It is hard to overstate the medical importance and burden of vector-transmitted infectious diseases. Whether the metric used is mortality (malaria, for example, kills 1 million to 2 million people annually, most of them children under 5 years of age), morbidity (more than 70 million disability-adjusted life-years of healthy living are lost to malaria, Chagas’ disease, leishmaniasis, dengue fever, lymphatic filariasis, and the encephalitis viruses), or something as difficult to quantify as anxiety in a population (activities in outdoor playgrounds and high schools, for example, were moved or suspended along the south shore of Massachusetts this past fall because of concern raised by three cases of eastern equine encephalitis), the burden of these infections is enormous.
The key elements involved in human vectorborne infectious diseases are the infectious microorganism (virus, bacterium, or parasite), the vector (mosquito, tick, or fly), and the reservoir from which the vector obtains the infection (see Figure 1). Control strategies for these diseases should be informed by an understanding of the complex dynamics of vector–host interactions and the ways in which the environments of both the vector and host intersect to produce human disease. Models, including the Ross–Macdonald model (see Figure 1),1 have been developed to permit prediction of the effects of different approaches. For example, when the reservoir is accessible, the elements of the model involving the reservoir host can potentially be manipulated in a way that has a substantial influence on organism transmission and therefore disease burden. In contrast, when the reservoir cannot be influenced, approaches to mediating the transmission of vectorborne diseases to humans are almost exclusively dependent on affecting the relative abundance and life expectancy of the insect vector.
Traditional approaches to the control of such infections have targeted two broad strategies: vaccination or chemical prophylaxis for at-risk humans, or reduction and avoidance of vectors. Vaccination has had some success for a number of vectorborne infections, including yellow fever and Japanese encephalitis. However, despite years of research and investment, vaccines for important vectorborne infections such as malaria and dengue fever remain elusive. The use of antimicrobial prophylaxis for malaria is effective for travelers to areas where the disease is endemic but has not proven practical for residents of such areas and has led to the development of resistance to the antimicrobial agents.
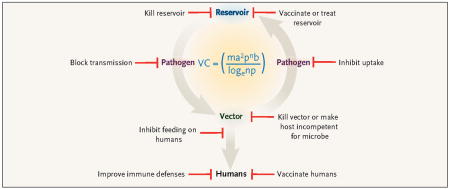
In this issue of the journal, Sundar and colleagues (pages 2571–2581) report on a control strategy they tested for sandfly-transmitted visceral leishmaniasis in Bihar, India, that extends the concept of antimicrobial administration by taking advantage of the local vector–host dynamics to target eradication of the reservoir (see Figure 2B). Since, on the Indian subcontinent, the reservoir for Leishmania donovani is limited solely to humans (unlike the zoonotic reservoirs in South America), these investigators reasoned that mass outpatient treatment of the human reservoir population with an affordable, effective, intramuscularly administered amino-glycoside (paromomycin), coupled with a program to reduce the sandfly vector population, could be an effective control strategy for this potentially lethal disease. Their results, indicating a 94.6% cure rate that was similar to that of intravenously administered amphotericin B, has prompted the adoption by the Indian government of widespread paromomycin treatment as part of a public health program to eliminate visceral leishmaniasis from the region.
Reservoir reduction may also be a viable strategy for the control of infections for which the primary reservoir is not human. Tsao et al.2 have shown that vaccination of wild mice, a major reservoir of Borrelia burgdorferi (the causative agent of Lyme disease), with injected recombinant outer-surface protein A can significantly reduce carriage of the organism by ticks in the subsequent year (see Figure 2C). Several research groups are now developing mechanisms for delivering vaccines to animal reservoirs, using oral baits. Oral baiting for wild-life vaccination has proven very effective in combating the non-vectorborne disease rabies. Other vectorborne infections for which wildlife vaccination strategies are being tested include Yersinia pestis (plague) and hantavirus.
Although reservoir-reduction strategies are becoming more prominent, most previous infection-control strategies have focused on the vector. Vector-targeted strategies are particularly attractive, since the vectorial capacity to transmit infectious diseases to humans is related to vector density and, in an exponential way, to vector survival. Perhaps the best-known example of a successful vector-reduction strategy is the U.S. campaign to eradicate yellow fever and malaria during the construction of the Panama Canal. In that case, a comprehensive plan that included drainage of standing pools of water, cutting of grass and brush, oiling of ponds and swamps to kill larvae, and capture of indoor mosquitoes resulted in the eradication of yellow fever and a substantial reduction in cases of malaria. In the 1930s and 1940s, similar efforts toward mosquito control in the southeastern United States, as part of a program of the Tennessee Valley Authority, led to the near-eradication of endemic malaria in the United States. Insecticidal spraying with dichlorodiphenyltrichloroethane (DDT) was begun in this country in the late 1940s as part of the National Malaria Eradication Program and helped to eliminate the few remaining cases of malaria in the United States. However, although initially hailed as a panacea, spraying with DDT has not been effective at eradicating malaria worldwide. Well-publicized problems with environmental toxicity, the possibility of human carcinogenesis, and the development of resistance among insects have led to the withdrawal of DDT from widespread use.
Exciting new strategies are targeting novel components of the vector–pathogen interaction (see Figure 2D). For example, a small peptide molecule (SM1) has been shown to bind to mosquito salivary and midgut cells and to impair plasmodium development and subsequent transmission from this insect vector. Whereas plasmodium has usually been thought of as causing disease in humans, it has become apparent that mosquitoes themselves have reason to avoid becoming infected with this parasite, since it decreases fertility. In mixed caged populations of mosquitoes, those expressing the plasmodium-resistant SM1 gradually replace wild-type, disease-transmitting mosquitoes,3 raising the possibility that a genetically altered, malaria-resistant mosquito could be introduced to reduce transmission.
Targeting of the vector–human interaction with vaccines that protect against vector feeding is another new approach. Vaccination with a midgut protein of the boophilus tick, Bm86 antigen, has been shown to be effective in preventing these ticks from feeding on cattle4 and has been approved for commercial use. Vaccination may also be able to prevent the transmission of pathogens either by decreasing the feeding time or by recruiting a vigorous immune defense to the site of the tick bite. The BM86 vaccine has reduced the incidence of babesiosis in vaccinated cattle, and vaccination with a different tick salivary protein, 64TRP, has been shown to prevent the transmission of tickborne encephalitis as effectively as a pathogen-targeted vaccine. Similarly, vaccination with a sandfly salivary protein can prevent the transmission of leishmania.5 Vaccination against insect and arthropod vectors may be used alone as a strategy for protecting humans or combined with attempts at reservoir eradication.
As we enter the postgenomic era for many of the pathogens, vectors, and reservoirs of human vectorborne diseases, we are gaining a new understanding of genome–genome intersections that are critical to the maintenance of infectious cycles. The availability of new molecular tools such as small interfering RNA (siRNA) and microarrays are allowing scientists to rapidly identify and test promising new candidates for disease-interruption strategies. These strategies offer great hope that targeting specific interactions between a pathogen and either its vector or its host may lead to new approaches that can reduce human disease with minimal disturbance of the delicate ecosystems in which they persist.
Contributor Information
Mark S. Klempner, Dr. Klempner is a professor of medicine and microbiology and associate provost for research at Boston University School of Medicine, Boston, and an associate editor of the journal
Thomas R. Unnasch, Dr. Unnasch is a professor of medicine at the Department of Global Health, College of Public Health, University of South Florida, Tampa, FL
Linden Hu, Dr. Hu is an associate professor of medicine at Tufts University School of Medicine, Boston
References
- 1.Garret-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito’s vectorial capacity. Nature. 1964;204:1173–5. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- 2.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci USA. 2004;101:18159–64. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci USA. 2007;104:5580–3. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labuda M, Trimnell AR, Lickova M, Kazimirova M, Davies GM, Lissina O, Hails RS, Nuttall PA. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006;2(4):e27. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;193:331–42. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]


