Abstract
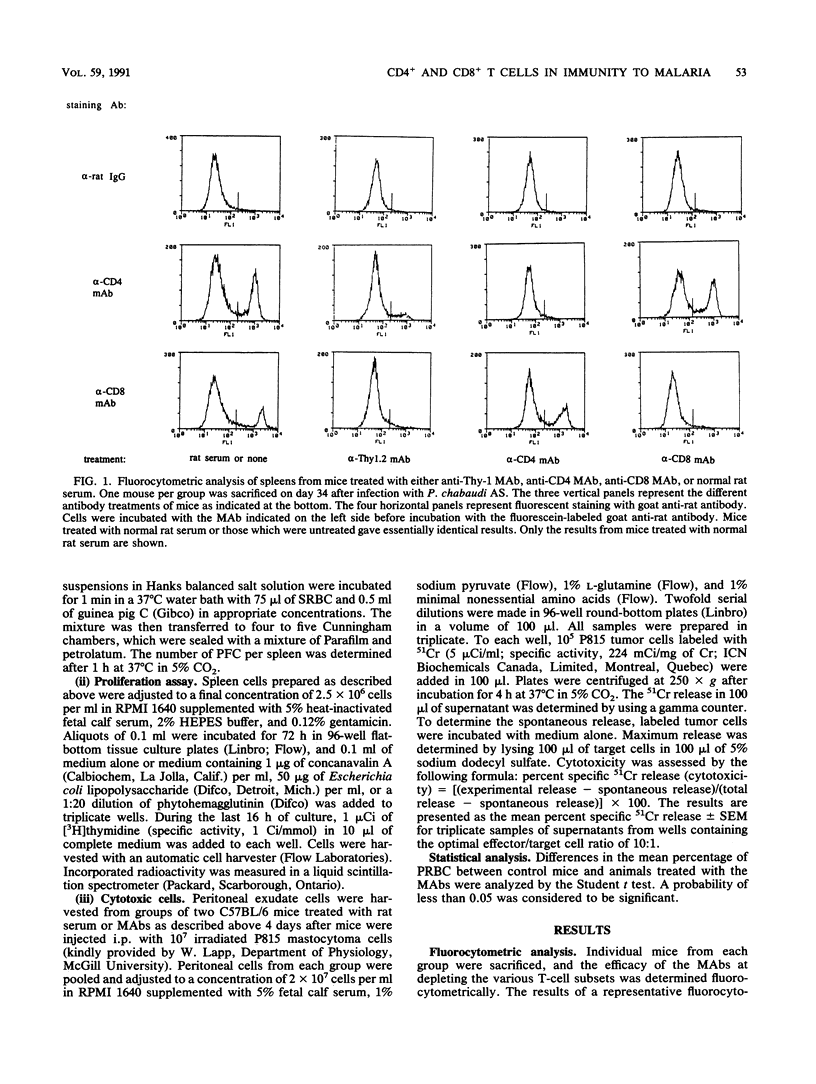
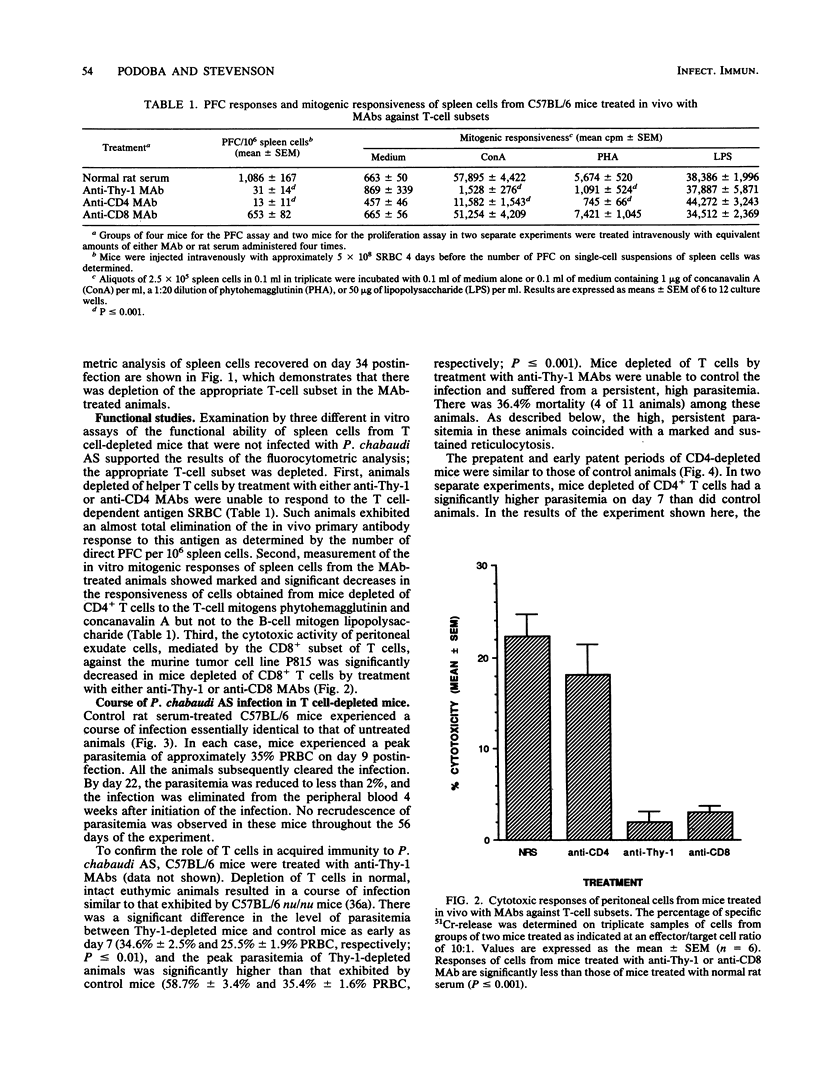
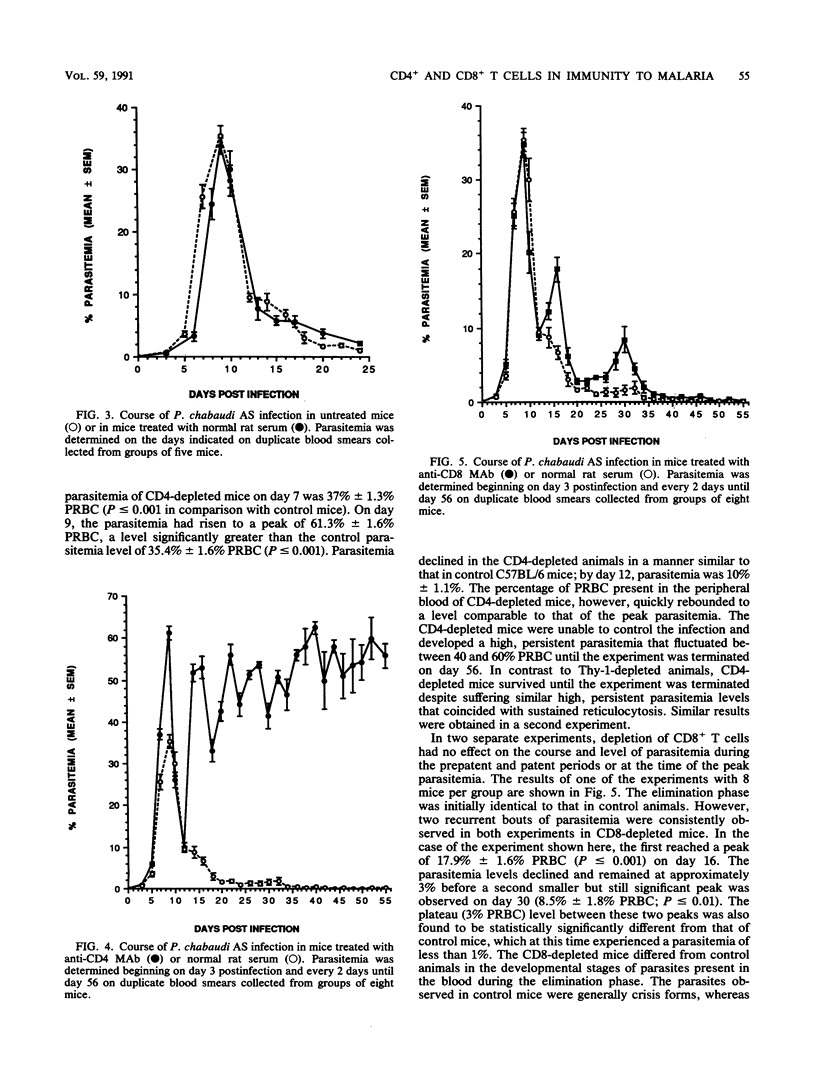
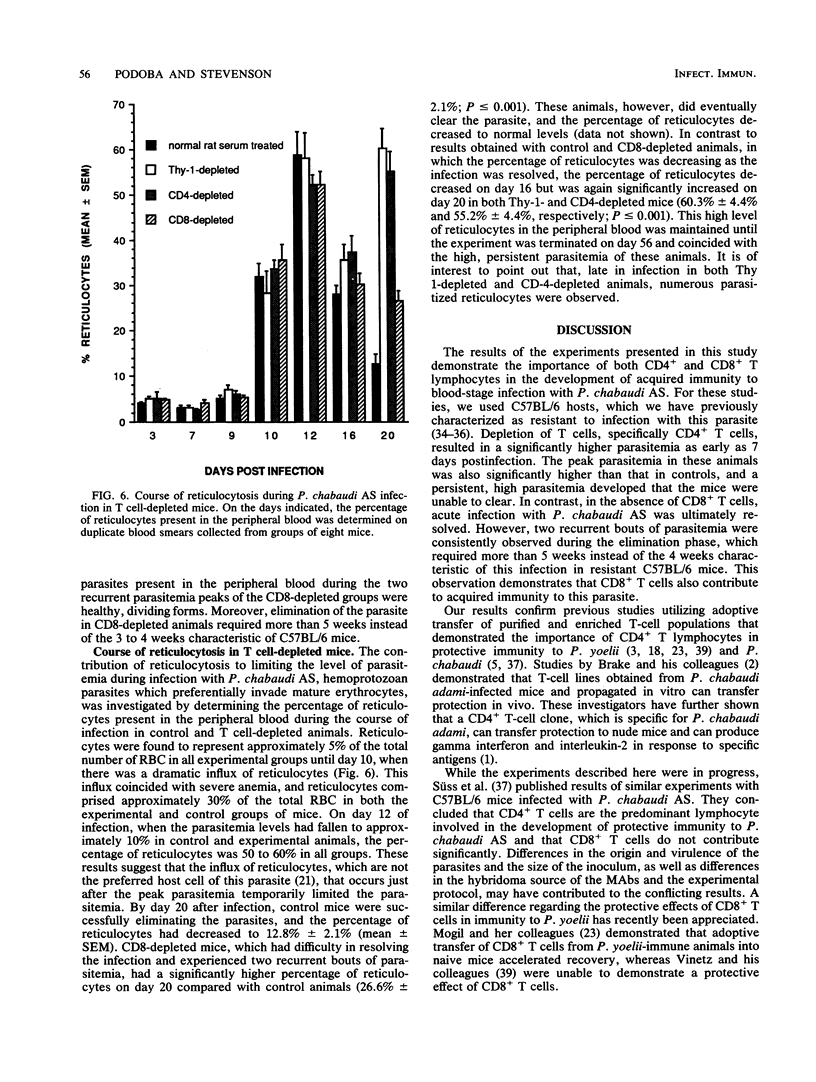
In the present study, the contribution of CD4+ and CD8+ T lymphocytes to acquired immunity to blood-stage infection with the murine malaria species Plasmodium chabaudi AS was investigated. C57BL/6 mice, which are genetically resistant to infection with this hemoprotozoan parasite and exhibit a transient course of infection, were treated intraperitoneally with monoclonal antibodies to T-cell epitopes, either anti-Thy-1, anti-CD4, or anti-CD8. After intraperitoneal infection with 10(6) parasitized erythrocytes, control C57BL/6 mice exhibited a peak parasitemia on day 9 of approximately 35% parasitized erythrocytes and eliminated the infection within 4 weeks. Mice depleted of Thy-1+ or CD4+ T cells had significantly higher parasitemias on day 7 as well as significantly higher peak parasitemias. These mice were unable to control the infection and developed a persistent, high parasitemia that fluctuated between 40 and 60% until the experiment was terminated on day 56 postinfection. Depletion of CD8+ T lymphocytes was found to have no effect on the early course of parasitemia or on the level of peak parasitemia. However, mice depleted of CD8+ T cells experienced two recurrent bouts of parasitemia during the later stage of the infection and required more than 5 weeks to eliminate the parasites. After the peak parasitemia, which occurred in control and experimental animals on day 9, there was a sharp drop in parasitemia coinciding with a wave of reticulocytosis. Therefore, the contribution of the influx of reticulocytes, which are not the preferred host cell of this hemoprotozoan parasite, to limiting the parasitemia was also examined by determining the course of reticulocytosis during infection in control and T cell-depleted animals. Early in infection, there was a marked and comparable reticulocytosis in the peripheral blood of control and T cell-depleted mice; the reticulocytosis peaked on day 12 and coincided with the dramatic and sudden reduction in parasitemia occurring in all groups. In both control and CD8-depleted mice the percentage of reticulocytes decreased as the infection was resolved, whereas in CD4-depleted mice marked reticulocytosis correlated with high, persistent parasitemia. These results thus demonstrate that both CD4+ and CD8+ T cells are involved in acquired immunity to blood-stage P. chabaudi AS and that the influx of reticulocytes into the blood that occurs just after the peak parasitemia may contribute temporarily to limiting the parasitemia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M. T-cell-mediated immune response in murine malaria: differential effects of antigen-specific Lyt T-cell subsets in recovery from Plasmodium yoelii infection in normal and T-cell-deficient mice. Infect Immun. 1985 Mar;47(3):737–743. doi: 10.1128/iai.47.3.737-743.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Hills L. A. T cells and protective immunity to Plasmodium berghei in rats. Infect Immun. 1976 Oct;14(4):858–871. doi: 10.1128/iai.14.4.858-871.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Long C. A., Weidanz W. P. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986 Jun;52(3):637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Parke L. A., Weidanz W. P. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990 Sep;58(9):2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Coleman R. M., Rencricca N. J., Stout J. P., Brissette W. H., Smith D. M. Splenic mediated erythrocyte cytotoxicity in malaria. Immunology. 1975 Jul;29(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Gravely S. M., Kreier J. P. Adoptive transfer of immunity to Plasmodium berghei with immune T and B lymphocytes. Infect Immun. 1976 Jul;14(1):184–190. doi: 10.1128/iai.14.1.184-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Long C. A., Weidanz W. P. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect Immun. 1985 Jun;48(3):853–858. doi: 10.1128/iai.48.3.853-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Harris R., Zervas J. D. Reticulocyte HL-A antigens. Nature. 1969 Mar 15;221(5185):1062–1063. doi: 10.1038/2211062a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Janeway C. A., Jr, Kemp J. D. Experimental malaria in the CBA/N mouse. J Immunol. 1979 Dec;123(6):2532–2539. [PubMed] [Google Scholar]
- Jayawardena A. N., Mogil R., Murphy D. B., Burger D., Gershon R. K. Enhanced expression of H-2K and H-2D antigens on reticulocytes infected with Plasmodium yoelii. Nature. 1983 Apr 14;302(5909):623–626. doi: 10.1038/302623a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Murphy D. B., Janeway C. A., Gershon R. K. T cell-mediated immunity in malaria. I. The Ly phenotype of T cells mediating resistance to Plasmodium yoelii. J Immunol. 1982 Jul;129(1):377–381. [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kourilsky F. M., Silvestre D., Levy J. P., Dausset J., Nicolai M. G., Senik A. Immunoferritin study of the distribution of HL-A antigens on human blood cells. J Immunol. 1971 Feb;106(2):454–466. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Mogil R. J., Patton C. L., Green D. R. Cellular subsets involved in cell-mediated immunity to murine Plasmodium yoelii 17X malaria. J Immunol. 1987 Mar 15;138(6):1933–1939. [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Orago A. S., Solomon J. B. Antibody-dependent and -independent cytotoxic activity of spleen cells for Plasmodium berghei from susceptible and resistant rats. Immunology. 1986 Oct;59(2):283–288. [PMC free article] [PubMed] [Google Scholar]
- Oster C. N., Koontz L. C., Wyler D. J. Malaria in asplenic mice: effects of splenectomy, congenital asplenia, and splenic reconstitution on the course of infection. Am J Trop Med Hyg. 1980 Nov;29(6):1138–1142. doi: 10.4269/ajtmh.1980.29.1138. [DOI] [PubMed] [Google Scholar]
- Ott K. J. Influence of reticulocytosis on the course of infection of Plasmodium chabaudi and P. berghei. J Protozool. 1968 May;15(2):365–369. doi: 10.1111/j.1550-7408.1968.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Matile H., Pink J. R. Plasmodium falciparum-specific human T cell clones: evidence for helper and cytotoxic activities. Eur J Immunol. 1987 Feb;17(2):187–192. doi: 10.1002/eji.1830170206. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Richard J., Pink L. Human T lymphocyte clones specific for malaria (Plasmodium falciparum) antigens. EMBO J. 1985 Dec 30;4(13B):3819–3822. doi: 10.1002/j.1460-2075.1985.tb04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Ghadirian E., Phillips N. C., Rae D., Podoba J. E. Role of mononuclear phagocytes in elimination of Plasmodium chabaudi AS infection. Parasite Immunol. 1989 Sep;11(5):529–544. doi: 10.1111/j.1365-3024.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Nesbitt M. N., Skamene E. Chromosomal location of the gene determining resistance to Plasmodium chabaudi AS. Curr Top Microbiol Immunol. 1988;137:325–328. doi: 10.1007/978-3-642-50059-6_48. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Skamene E. Murine malaria: resistance of AXB/BXA recombinant inbred mice to Plasmodium chabaudi. Infect Immun. 1985 Feb;47(2):452–456. doi: 10.1128/iai.47.2.452-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss G., Eichmann K., Kury E., Linke A., Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988 Dec;56(12):3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres B. A., Farrar W. L., Johnson H. M. Interleukin 2 regulates immune interferon (IFN gamma) production by normal and suppressor cell cultures. J Immunol. 1982 May;128(5):2217–2219. [PubMed] [Google Scholar]
- Vinetz J. M., Kumar S., Good M. F., Fowlkes B. J., Berzofsky J. A., Miller L. H. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. J Immunol. 1990 Feb 1;144(3):1069–1074. [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]



