Abstract
We previously showed that activation of Gi/o-coupled histamine H3-receptors (H3R) is cardioprotective since it attenuates excessive norepinephrine release from cardiac sympathetic nerves. This action is characterized by a marked decrease in intraneuronal Ca2+ ([Ca2+]i), as Gαi impairs the adenylyl cyclase-cAMP-PKA pathway, and this decreases Ca2+ influx via voltage-operated Ca2+ channels (VOCC). Yet, the Gi/o-derived βγ dimer could directly inhibit VOCC, and the subsequent reduction in Ca2+ influx would be responsible for the H3R-mediated attenuation of transmitter exocytosis. Here, we tested this hypothesis in nerve-growth factor-differentiated rat pheochromocytoma cells (PC12) stably transfected with H3R (PC12-H3) and with the Gβγ scavenger β-ARK1-(495−689)-polypeptide (PC12-H3/β-ARK1). Thus, we evaluated the effects of H3R activation directly on: 1) Ca2+ current (ICa) using the whole-cell patch-clamp technique, and 2) K+-induced exocytosis of endogenous dopamine. H3R activation attenuated both peak ICa and dopamine exocytosis in PC12-H3, but not in PC12-H3/β-ARK1 cells. Moreover, a membrane permeable phosducin-like Gβγ scavenger also prevented the anti-exocytotic effect of H3R activation. In contrast, the H3R-induced attenuation of cAMP accumulation and dopamine exocytosis in response to forskolin were the same in both PC12-H3 and PC12-H3/β-ARK1 cells. Our findings reveal that while Gαi participates in the H3-mediated anti-exocytotic effect when the adenylyl cyclase-cAMP-PKA pathway is stimulated, a direct Gβγ-induced inhibition of VOCC, resulting in an attenuation of ICa plays a pivotal role in the H3R-mediated decrease in [Ca2+]i and associated cardioprotective anti-exocytotic effects. The discovery of this H3R signaling step may offer new therapeutic approaches to cardiovascular diseases characterized by hyperadrenergic activity.
Introduction
Sympathetic nerve endings in the mouse (Koyama et al., 2003), guinea pig (Endou et al., 1994) and human hearts (Imamura et al., 1995) express histamine H3-receptors (H3R). H3R activation results in attenuation of excessive norepinephrine release in myocardial ischemia, a recognized cardioprotective effect, since it alleviates arrhythmic cardiac dysfunction (Levi and Smith, 2000).
We previously reported that imetit, a selective H3R agonist (Garbarg et al., 1992), reduces norepinephrine exocytosis evoked by depolarization of cardiac sympathetic nerve endings (Imamura et al., 1994; Imamura et al., 1995), an action associated with a marked decrease in intraneuronal Ca2+ ([Ca2+]i) (Silver et al., 2002). It is conceivable that H3R activation may decrease ([Ca2+]i) by inhibiting Ca2+ influx through voltage-operated Ca2+ channels (VOCC) in sympathetic nerve terminals. H3R-mediated inhibition of N-type Ca2+ channel current has been claimed to occur in histaminergic neurons from the rat hypothalamus; an unverified claim, however, since selective H3R antagonists were not shown to block this effect (Takeshita et al., 1998).
Although N-type Ca2+ channels are the dominant Ca2+ entry pathway triggering sympathetic transmitter release (Lipscombe et al., 1989; Zhu and Yakel, 1997), it is possible that entry of Ca2+ through L-type Ca2+ channels may also be important in norepinephrine exocytosis and be inhibited by H3R activation. Indeed, we found that H3R activation synergizes with both N- and L-type Ca2+ channel blockers to reduce K+-induced norepinephrine release from cardiac synaptosomes (Seyedi et al., 2005).
H3R-mediated attenuation of norepinephrine exocytosis from cardiac sympathetic nerves involves an H3R-Gi/o coupling, adenylyl cyclase inhibition by Gαi, decreased cAMP formation and diminished PKA activity (Seyedi et al., 2005). Inasmuch as a decrease in PKA activity is likely to decrease phosphorylation of VOCC, which would lead to a decrease in voltage-activated calcium current (ICa), it is plausible that the H3R-mediated decrease in norepinephrine exocytosis results from a decreased Ca2+ influx via VOCC due to diminished activity of the adenylyl cyclase-cAMP-PKA pathway.
On the other hand, Gβγ is known to decrease adenylyl cyclase activity (Taussig et al., 1993) and it is therefore possible that in addition to the attenuation of adenylyl cyclase by Gαi (Seyedi et al., 2005), Gβγ will also play a role in the H3R-mediated decrease in cAMP. Yet, since the Gβγ dimer is known to directly inhibit VOCC (Ikeda, 1996; Herlitze et al., 1996), we questioned whether the H3R-mediated attenuation of norepinephrine exocytosis may also result from an inhibition of Ca2+ influx by a Gβγ action.
Accordingly, the purpose of our investigation was to determine the role of a Gβγ-induced inhibition of VOCC in the H3R-mediated attenuation of norepinephrine exocytosis. To accomplish this, we evaluated the effects of H3R activation on ICa and endogenous transmitter exocytosis in nerve-growth factor (NGF)-differentiated rat pheochromocytoma cells (PC12) stably transfected with H3R and the Gβγ scavenger β-ARK1-(495−689)-polypeptide (Koch et al., 1994; Dickenson and Hill, 1998). NGF-differentiated PC12 cells were chosen as a functional model of sympathetic neurotransmission because their phenotype closely resembles that of sympathetic neurons (Dichter et al., 1977; Taupenot, 2007). Our results demonstrate a pivotal involvement of the Gβγ subunit in H3R-mediated attenuation of neuronal ICa and consequent neurotransmitter exocytosis.
Methods
Cell Culture
The rat pheochromocytoma PC12 cell line was maintained in Dulbecco's Modified Eagle's Medium (DMEM) plus 10% fetal bovine serum (FBS), 5% donor horse serum (DHS), 1% L-glutamine, and antibiotics at 37°C in 5% CO2. The differentiating protocol involved plating PC12 cells on tissue culture plates coated with collagen (rat tail type-VII, Sigma) combined with exposure to low serum medium containing 1% FBS, 0.5% DHS, 1% L-glutamine, and antibiotics supplemented with 2.5S-NGF (Harlan Bioscience, Indianapolis, IN, USA). Culture medium and NGF were replenished every 2 days. PC12 cells were transfected with the human H3R (donated by Dr. T. W. Lovenberg) and the β-ARK1 (495−689) minigene (obtained from Dr. R.J. Lefkowitz) using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. PC12-H3 and PC12-H3/β-ARK1 cell lines were selected and maintained in selection media containing 500 μg/ml G418 sulfate (Mediatech, Herndon, VA) and/or zeocin (Invitrogen) respectively.
Reverse-Transcriptase PCR
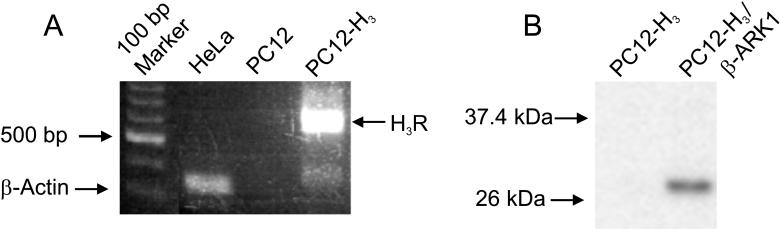
Total RNA was isolated from PC12 and PC12-H3 cells using the TRIzol RNA purification kit (Invitrogen). cDNAs were synthesized from total RNA using Superscript II reverse transcriptase (Invitrogen) and random primers as described by the manufacturer. PCR was used to detect the H3 mRNA expression with cDNA from either PC12 or PC12-H3 as templates, and with P1: 5'-CTCTGCAAGCTGTGGCTGGTGGTAGACTACCTACTGTGTG-3' and P2: 5'-CTTCTTGTCCCGCGACAGCCGAAAGCGCTGGGTGATGCTT-3' as primers (Invitrogen). PCRs were performed under conditions of 94°C, 40 s; 65°C, 40 s; 72°C, 2 min for 40 cycles. As control, total HeLa RNA was used as the template for amplification of a 353-bp segment of β-actin mRNA in a parallel PCR reaction. The PCR products were run on a 1.5% agarose gel, stained with ethidium bromide and visualized under UV light.
Western blotting
Cell lysates (20 μg) prepared from PC12-H3 and PC12-H3/β-ARK1 cells were separated by SDS/PAGE 10−20% acrylamide gel and transferred to a PVDF membrane (Millipore, Billerica, MA). Following transfer, the membrane was washed with Tris-buffered saline (TBS) and blocked for 2 h at room temperature in blocking buffer (TBS containing 0.1% Tween 20, 5% (w/v) non-fat dry milk). Blots were then incubated overnight at 4°C with anti-β-ARK1 primary antibody (Epitomics, Burlingame, CA) at 1:500 dilution in 5% (w/v) bovine serum albumin (BSA) dissolved in TBS-Tween 20 (0.1%). The primary antibody was removed and the blot extensively washed with TBS/Tween 20. Blots were then incubated for 2 h at room temperature with horseradish peroxidase-coupled anti-rabbit IgG (Cell Signaling Technology, Beverly, MA) at a 1:3000 dilution in blocking buffer. Following removal of the secondary antibody, blots were extensively washed as above and developed using the Enhanced Chemiluminescence detection system (Pierce, Rockford, IL) followed by exposure to X-ray film (Biomax MR; Eastman Kodak, Rochester, NY).
cAMP measurement
Cellular cAMP accumulation was measured in PC12-H3 and PC12-H3/β-ARK1 cells seeded in 96-well plates and differentiated with NGF (100 ng/mL) for 5−7 days. After a 20-min treatment with the cAMP phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) (2 mM), cells were incubated for 5 min with or without the H3R agonist imetit (100 nM), either alone or in combination with the H3R antagonist CBP (50 nM). Cells were incubated with CBP for 5 min before the addition of imetit. Intracellular cAMP levels were then enhanced with forskolin (10 μM) for 20 min. The incubation buffer was immediately aspirated, cells were lysed and intracellular cAMP levels determined using a cAMP Biotrak EIA kit (Amersham Biosciences Inc., Piscataway, NJ) following the manufacturer's protocol. All drugs were constituted in HEPES-buffered Na+ Ringer's solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES, 2 mM CaCl2, 1 mM MgCl2, pH 7.4).
Dopamine assay
PC12-H3 and PC12-H3/β-ARK1 cells cultured in 12-well plates and differentiated with NGF (100 ng/ml) for 5−7 days were incubated with drugs for 5 min. When CBP or the anti-βγ peptide was used, cells were incubated with these compounds for 5 min before incubation with imetit. For PTX treatment, cells were incubated for 24 h with PTX (200 ng/ml) prior to assay. Dopamine exocytosis was elicited by incubating samples for 5 min with K+ 100 mM (osmolarity was maintained constant by adjusting the NaCl concentration), phorbol 12-myristate 13-acetate (PMA, 300 nM) or for 20 min with forskolin (10 μM). At the end of the incubation period, aliquots of the supernatant and cell lysates (after a 30-min treatment with 0.3% Triton X-100) were taken from each well and analyzed for dopamine content by high-performance liquid chromatography with electrochemical detection as previously described (Seyedi et al., 2005) with a 6.0-min retention time.
Electrophysiology
Whole-cell voltage-clamp studies were performed on PC12 cells. The cells were transferred to 22 × 22 mm glass coverslips coated with poly-L-lysine and collagen and differentiated with NGF (50 ng/ml) for 3−7 days before use in voltage-clamp experiments. Bath solution was (in mM): 20 BaCl2, 125 N-methyl-D-glucamine, 10 HEPES (pH 7.5). Pipettes had resistances of 6−9 MΩ when filled with intracellular solution containing (in mM): 100 N-methyl-D-glucamine, 20 TEACl, 10 EGTA, 2 MgCl2, 10 glucose, 2 Na2ATP, 10 HEPES (pH 7.35). Fragments of coverslip were transferred to a recording chamber and the medium rinsed off with bath solution. Following adoption of whole-cell configuration, currents were recorded either in the absence (baseline) or presence of drugs. Cells were held at −40 mV and subjected to 200 ms test pulses from −40 mV to +40 mV in 10 mV increments every 30 s. Recordings were performed at 22°C to 25°C using an IX50 inverted microscope (Olympus, Tokyo, Japan), a Multiclamp 700A Amplifier, a Digidata 1300 Analog/Digital converter and pClamp9 software (Axon Instruments, Foster City, CA). Recordings were performed at 10 kHz sampling frequency, with no filtering prior to analysis, and were subsequently low-pass Bessel filtered for presentation purposes. All recordings with leak currents >20 pA or an access resistance >25 MΩ were discarded.
Current-voltage relationships were obtained by measuring the peak current during depolarizing pulses. Data analysis was performed using Clampfit 9 (Axon Instruments). Current density was calculated by dividing the current (pA) recorded from each individual cell by the capacitance (pF) of that cell. ICa activation was described by fitting a single exponential function to the current trace from the beginning of the pulse to the point where a steady-state level was reached so as to obtain the time constant of activation (τact).
Reagents
CBP, imetit, IBMX, NGF, ω-CTX, PMA and PTX were prepared in aqueous solution while DMSO was the vehicle for IBMX, forskolin and nifedipine. The anti-βγ peptide was obtained from Anaspec (San Jose, CA), imetit from Tocris (Ellisville, MO) and PMA from LC Laboratories (Woburn, MA). All other chemicals were reagent grade and purchased from Sigma Aldrich (St. Louis, MO).
Statistics
cAMP levels are expressed as mean absolute values ± SEM. Dopamine release values are expressed as mean percent increases above basal level ± SEM. Current density is expressed as mean ± SEM, with n specifying the number of independent experiments. Statistical significance was assessed by Student's t test or one-way ANOVA followed by post hoc testing (Dunnett's test) as indicated in the appropriate figure legend. Significance was asserted if p < 0.05.
Results
We first ascertained by RT-PCR the stable expression of human H3R in PC12 cells. A segment of ∼700 bp was amplified demonstrating the presence of H3R in PC12-H3 cells; this band was absent in non-transfected PC12 cells (Fig. 1A). The transfected H3R were Gi/o-coupled as demonstrated by the loss of their anti-exocytotic activity after pretreatment with PTX (100 ng/ml; Fig. 2C and 4). Stable expression of β-ARK1-(495−689)-polypeptide in PC12 cells was determined by Western blotting. A band of ∼27 kDa confirmed the expression of β-ARK1 in PC12-H3/β-ARK1 cells, but not in PC12-H3 cells (Fig. 1B).
Figure 1.
Detection of H3R and over-expression of β-ARK1(495−689) minigene in PC12-H3 cells. (A) Reverse-transcriptase PCR. cDNA was synthesized from total RNA prepared from PC12 and PC12-H3 cells and used as template in a PCR reaction. The PCR products were run on a 1.5 % agarose gel and detected under UV light. As controls, β-Actin primers were used in a parallel PCR reaction for amplification of a 353-bp segment. (B) Western blot analysis of β-ARK1(495−689) minigene. Cell lysates (20 μg) isolated from PC12-H3 and PC12-H3/β-ARK1 cells were resolved by SDS-PAGE and transferred to PVDF membrane. Blotting of the membrane with anti-β-ARK1 monoclonal antibody (1:500) revealed a ∼27 kDa specific band. Positions of standard molecular-mass markers of 26 and 37.4 kDa are shown on the left.
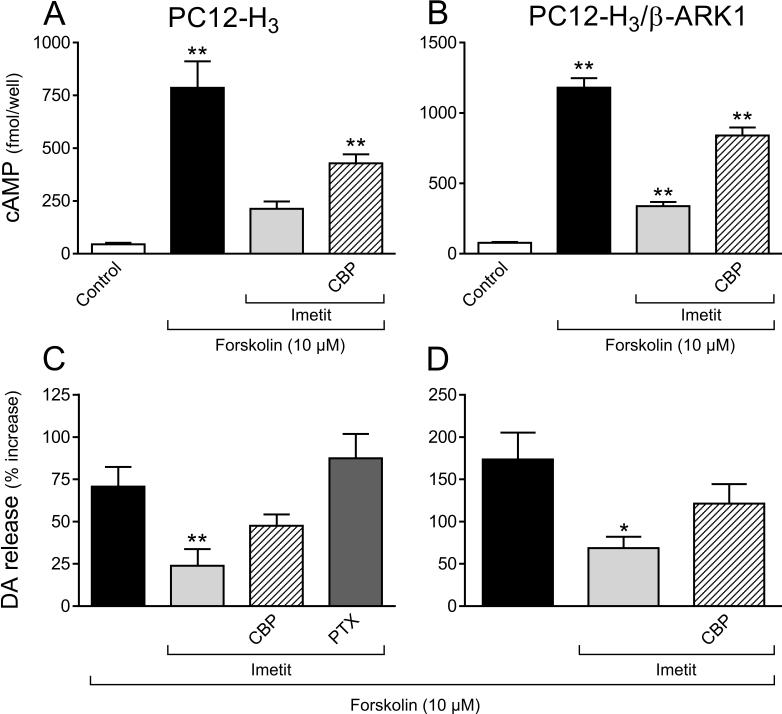
Figure 2.
H3R activation reduces intracellular cAMP accumulation and dopamine exocytosis elicited by forskolin (10 μM) in NGF-differentiated rat pheocromocytoma cells stably transfected with human H3R (PC12-H3) and in PC12 cells transfected with both H3R and the Gβγ scavenger β-ARK1 (PC12-H3/β-ARK1). Panels A and B: intracellular cAMP accumulation (absolute values); panels C and D: dopamine release, in the absence or presence of the H3R agonist imetit (100 nM), either alone or in combination with the H3R antagonist clobenpropit (CBP, 50 nM), or after pre-treatment with PTX (200 ng/ml for 24 h). Dopamine release is expressed in percent increase above basal level. Bars are means ± SEM (n = 15−19 for A and B and n = 10−15 for C and D). Significantly different from control or forskolin (*p < 0.05 and **p < 0.01 by ANOVA followed by post hoc Dunnett's test).
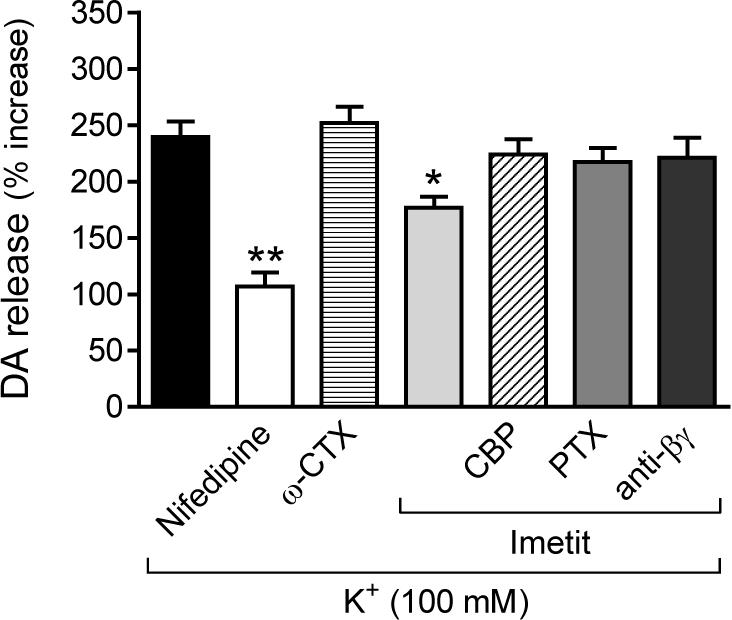
Figure 4.
H3R activation attenuates endogenous dopamine exocytosis elicited by depolarization with K+ (100 mM) in NGF-differentiated rat pheocromocytoma cells transfected with human H3R (PC12-H3). Dopamine exocytosis is inhibited by nifedipine (5 μM), an L-type calcium channel blocker, but not by ω-CTX (100 nM), an N-type calcium channel blocker. The anti-exocytotic effect of imetit (100 nM) is prevented by the H3R antagonist clobenpropit (CBP; 50 nM), a membrane-permeable phosducin-like anti-βγ peptide (1 μM), and by pre-treatment with PTX (200 ng/ml for 24 hr). Bars are means ± SEM of percent increases in dopamine release above control (n = 18−21). Significantly different from K+ alone (*p < 0.05 and **p < 0.01 by ANOVA followed by post hoc Dunnett's test).
We next verified the functionality of transfected H3R. Inasmuch as H3R are known to be negatively coupled to adenylyl cyclase via Gαi (Lovenberg et al., 1999), we determined that H3R activation did indeed reduce the formation of cAMP in PC12-H3 cells in response to forskolin. In the presence of the selective agonist imetit (100 nM) (Garbarg et al., 1992) a ∼20-fold forskolin-induced (10 μM) increase in intracellular cAMP concentration was attenuated by 70% (Fig. 2A). Clobenpropit (50 nM), a selective H3R antagonist (Van der Goot et al., 1992), reduced the effect of imetit by ∼60% (Fig. 2A). This confirmed that the Gi/o signaling cascade associated with transfected H3R was functional.
Inasmuch as some forms of adenylyl cyclase are inhibited by the Gβγ dimer (Tang and Gilman, 1991), we questioned whether the decrease in intracellular cAMP concentration in the presence of imetit was due to a Gβγ-induced diminution of cAMP-PKA activity. To evaluate this possibility, we determined cAMP accumulation and endogenous dopamine exocytosis (Chen and Westfall, 1994) in response to forskolin, in control and H3R-activated conditions, in PC12-H3 cells stably transfected with the Gβγ scavenger β-ARK1 polypeptide (Koch et al., 1994) and compared them to those measured in PC12-H3 cells. In PC12-H3/β-ARK1 cells forskolin (10 μM) elicited a ∼15-fold increase in intracellular cAMP concentration, which was comparable to that observed in PC12-H3 cells (compare panels A and B in Fig. 2). In the presence of imetit (100 nM) the increase in cAMP formation was attenuated by ∼70%; clobenpropit (50 nM) reduced the effect of imetit by ∼60% (Fig. 2B). Thus, H3R activation produced effects of similar magnitude in PC12-H3 and PC12-H3/β-ARK1 cells (compare panels A and B in Fig. 2), indicating that the Gβγ complex does not have inhibitory effects on the form of adenylyl cyclase present in PC12 cells. Furthermore, forskolin (10 μM) elicited a ∼2- and ∼3-fold increase in dopamine release in PC12-H3 and PC12-H3/β-ARK1 cells, respectively (Fig. 2C and D). H3R activation with imetit also significantly attenuated dopamine exocytosis (by ∼60%) in both PC12-H3 and PC12-H3/β-ARK1 cells; this effect was inhibited by H3R blockade with clobenpropit (Fig. 2C and D). Thus, in differentiated PC12 cells stimulated with forskolin, the H3R-mediated decrease in cAMP and associated anti-exocytotic effect derive mostly from an inhibition of adenylyl cyclase by Gαi and not by Gβγ.
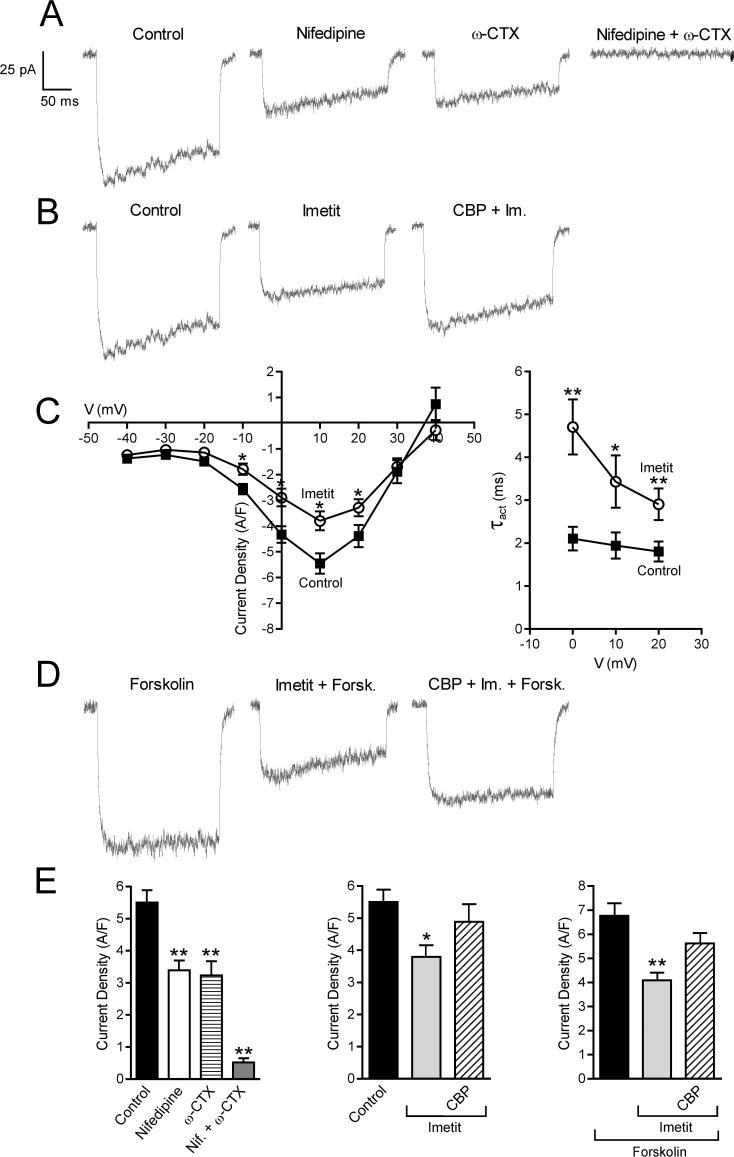
We next measured peak ICa in PC12-H3 cells using the conventional whole-cell patch-clamp technique (Fig. 3). The ICa activated at voltages equal or positive to −20 mV and reached a peak at +10 mV. Peak ICa was markedly reduced by incubation with ω-conotoxin GVIA (ω-CTX; 100 nM) or nifedipine (5 μM), indicating that both N- and L-type Ca2+ channels contribute to ICa in these cells (Fig. 3A and E). Indeed, combined treatment of the cells with nifedipine and ω-CTX blocked 90% of the ICa (Fig. 3A). When PC12-H3 cells were incubated with imetit (100 nM), peak ICa was significantly attenuated; this response was antagonized by clobenpropit (50 nM) (Fig. 3B, C and E). Stimulation of adenylyl cyclase with forskolin (10 μM) enhanced peak ICa by ∼25%; again, this current was markedly reduced by imetit, and clobenpropit antagonized the effect of imetit (Fig. 3D and E).
Figure 3.
Calcium current traces and peak current density recorded in NGF-differentiated PC12-H3 cells by stepping from the holding potential of −40 mV to the test potential of +10 mV. Panel A and E (Left): calcium current is inhibited by selective blockers of N- and L-type Ca2+ channels, i.e., ω-CTX (100 nM) and nifedipine (5 μM), both alone (n = 20) and in combination (n = 5). Panel B and E (Center) H3R activation with imetit (100 nM) attenuates calcium current, an effect prevented by the H3R antagonist clobenpropit (CBP; 50 nM). Panel C (Left): I-V curve in the absence and presence of imetit (100 nM) (means ± SEM; n = 20; *p < 0.05, from control by Student's t test). Panel C (Right): τact-voltage relationship. Imetit produced a slowing of current activation indicated by an increase in τact (means ± SEM; n = 12; *p < 0.05, **p < 0.01 from control by Student's t test). Panel D: Stimulation of adenylyl cyclase with forskolin (10 μM) increases calcium current. Incubation with imetit markedly reduces the forskolin-stimulated calcium current; CBP antagonizes this effect. Bars represent means (± SEM; n = 20; *p < 0.05, **p < 0.01 from control or forskolin by ANOVA followed by post hoc Dunnett's test).
We next depolarized PC12-H3 cells with K+ (100 mM) and determined the exocytosis of endogenous dopamine. Depolarization with K+ elicited a ∼2.5-fold increase in dopamine release, a response curtailed by ∼70% by the L-type Ca2+ channel antagonist nifedipine (5 μM), but not by the N-type channel antagonist ω-CTX (100 nM)(Fig. 4). H3R activation with imetit also significantly attenuated dopamine exocytosis (∼30%); this effect was abolished by H3R blockade with clobenpropit, a further confirmation of the functionality of transfected H3R (see Fig. 2A). Thus, H3R activation attenuated both peak ICa and dopamine exocytosis, suggesting that H3R-mediated anti-exocytotic effects could result from a reduced Ca2+ influx via VOCC.
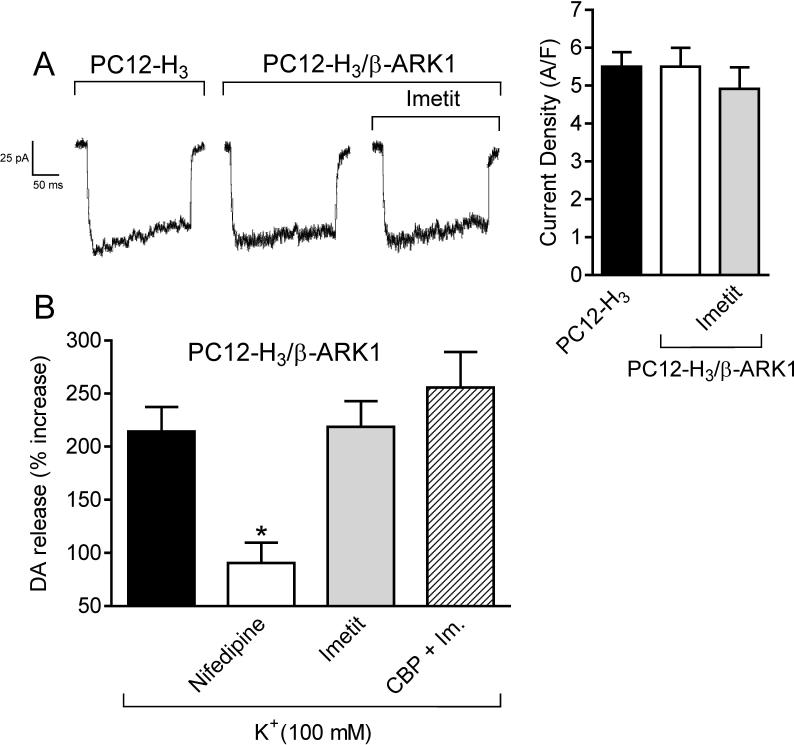
Since H3R are Gi/o-coupled and Gβγ subunits are known to directly inhibit VOCC function (Ikeda, 1996; Herlitze et al., 1996), we next assessed whether the Gβγ dimer plays a role in the decrease in ICa and associated anti-exocytotic effect observed with H3R activation. Peak ICa in PC12-H3/β-ARK1 cells did not differ from that recorded in PC12-H3 cells (Fig. 5A), suggesting that Gβγ was not constitutively active in these cells. In contrast to what was observed in PC12-H3 cells, imetit failed to reduce peak ICa in PC12-H3/β-ARK1 cells, indicating that the Gβγ dimer plays a role in the H3R-mediated reduction of ICa (compare Figs. 3B and 5A). Furthermore, the slowing of current activation, as demonstrated by an increase in τact in the presence of imetit, supports a direct Gβγ-induced inhibition of VOCC (Fig. 3C) (Stephens et al., 1998). Additionally, whereas the anti-exocytotic effect of nifedipine was preserved in PC12-H3/β-ARK1 cells, imetit failed to affect dopamine exocytosis (Fig. 5B), nor did imetit modify dopamine exocytosis in PC12-H3 cells incubated with a membrane-permeable phosducin-like anti-βγ peptide (1μM) (Chang et al., 2000) (Fig. 4). Collectively, these findings indicate that the H3R-mediated anti-exocytotic effect derives from the Gβγ-dependent inhibition of ICa and not from a βγ-induced inhibition of adenylyl cyclase.
Figure 5.
Panel A: Calcium current traces and peak current density, recorded in NGF-differentiated PC12-H3 by stepping from the holding potential of −40 mV to the test potential of +10 mV, do not differ from calcium currents evoked in PC12-H3 cells transfected with the Gβγ scavenger β-ARK1 (PC12-H3/β-ARK1). Notably, H3R activation with imetit (100 nM) fails to reduce peak calcium current in PC12-H3/β-ARK1 cells. Bars represent means (± SEM; n = 20). Panel B: endogenous dopamine release was measured in PC12-H3/β-ARK1 cells in response to K+ (100 mM). Dopamine exocytosis was inhibited by nifedipine (5 μM), but not by imetit (100 nM), either alone or in combination with clobenpropit (CBP, 50 nM). Bars are means ± SEM of percent increases in dopamine release above control (n = 20−25). Significantly different from K+ alone (*p < 0.05 by ANOVA followed by post hoc Dunnett's test).
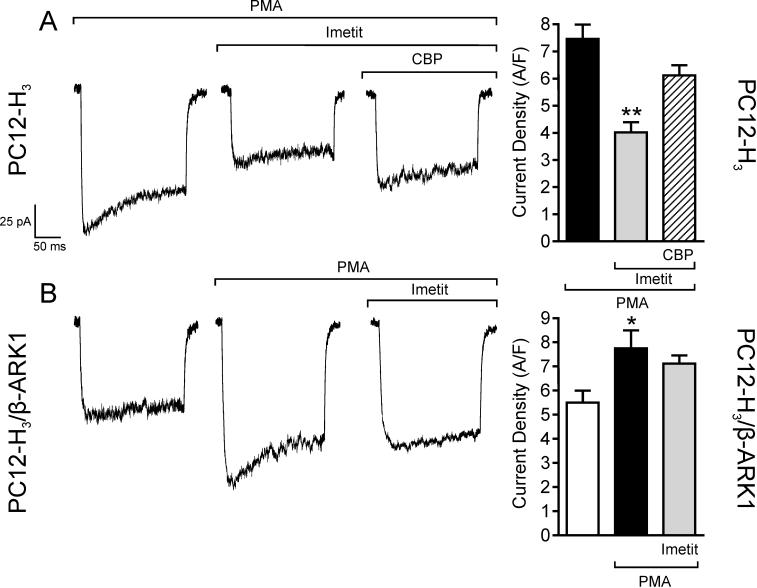
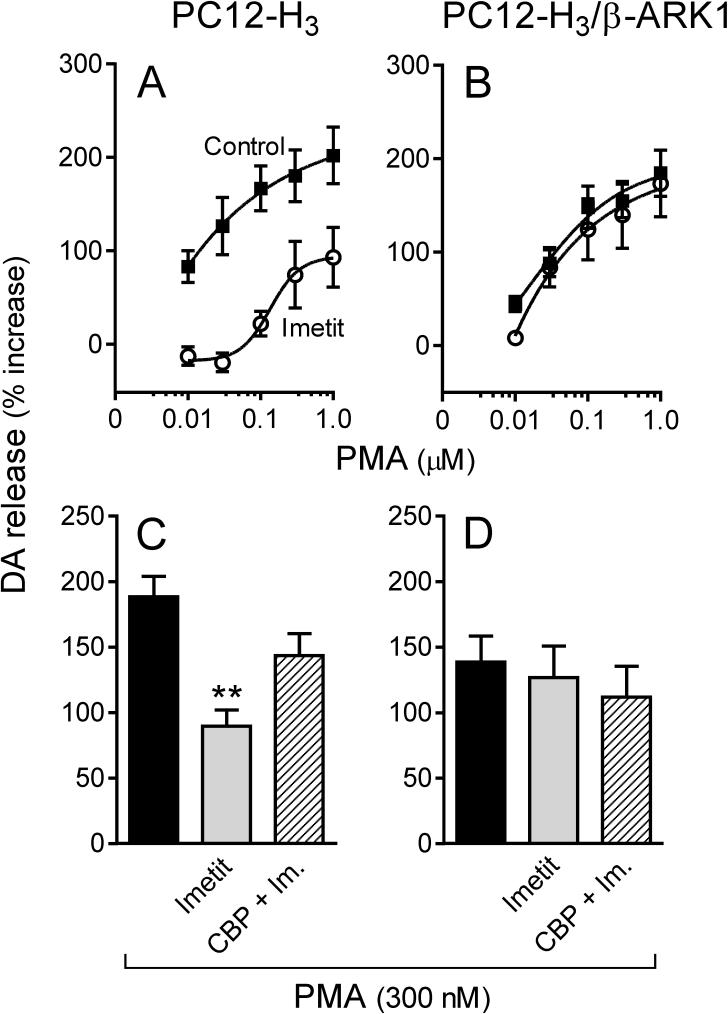
Activation of protein kinase C (PKC) upon application of phorbol esters is known to increase ICa (Zamponi et al., 1997), an action negatively modulated by Gβγ subunits (Ikeda, 1996; Herlitze et al., 1996). Accordingly, we next assessed whether H3R stimulation would attenuate PKC-activated ICa and associated increase in dopamine exocytosis via a Gβγ-mediated effect. Incubation of PC12-H3 cells with phorbol 12-myristate 13-acetate (300 nM; PMA) enhanced peak ICa by ∼30% (compare Fig. 5A and Fig. 6A). In the presence of imetit (100 nM), PMA-stimulated ICa was reduced by ∼45% and this response was antagonized by clobenpropit (50 nM)(Fig. 6A). PMA also elicited a concentration-dependent increase in dopamine release: in the 10 nM-1 μM PMA concentration range, dopamine release was increased ∼2−3-fold above control in both PC12-H3 and PC12-H3/β-ARK1 cells (Fig. 7A and B). In the presence of imetit (100 nM) the concentration-response curve for PMA was shifted to the right by two orders of magnitude in PC12-H3 cells (Fig. 7A). Incubation with clobenpropit (50 nM) antagonized the effect of imetit (Fig. 7C).
Figure 6.
Activation of PKC with the phorbol ester PMA (300 nM) increases peak calcium current and current density in both PC12-H3 (Panel A) and PC12-H3/β-ARK1 cells (Panel B). In PC12-H3 cells, incubation with imetit (100 nM) significantly reduces peak calcium current, an effect inhibited by CBP (50 nM). Notably, the imetit-induced calcium current reduction in PC12-H3 cells is not present in PC12-H3/β-ARK1 cells. Bars represent means (± SEM; n = 20; *p < 0.05 and **p < 0.01 by ANOVA followed by post hoc Dunnett's test).
Figure 7.
Activation of H3R attenuates endogenous dopamine exocytosis elicited by PKC activation with PMA (300 nM) in PC12-H3 cells but not in PC12-H3/β-ARK1 cells. Panels A and B: concentration-response curves for PMA-induced dopamine release in PC12-H3 and PC12-H3/β-ARK1 cells, respectively. Activation of H3R with imetit (100 nM) significantly reduced PMA-induced dopamine release in PC12-H3 cells (A), an effect that was antagonized by CBP (50 nM; panel C). In PC12-H3/β-ARK1 cells (B), H3R activation with imetit (100 nM) failed to affect the PMA-induced dopamine release. Panels C and D represent dopamine release elicited by activation of PKC with PMA (300 nM) in the absence or presence of imetit (100 nM), either alone or in combination with clobenpropit (CBP, 50 nM). Bars are means ± SEM (n = 10 for C and n = 15 for D). Significantly different from PMA alone (**p < 0.01 by ANOVA followed by post hoc Dunnett's test). Dopamine release is expressed as percent increase above basal level.
Application of PMA (300 nM) to PC12-H3/β-ARK1 cells enhanced peak ICa by ∼30% (Fig. 6B). Notably, activation of H3R with imetit in PC12-H3/β-ARK1 cells failed to attenuate the PMA-stimulated ICa (Fig. 6B). Moreover, in PC12-H3/β-ARK1 cells, H3R activation did not modify PMA-induced dopamine exocytosis (Fig. 7B). Indeed, the PMA concentration-response curve for the promotion of dopamine exocytosis was the same whether in the presence or absence of imetit (Fig. 7B), and the magnitude of the exocytotic response to the 300 nM concentration of PMA did not differ from that recorded in the presence of imetit, either alone or together with clobenpropit (Fig. 7D). Thus, stimulation of PKC in PC12-H3 and PC12-H3/β-ARK1 cells confirmed that a Gβγ-dependent inhibition of ICa plays a pivotal role in the anti-exocytotic effect of H3R activation.
Discussion
Our findings indicate that Gβγ subunits, liberated from Gαβγ trimers upon H3R activation attenuate neuronal ICa and consequent neurotransmitter exocytosis. This establishes a novel mechanism of H3R transduction capable of preventing excessive norepinephrine release, a recognized cardioprotective action (Levi et al., 2007).
In our investigation we utilized the PC12 rat pheochromocytoma cell line, which acquires a sympathetic nerve phenotype when treated with NGF (Dichter et al., 1977; Taupenot, 2007). Having stably transfected PC12 cells with human H3R, a PTX-sensitive Gi/o-coupled receptor (see Figs. 2 and 4), we set out to demonstrate - using the whole-cell patch clamp technique - the presence of an ICa likely responsible for the exocytosis of dopamine, the endogenous transmitter in these cells (Chen and Westfall, 1994). Indeed, by stepping to different test potentials in the −40 to +40 mV range, we identified a current in PC12-H3 cells with typical ICa characteristics (i.e., shape of the I/V curve, its persistence in the absence of Na+ in the bathing solution and its sensitivity to 0.1 mM CdCl2) which was also inhibited by the L- and N-type Ca2+ channel blockers nifedipine and ω-CTX, respectively. Moreover, this ICa was amplified by stimulation of adenylyl cyclase with forskolin and by PKC activation with a phorbol ester, responses which are typical of neuronal Ca2+ channel currents (Bean et al., 1984; Yang and Tsien, 1993). Notably, H3R activation with the selective agonist imetit (Garbarg et al., 1992) markedly reduced the ICa in control and both adenylyl cyclase- or PKC-stimulated conditions, an action inhibited by H3R blockade with the selective antagonist clobenpropit (Van der Goot et al., 1992). These findings reveal that the decrease in intracellular Ca2+ which we had previously described to occur in response to H3R activation (Silver et al., 2002; Seyedi et al., 2005) originates from a reduction in Ca2+ influx via VOCC.
To determine whether the H3R-mediated decrease in ICa is due to inhibition of VOCC by the Gi/o-derived Gβγ dimer, we transfected PC12-H3 cells with the Gβγ scavenger β-ARK1 (Koch et al., 1994). H3R activation in PC12-H3/β-ARK1 cells failed to inhibit ICa, unequivocally demonstrating the pivotal role played by the Gβγ dimer in the H3R-mediated inhibition of VOCC. Clearly, the Gβγ dimer-induced VOCC inhibition is also of major importance for the anti-exocytotic effects of H3R activation. Indeed, we found that the imetit-induced inhibition of dopamine exocytosis in PC12-H3 cells was prevented not only by a membrane-permeable phosducin-like anti-βγ peptide (Chang et al., 2000), but also in cells transfected with the Gβγ scavenger β-ARK1. Moreover, the Gβγ scavenger prevented the H3R-mediated attenuation of dopamine exocytosis elicited by PKC activation with the phorbol ester PMA.
Interestingly, whereas nifedipine and ω-CTX each inhibited ICa, only nifedipine attenuated dopamine release. Thus, although both L- and N-type Ca2+ channels are present in PC12-H3 cells, only the L-type appears to be involved in dopamine exocytosis. In fact, calcium entering through the L-type channel is the likely predominant stimulus for dopamine release in PC12 cells (Avidor et al., 1994; Kanwal et al., 1997). Furthermore, although the Gβγ dimer is generally viewed as an N-type Ca2+ channel inhibitor (Ikeda, 1996; Herlitze et al., 1996; Catterall, 2000), a Gβγ-induced inhibition of the L-type Ca2+ channel has also been recognized (Ivanina et al., 2000). In contrast to dopamine release in PC12 cells, both L- and N-type Ca2+ channels participate in neurotransmitter release from cardiac sympathetic nerve endings. Indeed, we had found that the H3R agonist imetit synergizes with either nifedipine or ω-CTX to attenuate norepinephrine exocytosis from cardiac synaptosomes (Seyedi et al., 2005).
Expression of the β-ARK1 polypeptide failed to modify the H3R-mediated attenuation of the forskolin-induced increase in intracellular cAMP and associated dopamine exocytosis (see Fig. 2). Although a βγ-induced inhibition of adenylyl cyclase has been described in insect ovarian cells (Taussig et al., 1993), our findings clearly indicate that the Gβγ dimer liberated upon H3R activation is not responsible for the reduction of adenylyl cyclase activity in differentiated PC12-H3 or PC12-H3/β-ARK1 cells. Therefore, a direct βγ-induced inhibition of ICa at the VOCC level is likely to play a key role in the H3R-mediated anti-exocytotic effect. The Gβγ dimer is also liberated upon PGE2-induced activation of EP3R, another Gi/o-coupled receptor with anti-exocytotic properties. PGE2 formation is the final step of the MAPKinase signaling cascade initiated by H3R activation, culminating in the attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings (Levi et al., 2007). This substantiates the present findings on the pivotal role of the Gβγ dimer as an inhibitor of ICa and consequent exocytosis.
We had previously reported that the anti-exocytotic effect of H3R activation is associated with a decrease in intraneuronal Ca2+ concentration in SH-SY5Y human neuroblastoma cells stably transfected with H3R (Silver et al., 2002). We hypothesized that the decrease in [Ca2+]i was due to a Gαi-induced inhibition of adenylyl cyclase, ultimately resulting in a decreased PKA-induced phosphorylation of VOCC (Seyedi et al., 2005). The novelty of the present findings is that a direct Gβγ-induced inhibition of VOCC, resulting in an attenuation of ICa, plays a pivotal role in the H3R-mediated decrease in [Ca2+]i and associated anti-exocytotic effects. The identification of this final step in the H3R transduction cascade has broad implications in the development of new therapeutic strategies in cardiovascular diseases characterized by hyperadrenergic activity, such as myocardial ischemia and congestive heart failure.
Acknowledgments
The first two authors, Christopher Morrey and Rima Estephan contributed equally to this research.
This work was supported by research grants from the National Institutes of Health HL34215, HL73400, HL7423, HL46403, HL47073 and HL79275.
Non-standard abbreviations
- β-ARK1
β-adrenergic receptor kinase 1
- [Ca2+]i
intraneuronal Ca2+
- H3R
histamine H3-receptors
- ICa
Ca+ current
- NGF
nerve-growth factor
- RT-PCR
reverse-transcriptase polymerase chain reaction
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PTX
pertussis toxin
- τact
time constant of activation
- VOCC
voltage-operated Ca2+-channels
- ω-CTX
ω-conotoxin GVIA.
References
- Avidor B, Avidor T, Schwartz L, De Jongh KS, Atlas D. Cardiac L-type Ca2+ channel triggers transmitter release in PC12 cells. FEBS Lett. 1994;342:209–213. doi: 10.1016/0014-5793(94)80502-4. [DOI] [PubMed] [Google Scholar]
- Bean BP, Nowycky MC, Tsien RW. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chang M, Zhang L, Tam JP, Sanders-Bush E. Dissecting G Protein-coupled Receptor Signaling Pathways with Membrane-permeable Blocking Peptides. J Biol Chem. 2000;275:7021–7029. doi: 10.1074/jbc.275.10.7021. [DOI] [PubMed] [Google Scholar]
- Chen X, Westfall TC. Modulation of intracellular calcium transients and dopamine release by neuropeptide Y in PC-12 cells. Am J Physiol. 1994;266:C784–C793. doi: 10.1152/ajpcell.1994.266.3.C784. [DOI] [PubMed] [Google Scholar]
- Dichter MA, Tischler AS, Greene LA. Nerve growth factor-induced increase in electrical excitability and acetylcholine sensitivity of a rat pheochromocytoma cell line. Nature. 1977;268:501–504. doi: 10.1038/268501a0. [DOI] [PubMed] [Google Scholar]
- Dickenson JM, Hill SJ. Involvement of G-protein betagamma subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol. 1998;355:85–93. doi: 10.1016/s0014-2999(98)00468-3. [DOI] [PubMed] [Google Scholar]
- Endou M, Poli E, Levi R. Histamine H3-receptor signaling in the heart: possible involvement of Gi/Go proteins and N-type Ca2+ channels. J Pharmacol Exp Ther. 1994;269:221–229. [PubMed] [Google Scholar]
- Garbarg M, Arrang JM, Rouleau A, Ligneau X, Tuong MD, Schwartz JC, Ganellin CR. S-[2-(4-imidazolyl)ethyl]isothiourea, a highly specific and potent histamine H3 receptor agonist. J Pharmacol Exp Ther. 1992;263:304–310. [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Imamura M, Poli E, Omoniyi AT, Levi R. Unmasking of activated histamine H3-receptors in myocardial ischemia: their role as regulators of exocytotic norepinephrine release. J Pharmacol Exp Ther. 1994;271:1259–1266. [PubMed] [Google Scholar]
- Imamura M, Seyedi N, Lander HM, Levi R. Functional identification of histamine H3-receptors in the human heart. Circ Res. 1995;77:206–210. doi: 10.1161/01.res.77.1.206. [DOI] [PubMed] [Google Scholar]
- Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by Gbeta gamma and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- Kanwal S, Elmquist BJ, Trachte GJ. Atrial natriuretic peptide inhibits evoked catecholamine release by altering sensitivity to calcium. J Pharmacol Exp Ther. 1997;283:426–433. [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- Koyama M, Seyedi N, Fung-Leung WP, Lovenberg TW, Levi R. Norepinephrine release from the ischemic heart is greatly enhanced in mice lacking histamine H3 receptors. Mol Pharmacol. 2003;63:378–382. doi: 10.1124/mol.63.2.378. [DOI] [PubMed] [Google Scholar]
- Levi R, Seyedi N, Schaefer U, Estephan R, Mackins CJ, Tyler E, Silver RB. Histamine H3-receptor signaling in cardiac sympathetic nerves: Identification of a novel MAPK-PLA2-COX-PGE2-EP3R pathway. Biochem Pharmacol. 2007;73:1146–1156. doi: 10.1016/j.bcp.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi R, Smith NCE. Histamine H3-receptors: A new frontier in myocardial ischemia. J Pharmacol Exp Ther. 2000;292:825–830. [PubMed] [Google Scholar]
- Lipscombe D, Kongsamut S, Tsien RW. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- Seyedi N, Mackins CJ, Machida T, Reid AC, Silver RB, Levi R. Histamine H3-receptor-induced attenuation of norepinephrine exocytosis: A decreased protein kinase A activity mediates a reduction in intracellular calcium. J Pharmacol Exp Ther. 2005;312:1–9. doi: 10.1124/jpet.104.072504. [DOI] [PubMed] [Google Scholar]
- Silver RB, Poonwasi KS, Seyedi N, Wilson SJ, Lovenberg TW, Levi R. Decreased intracellular calcium mediates the histamine H3-receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings. Proc Natl Acad Sci USA. 2002;99:501–506. doi: 10.1073/pnas.012506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Brice NL, Berrow NS, Dolphin AC. Facilitation of rabbit alpha1B calcium channels: involvement of endogenous Gbetagamma subunits. J Physiol. 1998;509(Pt 1):15–27. doi: 10.1111/j.1469-7793.1998.015bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita Y, Watanabe T, Sakata T, Munakata M, Ishibashi H, Akaike N. Histamine modulates high-voltage-activated calcium channels in neurons dissociated from the rat tuberomammillary nucleus. Neuroscience. 1998;87:797–805. doi: 10.1016/s0306-4522(98)00152-3. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Taupenot L. Analysis of regulated secretion using PC12 cells. Curr Protoc Cell Biol. 2007;Chapter 15:Unit15. doi: 10.1002/0471143030.cb1512s36. [DOI] [PubMed] [Google Scholar]
- Taussig R, Quarmby LM, Gilman AG. Regulation of purified type I and type II adenylylcyclases by G protein beta gamma subunits. J Biol Chem. 1993;268:9–12. [PubMed] [Google Scholar]
- Van der Goot H, Schepers MJP, Sterk GJ, Timmerman H. Isothiourea analogues of histamine as potent agonists or antagonists of the histamine H3-receptor. Eur J Med Chem. 1992;27:511–517. [Google Scholar]
- Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yakel JL. Modulation of Ca2+ currents by various G protein-coupled receptors in sympathetic neurons of male rat pelvic ganglia. J Neurophysiol. 1997;78:780–789. doi: 10.1152/jn.1997.78.2.780. [DOI] [PubMed] [Google Scholar]