humans, dogs, and cats in suburban neighborhoods (n = 8) in Raleigh, NC, during the 2003 mosquito season
| Mean feeding index ± SEa |
|||
|---|---|---|---|
| Cats vs. dogs | Cats vs. humans | Dogs vs. humans | |
| Per residence | |||
| Host abundance | |||
| Neighborhood-wideb | 1.0 ± 0.2a | 6.3 ± 1.3b | 4.4 ± 0.9b |
| Residence-specificc | 0.4 ± 0.1a | 2.6 ± 2.2b | 5.4 ± 1.1b |
| Host abundance time-weighted | |||
| Neighborhood-wideb | 0.5 ± 0.1b | 0.2 ± 0.04a | 0.7 ± 0.2b |
| Residence-specificc | 0.1 ± 0.02a | 0.1 ± 0.02a | 0.7 ± 0.2b |
| Per hectare | |||
| Host abundance | |||
| Neighborhood-wideb | 0.5 ± 0.1a | 2.6 ± 0.5b | 4.1 ± 0.8c |
| Residence-specificc | 0.3 ± 0.1a | 0.4 ± 0.1a | 1.3 ± 0.3b |
| Host abundance time-weighted | |||
| Neighborhood-wideb | 0.5 ±3 0.04b | 0.2 ± 0.1a | 0.4 ± 0.1b |
| Residence-specificc | 0.1 ± 0.02a | 0.2 ± 0.03a | 1.2 ± 0.2b |
Means within the same row followed by the same letter are not significantly different at P=0.05 by Tukey’s HSD test.
All residences in neighborhoods were used in calculating host-feeding indices.
Only residences where host-specific bloodmeals were collected were used in calculating host-feeding indices.
When we analyzed HFI values for all residences on a per hectare basis, a preference for cats and dogs relative to humans and a preference for cats relative to dogs was observed (Table 4). We found significant differences between mean HFI values for the three pairs of hosts (F = 10.06; df = 2, 21; P = 0.001). Similar to the results of HFI on a per residence basis, the mean HFIcats versus humans and mean HFIdogs versus humans were significantly higher (P < 0.05) when all residences were considered. Analyses that included residences where host-specific blood-fed Ae. albopictus were collected, indicated that Ae. albopictus preferred to feed equally on dogs, but more on these hosts than on cats (Table 4). Again, there was a significant difference between the feeding indices for the three pairs of hosts (F = 11.81; df = 2, 21; P = 0.0004). The mean HFIdogs versus humans was significantly higher (P < 0.05) than other HFIs when residences where host-specific blood-fed Ae. albopictus were collected were considered.
Subsequent analyses were carried out incorporating estimates of the time spent out of doors into HFIT values. When all residences were included in analyses, we found more mosquitoes feeding on humans relative to dogs and cats and more feeding on dogs compared with cats (Table 4). Analyses exclusively including residences where host-specific blood-fed Ae. albopictus also were collected showed that Ae. albopictus took a greater number of feedings from humans than expected relative to dogs and cats and a greater number of bloodmeals from dogs than cats (Table 4). There was a marginally significant difference between the mean HFIT values for the three pairs of hosts including all residences (F = 3.30; df = 2, 21; P = 0.057) and a significant difference at residences where blood-fed mosquitoes were collected (F = 16.63; df = 2, 21; P < 0.0001). The mean HFIT-dogs versus humans was significantly higher (P < 0.05) than other HFITs when residences where host-specific blood-fed Ae. albopictuswere collected were considered.
The HFIT also was calculated based on a per hectare basis. Similar to the analyses on a per residence basis, when all residences were included in analyses, Ae. albopictusexhibited a predilection to feed on humans relative to dogs and cats and a preference to feed on dogs relative to cats (Table 4). Analyses solely including residences where host-specific blood-fed Ae. albopictus were collected showed that Ae. albopictus took a greater number of feedings from humans and dogs relative to cats; however, more feedings were taken from dogs relative to humans (Table 4). There was a significant difference between the mean HFIT values for the three pairs of hosts when all residences (F= 3.57; df = 2, 21; P= 0.046) and only residences where blood-fed mosquitoes were collected (F = 18.95; df = 2, 21; P < 0.0001) were included in analyses. The mean HFIT-dogs versus humans and HFIT-cats versus dogs were significantly higher (P < 0.05) than other HFITs when residences where host-specific blood-fed Ae. albopictus were collected and where all residences were considered, respectively.
Discussion
Ae. albopictus has been reported previously to exhibit an opportunistic host-feeding pattern with a predilection to feed on mammalian hosts (Tempelis et al. 1970, Sullivan et al. 1971, Savage et al. 1993, Niebylski et al. 1994). Our results support these findings because >80% of the blood-fed Ae. albopictus that we tested fed on mammals. Furthermore, Ae. albopictus fed more frequently on humans than on other mammals, which reflects the anthropophilic nature of this mosquito species or the greater abundance and availability of humans hosts. We used a PCR-DNA profiling technique to investigate the feeding behavior of Ae. albopictus on humans. We estimated that 20% of human bloodmeals were taken from two or more people. In addition, we found that some individual humans may have been fed on by more than one mosquito, suggesting that some humans are more attractive or vulnerable to attack by Ae. albopictus. Nonrandom feeding activity would be expected to result in spatial variation in the successful acquisition of bloodmeals from humans and in the resultant distribution of blood-fed mosquitoes in the landscape. However, our findings need to be interpreted cautiously. The mosquitoes in each of two of the six pairs were collected at two different residences that were separated by distances of ≈2 and 4 km and on dates that were 13 and 1 mo apart, respectively, so it is likely that the two mosquitoes in each pair had fed on two different humans that had matching allelic profiles. The remaining four pairs of mosquitoes were each collected at a different residence, but the two mosquitoes in each pair were collected at the same home within the same week, suggesting that the mosquitoes in each pair had fed on the same human. However, in their investigation of the feeding behavior of Aedes aegypti (L.), Chow-Shaffer et al. (2000) reported that the CTT STR triplex amplified the same allelic pattern from some family members. In our study to resolve the question of whether some humans are preferentially fed on by Ae. albopictus, we would need to include additional microsatellite markers with the CTT STR triplex to reduce the probabilities of amplifying matching allelic profiles from two different humans. Regarding mosquito feeding on cats, the abundance of this host was lower at residences where we collected Ae. albopictus that had fed on cats. In general cats are free-roaming and nocturnally active, and we speculate that in daytime these hosts rest in habitats away from their residence or that after blood feeding on cats, engorged mosquitoes congregated in vegetation around residences where cats were absent or low in abundance.
Ae. triseriatus also fed primarily on mammals (71%) but mostly upon squirrels (54%). This mosquito has been found previously to feed predominantly on mammals, such as dogs (Szumlas et al. 1996b), deer (Burkot and DeFoliart 1982), and chipmunks and gray squirrels (Nasci 1985). However, Irby and Apperson (1988) reported Oc. triseriatus to fed mainly (75%) on turtles. All of these studies were carried out in woodlands areas as opposed to suburban landscapes where our investigation was conducted. We found Ae. vexans also exhibits highly mammalophilic host-feeding habits. In previous research, Ae. vexans has been reported to feed predominantly on large mammals, such as deer, dogs, horses, and cows (Tempelis 1975, Burkot and DeFoliart 1982, Irby and Apperson 1988). Variations in reported feeding patterns of all three mosquito species undoubtedly resulted from geographic differences in the composition and abundance or availability of mammalian populations.
We found that Ae. albopictus fed occasionally upon avian hosts (7%), and our findings are congruent with those of other studies reporting the use of birds as hosts in Missouri (17%) (Savage et al. 1993) and Hawaii (6%) (Tempelis et al. 1970). PCR-HDA revealed that Ae. albopictus had mainly fed on confined domestic fowl rather than free-ranging wild birds. These findings further indicate that Ae. albopictus feeds opportunistically on locally abundant and available hosts.
Serological analyses showed that Ae. vexans took bloodmeals infrequently from birds (6%). The majority of avian bloodmeals were taken from ground-dwelling birds, specifically domestic chickens and northern cardinal. Similar results were reported by Gunstream et al. (1971) for host-feeding studies completed in southeastern California. They determined using a precipitin technique that ≈5% of blood-fed Ae. vexans had fed on birds, mainly domestic chickens.
Previous studies of the host-feeding patterns of Ae. albopictus (Savage et al. 1993, Niebylski et al. 1994) did not consider the relative abundance and availability of hosts. Consequently, it is difficult to draw any firm conclusions about how local host abundance affected feeding patterns of Ae. albopictus in these studies. The feeding index proposed by Kay et al. (1979) provides a method to compare the host preference of Ae. albopictus based on the of number of bloodmeals taken from specific hosts in relation to the estimated abundance of these hosts. Based on host abundance, we determined that Ae. albopictus preferred to feed on dogs and cats rather than humans. In contrast to our results, in periurban areas of the city of Tremembé, Sao Palo, Brazil, Gomes et al. (2003) used a feeding index based on host abundance to show that Ae. albopictus preferred to feed on humans relative to some domestic animals, including dogs. We derived time-weighted feeding indices, using estimates of the outdoor exposure of residents and their pets, and found that Ae. albopictus took a greater proportion of bloodmeals than would be expected from humans relative to dogs and cats. Our results support the assertions of Franco-Estrada and Craig (1995) that Ae. albopictus is highly anthropophilic but that host abundance and availability has a significant impact on the host-feeding patterns of this peri-domestic mosquito species.
Thus, host selection and blood-feeding habits of a mosquito species are one of many factors affecting the transmission of an arbovirus. The host-feeding pattern of Ae. albopictus seems to be a significant limiting factor for the vector potential of this mosquito species in the transmission of arboviruses in the continental United States. The mammalophilic feeding preference of Ae. albopictus suggests that this species may be a potential vector of arboviruses that involve a mammal-to-mammal virus transmission cycles. In this regard, Ae. albopictus has been identified as the primary vector in several dengue epidemics but only in the absence of Ae. aegypti (Gratz 2004). The high vector potential of Ae. aegypti for dengue virus transmission results in large part because this mosquito feeds almost exclusively on humans and takes more than one human bloodmeal during a gonotrophic cycle (Chow-Shaffer et al. 2000; Scott et al. 2000). The blood-feeding behavior of Ae. aegypti is also an important determinant of the spatial distribution of dengue as well, because blood feeding is nonrandom and occurs on a small proportion of available humans (de Benedictis et al. 2003). In comparison, we found that ≈20% of human bloodmeals were taken by Ae. albopictus from more than one person; additionally, we determined that some people were potentially fed upon repeatedly by Ae. albopictus. However, only 24% of bloodmeals taken from mammals were from humans, suggesting that the opportunistic feeding behavior of Ae. albopictus substantially reduces its opportunity to acquire or transmit dengue viruses. A review of host-feeding studies conducted in dengue-endemic areas (Hawley 1988) indicates that Ae. albopictus exhibits broad feeding habits in host utilization, indicating that our results are relevant to areas where dengue viruses are actively transmitted.
Ae. albopictus is widely distributed throughout the southern Appalachian region of the mid-Atlantic United States (Moore 1999) where La Crosse virus is endemic (Szumlas et al. 1996a, Jones et al. 1999, Nasci et al. 2000). Ae. albopictus has been shown to be a competent laboratory vector of La Crosse virus (Cully et al. 1992). Although Ae. albopictus has been found to be naturally infected with La Crosse virus (Gerhardt et al. 2001), this mosquito species has been incriminated as a vector based solely on its occurrence and high abundance on the home grounds of La Crosse encephalitis case patients (Erwin et al. 2002). It is notable that 11% of Ae. albopictus bloodmeals were taken from gray squirrels, which are La Crosse virus-amplifying hosts (Yuill 1983). In comparison, Ae. triseriatus, the primary vector of La Crosse virus (Turell and LeDuc 1983), fed predominantly (54%) on gray squirrels in the suburban landscapes where we collected blood-fed Ae. albopictus. These findings suggest that the opportunistic feeding habits may be a factor contributing to the low vector potential of Ae. albopictus for La Crosse virus.
We found Ae. albopictus to feed occasionally on birds, which explains why this mosquito has been found to be naturally infected with West Nile virus (CDC 2005). Turell et al. (2001) established Ae. albopictus to be a highly efficient vector of West Nile virus under laboratory conditions. As Turell and others pointed out, however, Ae. albopictus would not be a suitable enzootic vector for West Nile virus because this mosquito species feeds infrequently on birds and does not engage in multiple feedings on more than one bird. Cx. pipiens is generally regarded to be an efficient enzootic vector of West Nile virus. In comparison with our results for Ae. albopictus, 96% of blood-fed Cx. pipiens mosquitoes (n = 73) collected from New York City were found to have fed on 11 different species of birds by Apperson et al. (2002). In addition, some Cx. pipiens mosquitoes had fed on more than one avian host. In our investigation, molecular analyses of some Ae. albopictus avian bloodmeals revealed that these mosquitoes also had bitten a mammalian host, principally a human. These findings suggest that Ae. albopictus could occasionally act as a bridge vector of West Nile virus, but it is an unlikely epidemic vector for this virus.
The opportunistic feeding habits of Ae. albopictus is a significant biological attribute, which allows this mosquito to take advantage of available hosts. Undoubtedly, the rapid spread of this invasive mosquito species in the United States can be attributed in large part to its opportunistic feeding habits, which allows Ae. albopictus to obtain bloodmeals and be reproductively active regardless of the species composition of host populations. We conclude, however, that the tendency of Ae. albopictus to feed on abundant and accessible mammalian vertebrates limits its vector potential for arboviral transmission in the continental United States.
Acknowledgments
We thank Gene Powell for field assistance and Drs. Coby Schal, Jules Silverman (Department of Entomology, North Carolina State University), Heather Cheshire (Department of Forestry, North Carolina State University), and Bruce Harrison (North Carolina Department of the Environment and Natural Resources) for comments on the manuscript. We are grateful to Tom Scott and Andy Fleisher for providing software that assisted us in analyzing DNA profiles of human bloodmeals. Our research was supported in part by funds provided by the Walter Reed Army Institute of Research, the U.S. Army Center for Health Promotion and Preventive Medicine, the North Carolina Pesticide Environmental Trust Fund, and a grant from the Centers for Disease Control and Prevention (project number CI00026).
References Cited
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Burkot TR, DeFoliart GR. Blood meal sources of Aedes triseriatus and Aedes vexans in a southern Wisconsin forest endemic for La Crosse encephalitis virus. Am J Trop Med Hyg. 1982;31:376–381. doi: 10.4269/ajtmh.1982.31.376. [DOI] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. West Nile virus: entomology. 2005 http://www.cdc.gov/ncidod/dvbid/westnile/mosquitoSpecies.htm.
- Chow-Shaffer E, Sina B, Hawley WA, de Benedictis J, Scott TW. Laboratory and field evaluation of polymerase chain reaction-based forensic DNA profiling for use in identification of human blood meal sources of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:492–502. doi: 10.1603/0022-2585-37.4.492. [DOI] [PubMed] [Google Scholar]
- Cully JR, Jr, Steit TG, Heard PB. Transmission of La Crosse virus by four strains of Aedes albopictus to and from the eastern chipmunk (Tamias striatus) J Am Mosq Control Assoc. 1992;8:237–240. [PubMed] [Google Scholar]
- de Benedictis J, Chow-Shaffer E, Costero A, Clark GG, Edman JD, Scott TW. Identification of the people from whom engorged Aedes aegypti took blood meals in Florida, Puerto Rico, using polymerase chain reaction-based DNA profiling. Am J Trop Med Hyg. 2003;68:437– 446. [PubMed] [Google Scholar]
- Detinova TS. Age-grouping methods in Diptera of medical importance. Monogr Ser World Health Organ No. 47. 1962 [PubMed] [Google Scholar]
- Dye C, Hasibeder G. Population dynamics of mosquito-borne disease: effects of flies which bite some people more frequently than others. Trans R Soc Trop Med Hyg. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- Erwin PC, Jones TF, Gerhardt RR, Halford SK, Smith AB, Patterson LER, Gottfried KL, Burkhalter KL, Naci RS, Schaffner W. La Crosse encephalitis in eastern Tennessee: clinical, environmental, and entomological characteristics from a blinded cohort study. Am J Epidemiol. 2002;155:1060–1065. doi: 10.1093/aje/155.11.1060. [DOI] [PubMed] [Google Scholar]
- Franco-Estrada JG, Craig GB., Jr Biology, disease relationships, and control of Aedes albopictus. Pan Am Health Organ Tech Paper No. 42 1995 [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807– 811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AC, Silva NN, Marques GRAM, Brito M. Host-feeding patterns of potential human disease vectors in the Paraiba Valley region, state of Sao Paulo, Brazil. J Vector Ecol. 2003;28:74–78. [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Gunstream SE, Chew RM, Hagstrum DW, Tempelis CH. Feeding patterns of six species of mosquitoes in arid southeastern California. Mosq News. 1971;31:99–101. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4(Suppl 1):1– 40. [PubMed] [Google Scholar]
- Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J Med Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- Jones TF, Craig AS, Nasci RS, Patterson LER, Erwin PC, Gerhardt RR, Ussery XT, Schaffner W. Newly Recognized Focus of La Crosse Encephalitis in Tennessee. Clin Infect Dis. 1999;28:93–97. doi: 10.1086/515087. [DOI] [PubMed] [Google Scholar]
- Kay BH, Boreham P, Edman JD. Application of the “feeding index” concept to studies of mosquito host-feeding patterns. Mosq News. 1979;39:68–72. [Google Scholar]
- Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Unnasch TR. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg. 2002;66:599– 604. doi: 10.4269/ajtmh.2002.66.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CG. Aedes albopictus in the United States: current status and prospects for further spread. J Am Mosq Control Assoc. 1999;15:221–227. [PubMed] [Google Scholar]
- Nasci RS. A lightweight battery-powered aspirator for collecting resting mosquitoes in the field. Mosq News. 1981;41:818–811. [Google Scholar]
- Nasci RS. Local variation in blood feeding by Aedes triseriatus and Aedes hendersoni (Diptera: Culicidae) J Med Entomol. 1985;22:619– 623. doi: 10.1093/jmedent/22.6.619. [DOI] [PubMed] [Google Scholar]
- Nasci RS, Moore CG, Biggerstaff BJ, Panella NA, Liu HQ, Karabatsos N, Davis BS, Brannon ES. La Crosse encephalitis virus habitat associations in Nicholas County, West Virginia. J Med Entomol. 2000;37:559–570. doi: 10.1603/0022-2585-37.4.559. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Savage HM, Nasci RS, Craig GB., Jr Blood hosts of Aedes albopictus in the United States. J Am Mosq Control Assoc. 1994;10:447– 450. [PubMed] [Google Scholar]
- Promega. Technical manual D004. Promega; Madison, WI: 2001. GenePrint® STR Systems (Silver Stain Detection) [Google Scholar]
- SAS Institute. SAS/STAT user’s guide for personal computers, version 8.0. SAS Institute; Cary, NC: 2000. [Google Scholar]
- Savage HM, Niebylski ML, Smith GC, Mitchell CJ, Craig GB., Jr Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J Med Entomol. 1993;30:27–34. doi: 10.1093/jmedent/30.1.27. [DOI] [PubMed] [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Ae. aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Slaff M, Apperson CS. Agric Ext Ser. North Carolina State Univ; Raleigh. Publ. No. AG-412: 1989. A key to the mosquitoes of North Carolina and the Mid-Atlantic states. [Google Scholar]
- Sullivan MF, Gould DJ, Maneechai S. Observations on the host range and feeding preference of Aedes albopictus (Skuse) J Med Entomol. 1971;8:713–716. doi: 10.1093/jmedent/8.6.713. [DOI] [PubMed] [Google Scholar]
- Szumlas DE, Apperson CS, Hartig PG, Francy DB, Karabatsos N. Seroepidemiology of La Crosse virus infection in humans in western North Carolina. Am J Trop Med Hyg. 1996a;54:332–337. doi: 10.4269/ajtmh.1996.54.332. [DOI] [PubMed] [Google Scholar]
- Szumlas D, Apperson CS, Powell EE, Hartig P, Francy DB, Karabotsos N. Relative abundance and species composition of mosquito populations (Diptera: Culicidae) in a La Crosse virus-endemic area in western North Carolina. J Med Entomol. 1996b;33:598– 607. doi: 10.1093/jmedent/33.4.598. [DOI] [PubMed] [Google Scholar]
- Tempelis CH. Host-feeding patterns of mosquitoes, with a review of advances of blood meals by serology. J Med Entomol. 1975;11:635– 653. doi: 10.1093/jmedent/11.6.635. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Hayes R, Hess A, Reeves WC. Blood-feeding habits of four species of mosquito found in Hawaii. Am Soc Trop Med Hyg. 1970;19:335–341. doi: 10.4269/ajtmh.1970.19.335. [DOI] [PubMed] [Google Scholar]
- Turell MJ, LeDuc JW. In: The role of mosquitoes in the natural history of California serogroup viruses. Calisher CH, Thompson WH, editors. California serogroup viruses; Liss, New York: 1983. pp. 43–55. [PubMed] [Google Scholar]
- Turell MJ, O’Guinn M, Dohm D, Jones J. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Wetton JH, Carter RE, Parkin DT, Walters D. Demographic study of a wild house sparrow population by DNA fingerprinting. Nature (Lond) 1987;327:147– 149. doi: 10.1038/327147a0. [DOI] [PubMed] [Google Scholar]
- Yuill TM. In: The role of mammals in the maintenance and dissemination of La Crosse virus. Calisher CH, Thompson WH, editors. California sero-group viruses; Liss, New York: 1983. [Received 22 November 2005; accepted 2 February 2006]. pp. 77–87. [PubMed] [Google Scholar]


