Abstract
Anti-glomerular basement membrane (anti-GBM) disease is an aggressive form of glomerulonephritis usually mediated by IgG autoantibodies to the non-collagenous (NC1) domain of α3(IV) collagen. Less is known about the target antigen(s) in atypical anti-GBM disease involving IgA autoantibodies. We report a new case of IgA anti-GBM disease in a patient with a history of proliferative lupus nephritis, who presented with increasing creatinine, proteinuria and hematuria, but no clinical or serological evidence of lupus recurrence. Renal biopsy demonstrated focal and segmental necrotizing glomerulonephritis with strong linear capillary loop IgA staining by immunofluorescence. Serology was negative for IgG or IgA autoantibodies against the α3NC1 domain. By immunoblotting, IgA from patient’s serum bound to 38–48 kDa antigens collagenase-solubilized from human GBM, but not to purified NC1 domains of GBM collagen IV. The target of patient’s IgA autoantibodies was thus identified as a novel GBM antigen, distinct from the α3NC1 domain or other known targets of anti-GBM IgA autoantibodies. Clinical resolution was attained upon conventional treatment with steroids and cyclophosphamide. The diversity of antigens recognized by anti-GBM IgA autoantibodies highlights the importance of renal biopsy for the reliable diagnosis of this rare condition, since conventional serological immunoassays would likely yield false negative results.
Keywords: Anti-glomerular basement membrane disease, systemic lupus erythematosus, autoimmune glomerulonephritis, IgA autoantibodies
BACKGROUND
Circulating and tissue-bound autoantibodies that target antigenic sites within the glomerular basement membrane (GBM) are the hallmark of anti-GBM disease, a rare but aggressive form of glomerulonephritis. Most patients have IgG autoantibodies against the non-collagenous (NC1) domain of α3(IV) collagen (the “Goodpasture autoantigen”), which occurs as a supramolecular α3α4α5(IV) collagen network with tissue-restricted distribution. Goodpasture autoantibodies bind to autoantigen in the GBM and alveolar basement membranes, causing rapidly progressive glomerulonephritis and pulmonary hemorrhage, respectively (1).
A rare form of anti-GBM glomerulonephritis mediated by IgA autoantibodies has been described in 11 patients, reviewed elsewhere (2). The specificity of IgA anti-GBM autoantibodies has seldom been characterized, and it is not known whether α3(IV) collagen is a target. One patient with recurrent anti-GBM disease had a monoclonal IgA1-kappa antibody targeting collagenase-sensitive epitopes within α1/α2(IV) collagen (3). Another patient with crescentic glomerulonephritis and subepidermal blisters developed IgA autoantibodies against the NC1 domains of α5 and α6(IV) collagen (4). Here, we describe a new case of anti-GBM IgA antibody disease, the first in a patient with a history of proliferative lupus nephritis. Analysis of the patient’s serum revealed IgA autoantibodies targeting novel antigenic determinants in the GBM, distinct from both the α3NC1 domain and known targets of anti-GBM IgA autoantibodies from other patients.
CASE PRESENTATION
A 74-year-old white woman with a remote history of biopsy-proven proliferative lupus nephritis (Class unspecified) in 1975, maintained on prednisone 5mg every other day with a baseline creatinine of 0.87 mg/dL (77 µmol/L), developed proteinuria (1.5 g/day), glomerular hematuria, and decreased kidney function with creatinine 1.25 mg/dL (110 µmol/L). Past medical history included: hypercholesterolemia, hypertension and gastroesophageal reflux. Medications were: prednisone, telmisartan, atorvastatin, cimetidine and alendronate. Review of systems was negative for a lupus flare. Physical examination was unremarkable. Ultrasound showed normal kidneys. Laboratory investigations revealed normal C3, C4, and negative ANA, antiphospholipid antibodies, pANCA, cANCA and anti-GBM antibodies (see below). Urine and serum protein electrophoresis showed no monoclonal IgA, kappa or lambda light chains.
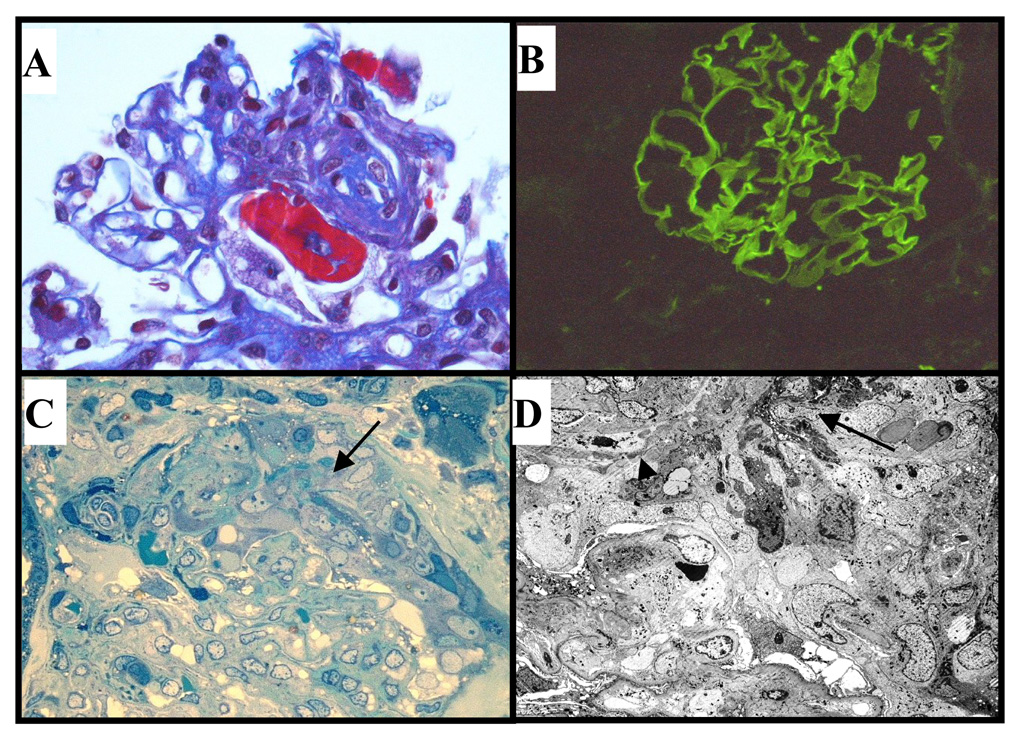
Renal biopsy yielded cortex containing 2 out of 5 globally sclerotic glomeruli. One glomerulus showed segmental fibrinoid necrosis (Fig. 1A) and another demonstrated a fibrous crescent with extensive segmental sclerosis, but no significant proliferation. There was mild focal chronic interstitial inflammation, patchy mild tubular atrophy and interstitial fibrosis. One small interlobular artery demonstrated no evidence of vasculitis and arterioles were normal. Immunofluorescence demonstrated strong (2–3+) linear capillary loop staining for human immunoglobulins, IgA (Fig. 1B) and lambda light chain, along with weak linear capillary loop IgG (1+), granular mesangial IgM (1+), segmental granular C3 (1+), and segmental fibrinogen (3+). Staining for kappa light chain, properdin and C1q were negative. Electron microscopy demonstrated a cellular crescent (Fig. 1C), and the capillary tuft underlying the crescent showed focal fibrinoid damage and endothelial cell swelling, with possible discontinuities of the GBM (Fig. 1D). No immune complex-type deposits were identified.
Figure 1. Diagnosis of IgA anti-GBM disease in the renal biopsy.
A. Light microscopy showing segmental fibrinoid necrosis (trichrome stain, x400). B. Direct immunofluorescence demonstrates strong (3+) linear capillary loop IgA (x200). C. Resin-embedded section showing active cellular crescent (arrow) composed of active cells with abundant cytoplasmic organelles and fibrinous exudate overlying compressed capillary tuft (toluidine blue, x400). D. Electron microscopy showing cellular crescent, focal fibrinoid damage (large arrow) and probable rupture of GBM (small arrow). Epithelial cell foot process fusion was present in relation to the crescent, but foot processes were largely intact in the other glomerulus (x800).
The patient received oral cyclophosphamide (1 mg/kg/day) and prednisone (1 mg/kg/day). Plasmapheresis was deferred due to limited proliferation on biopsy and gradual disease progression. Despite trimethoprim-sulfamethoxazole prophylaxis, the patient developed recurrent pneumonias requiring 2 hospital admissions and discontinuation of immunosuppression after 4 months. Nevertheless, the elevated creatinine 1.64 mg/dL (144 µmol/L) and proteinuria (6.1 g/d) improved and stabilized [creatinine 1.13 mg/dL (100 µmol/L); creatinine clearance 50 mL/min (.83 mL/s); proteinuria 1.1 g/d] by her last follow-up.
MATERIALS AND METHODS
Patient sera
Informed consent was obtained for analysis of patient’s serum. A previously characterized “reference” serum containing anti-GBM IgA autoantibodies to α1α2(IV) collagen (3) was used as positive control. Other human sera were used as negative controls.
Antigens
GBM was prepared from human kidney cortex as described (5), after sonication to disrupt the glomeruli. Antigens solubilized from human GBM by bacterial collagenase were fractionated on a DE-52 ion-exchange column; the 7S and NC1 domains of GBM collagen IV were separated from the DE52-unbound pool by gel filtration (5).
Indirect ELISA
was performed as described (3), measuring the binding of IgA from patient sera (diluted 1/10) to antigens (500 ng/well), including collagen IV NC1 domains purified from human GBM or recombinant (5), α1α2(IV) collagen and laminin from human placenta (Chemicon, Temecula, CA), and human fibronectin (Sigma Chemical Company, St. Louis, MO).
Western blot analysis
GBM-solubilized antigens were separated by SDS-PAGE under non-reducing conditions and transferred to Immobilon P membranes for immunoblotting with human sera (diluted 1/20), followed by alkaline phosphatase-conjugated anti-human IgA antibody and chromogenic substrate (3).
Indirect immunofluorescence staining
Acetone-fixed cryostat sections of human kidney (Human Tissue Acquisition Core, Vanderbilt University) were incubated with patient sera (diluted 1/10), and IgA binding was visualized using fluorescein isothiocyanate (FITC)-labelled anti-human IgA (Jackson ImmunoResearch Laboratories, West Grove, PA).
RESULTS
Patient’s serum did not contain IgG and IgA anti-GBM autoantibodies detectable by a commercial ELISA kit (Euroimmun EA1251-9601G; Lubeck Germany), using α3(IV) collagen NC1 domains from bovine kidneys as antigen. Further analysis of patient’s serum by ELISA showed negligible IgA and IgG reactivity toward NC1 domains from human GBM or recombinant α3,α4,α5 NC1 domains (<0.1 OD), and also no IgA immunoreactivity toward human α1α2(IV) collagen, human placenta laminin, human fibronectin, and bovine heparan sulphate proteoglycans (not shown). The positive control “reference” serum showed strong IgA binding (1.1 OD) to α1α2(IV) collagen from human placenta, as reported (3).
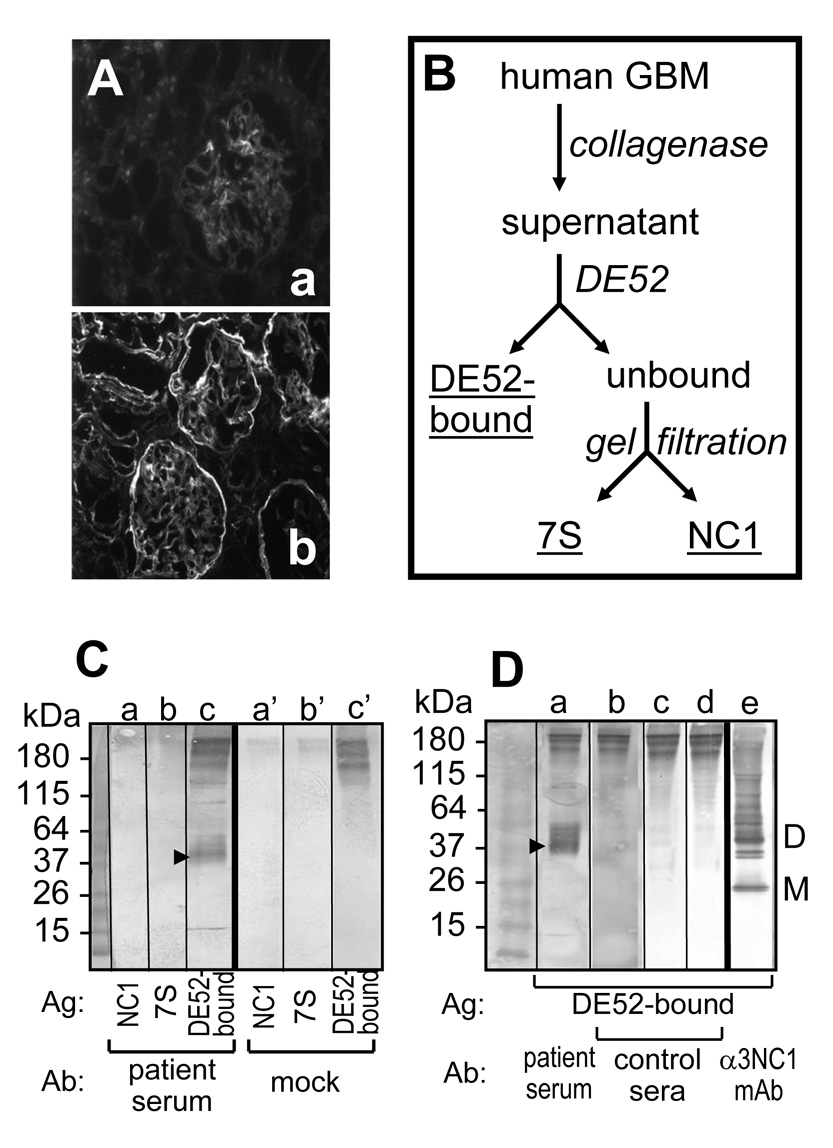
Indirect immunofluorescence staining of human kidneys with patient’s serum showed weak linear GBM binding of IgA (Fig. 2A,a), with or without acid-urea treatment to unmask putative cryptic epitopes. IgA from the “reference” serum stained basement membranes in a different pattern (Fig. 2A,b), indicating that anti-GBM IgA autoantibodies from the two patient sera have different specificity.
Figure 2. Analysis of IgA autoantibodies from patient serum.
A. IgA binding to normal human kidneys was evaluated by indirect immunofluorescence microscopy. Different IgA staining patterns were observed for the patient serum (a) and the reference serum (b). B. Antigens solubilized from human GBM by collagenase were fractionated by DE-52 ion-exhange and gel filtration chromatography. C. Western blot analysis of IgA binding to human collagen IV NC1 domains (a,a’), 7S domains (b,b’), and DE52-bound GBM antigens (c,c’). After incubation with patient serum (a–c) or mock control (a’–c’), the blots were developed using anti-human IgA secondary antibodies. D. Western blot analysis of IgA binding to DE52-bound GBM antigens (lanes a–e) after incubation with patient serum (a) and three control human sera (b–d); monomers (M) and dimers (D) of α3NC1 domains were stained with a specific mouse mAb (e).
The reactivity of serum IgA toward collagenase-solubilized antigens from human GBM (fractionated as shown in Fig. 2B) was evaluated by Western blot. Patient’s circulating IgA did not bind to NC1 (Fig. 2C,a) or 7S (Fig. 2C,b) domains of GBM collagen IV, but reacted with collagenase-solubilized antigens in the DE52-bound fraction (Fig. 2C,c). IgA mainly targeted a 38 kDa antigen, with weaker reactivity against 42–48 kDa bands. The antigen bands in the 38–48 kDa range were stained only by IgA autoantibodies from the patient’s serum (Fig. 2D,a), but not from other human sera (Fig. 2D,b–d). Although the identity of the 38–48 kDa antigen(s) targeted by IgA anti-GBM autoantibodies remains unknown, it is distinct from the Goodpasture autoantigen, detected in the DE52-bound fraction by immunoblotting with an α3NC1-specific mAb (Fig. 2D,e).
DISCUSSION
We report a new case of the rare IgA variant of anti-GBM disease and show that patient’s circulating IgA autoantibodies are directed against novel GBM antigens in the 38–48 kDa range. Clinical serology and subsequent immunoassays were negative for IgA or IgG autoantibodies against the NC1 domain of α3(IV) collagen, the major target of “Goodpasture” IgG autoantibodies. Moreover, patient’s IgA autoantibodies did not react with triple-helical epitopes within α1/α2(IV) collagen or the NC1 domains of α5/α6(IV) collagen, other known targets of circulating IgA anti-GBM autoantibodies (3, 4). These findings emphasize the diversity of antigens that can be targeted by anti-GBM IgA autoantibodies, contrasting with the narrow antigen and epitope specificity of anti-GBM IgG autoantibodies (6).
This is the first reported case of IgA anti-GBM disease in a patient with a history of lupus nephritis, raising the question of a possible connection between the two diseases. Lupus is associated with multiple autoantibodies, and some patient sera react with basement membrane components including laminin, collagen IV, entactin, heparan sulfate, and even α3NC1 domain (7). Co-occurrence of “classic” anti-GBM disease (though not the IgA variant) has been reported in some patients with active lupus nephritis (8, 9). Ongoing glomerular inflammation may alter GBM components, triggering an autoimmune reaction to modified antigens, as proposed for the association between anti-GBM disease and ANCA glomerulonephritis (10). This seems less likely in our patient, because the clinical and pathology findings excluded active lupus nephritis. Another alternative is a genetic predisposition for autoimmune disease, as IgA anti-GBM disease was also reported in association with another autoimmune condition, Crohn disease (2).
In conclusion, we report a case of IgA anti-GBM disease with circulating IgA autoantibodies targeting a novel GBM antigen, distinct from the NC1 domains of α3α4α5(IV) collagen or α1α2(IV) collagen. The glomerular lesions were not significantly different from those observed in patients with typical anti-GBM disease, and the patient responded well to conventional therapy with steroids and cyclophosphamide. The antigenic heterogeneity of anti-GBM IgA antibody disease demonstrates the necessity of renal biopsy for reliable diagnosis of this atypical presentation, as serological assays are likely to give false negative results.
ACKNOWLEDGEMENTS
Support: Julie Ho is funded by a Bristol Myers Squibb Cardiovascular Research Fellowship. This study was supported in part by National Institutes of Health grant P01 DK065123 to Dorin-Bogdan Borza. The Human Tissue Acquisition and Pathology Shared Resource at Vanderbilt University is supported by the NIH grant P30 CA68485.
Financial Disclosure: Dr. Ho is funded by a Bristol Myers Squibb Cardiovascular Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors (JH, IWG, JZ, FF, SC, DBB) declare no conflict of interest.
REFERENCES
- 1.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Mechanisms of Disease: Alport's syndrome, Goodpasture's Syndrome, and Type IV Collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 2.Shaer AJ, Stewart LR, Cheek DE, Hurray D, Self SE. IgA antiglomerular basement membrane nephritis associated with Crohn's disease: a case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis. 2003;41:1097–1109. doi: 10.1016/s0272-6386(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 3.Borza DB, Chedid MF, Colon S, Lager DJ, Leung N, Fervenza FC. Recurrent Goodpasture's disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis. 2005;45:397–406. doi: 10.1053/j.ajkd.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Ghohestani RF, Rotunda SL, Hudson B, et al. Crescentic glomerulonephritis and subepidermal blisters with autoantibodies to alpha5 and alpha6 chains of type IV collagen. Lab Invest. 2003;83:605–611. doi: 10.1097/01.lab.0000067497.86646.4d. [DOI] [PubMed] [Google Scholar]
- 5.Boutaud A, Borza DB, Bondar O, et al. Type IV collagen of the glomerular basement membrane: Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- 6.Borza DB. Autoepitopes and alloepitopes of type IV collagen: role in the molecular pathogenesis of anti-GBM antibody glomerulonephritis. Nephron Exp Nephrol. 2007;106:e37–e43. doi: 10.1159/000101791. [DOI] [PubMed] [Google Scholar]
- 7.Li QZ, Xie C, Wu T, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards NT, Lueck C, Davies DR, Jones NF, Al-Khadar A, Coode P. Anti-glomerular basement membrane antibody and linear glomerular immunofluorescence in a patient with systemic lupus erythematosus. Clin Nephrol. 1988;30:115–116. [PubMed] [Google Scholar]
- 9.Li CH, Li YC, Xu PS, Hu X, Wang CY, Zou GL. Clinical significance of anti-glomerular basement membrane antibodies in a cohort of Chinese patients with lupus nephritis. Scand J Rheumatol. 2006;35:201–208. doi: 10.1080/03009740500303181. [DOI] [PubMed] [Google Scholar]
- 10.Hellmark T, Niles JL, Collins AB, McCluskey RT, Brunmark C. Comparison of anti-GBM antibodies in sera with or without ANCA. J Am Soc Nephrol. 1997;8:376–385. doi: 10.1681/ASN.V83376. [DOI] [PubMed] [Google Scholar]