Summary
Protozoan parasites represent major public health challenges. Many aspects of their cell biology are distinct from their animal hosts, providing potential therapeutic targets. Toxoplasma gondii is a protozoan parasite that contains a divergent regulator of chromosome condensation 1 (TgRCC1) that is required for virulence and efficient nuclear trafficking. RCC1 proteins function as a guanine exchange factor for Ras-related nuclear protein (Ran), an abundant GTPase responsible for the majority of nucleocytoplasmic transport. Here we show that while there are dramatic differences from well–conserved RCC1 proteins, TgRCC1 associates with chromatin, interacts with Ran, and complements a mammalian temperature-sensitive RCC1 mutant cell line. During the investigation of TgRCC1, we observed several unprecedented phenotypes for TgRan, despite a high level of sequence conservation. The cellular distribution of TgRan is found throughout the parasite cell, whereas Ran in late branching eukaryotes is predominantly nuclear. Additionally, T. gondii tolerates at least low-level expression of dominant lethal Ran mutants. Wild type parasites expressing dominant negative TgRan grew similar to wild type in standard tissue culture conditions, but were attenuated in serum-starved host cells and mice. These growth characteristics paralleled the TgRCC1 mutant and highlight the importance of the nuclear transport pathway for virulence of eukaryotic pathogens.
Keywords: Toxoplasma, RCC1, Ran, nuclear transport, pathogenesis
Introduction
Apicomplexa is a phylum of protozoa whose members are intracellular parasites responsible for extensive disease worldwide. Notable pathogens include Plasmodium species (the causative agent of malaria), Cryptosporidium species (the causative agent of cryptosporidiosis, a water-borne diarrheal disease), and Toxoplasma gondii (the causative agent of toxoplasmosis). T. gondii infects approximately one third of the world's human population and is considered a model for the analysis of Apicomplexa (Tenter et al., 2000). In addition to their medical significance, these deep branching organisms are important to study because of their unique organelles and cell physiology (Dubey et al., 1998). Thus, along with identification of needed pharmaceutical targets against these parasites, an in depth understanding of their cell biology may shed light on the origins of many cellular processes.
To understand how T. gondii establishes an infection within the host, signature-tagged mutagenesis was modified to identify genetic determinants of virulence. One of the 34 virulence genes identified was a divergent ortholog of the regulator of chromosome condensation 1 (RCC1), named TgRCC1 (Frankel et al., 2007). RCC1 is an essential nuclear protein that functions as a guanine exchange factor (GEF) for the GTPase Ran to control various cellular processes including nuclear transport (Bischoff and Postingl, 1991; Bischoff and Postingl, 1991). GEF activity is the only known function for RCC1. The 73F9 mutant contains an insertion in the promoter of TgRCC1, resulting in a “knock-down” rather than a “knock-out”. In addition to its attenuation in a mouse model of infection, the 73F9 mutant is growth impaired in host cells depleted of serum. This nutrient limitation in vitro may mimic in vivo conditions. As expected, 73F9 parasites show a decrease in their ability to transport nuclear-bound cargo to the nucleus compared to wild type parasites (Frankel et al., 2007).
RCC1 proteins are highly conserved in eukaryotes and are comprised of seven tandem repeats of 50-60 amino acids (Renault et al., 1998; Renault et al., 2001). RCC1 folds into a seven-blade propeller structure to bind to histones H2A and H2B at one end, and interact with Ran at the other (Renault et al., 1998; Nemergut et al., 2001; Renault et al., 2001). Interestingly, no protozoan parasite appears to encode for a typical RCC1 protein. TgRCC1 is the most similar T. gondii protein to human RCC1, yet it contains just five RCC1 repeats. At 1155 amino acids, TgRCC1 is also much larger than the conventional RCC1 proteins of ∼420 amino acids. In addition, TgRCC1 contains a unique zinc finger motif at its N-terminus that is conserved in the nucleoporins Nup153 and Nup358, also called RanBP2. The zinc finger domain of RanBP2 was shown to bind to Ran (Yaseen and Blobel, 1999).
RCC1 is the only identified GEF for Ran, an abundant GTPase that belongs to the Ras superfamily and is primarily localized in the nucleus. Ran is bound to either GTP or GDP, and its nucleotide state is essential for its differential functions in nuclear transport, and nuclear envelope and spindle formation during mitosis (DiFiore et al., 2004). RCC1 is required to exchange GTP for GDP on Ran when it is translocated into the nucleus. Conversely, in the cytoplasm, Ran GTPase Activating Protein (RanGAP) is needed to hydrolyze GTP to GDP, creating a necessary gradient of RanGTP in the nucleus and RanGDP in the cytosol. This gradient serves as the sole energy source driving the directionality and movement of nucleocytoplasmic transport (Moore, 1998; Macara, 2001). Mutations of characterized, conserved residues within Ras and other family members have allowed for the functional analysis of Ran. For example, the loss of function mutation, T24N, causes Ran to have effectively no affinity for GTP, resulting in Ran bound to either GDP or no nucleotide at all (Klebe et al, 1995). Additionally, Ran T24N has been shown to bind strongly to and inactivate RCC1 (Dasso et al., 1994; Klebe et al, 1995). The gain of function mutation, G19V, causes Ran to be locked in the GTP state due to its inability to be activated by RanGAP, thus eliminating GTPase activity (Lounsbury et al., 1996). Not surprisingly, both T24N and G19V are dominant lethal mutations (Carey et al., 1996).
In this report, we functionally characterize the divergent RCC1 ortholog, TgRCC1 and its interacting partner, TgRan. TgRCC1 interacts with chromatin and TgRan, even when its zinc finger motif is mutated. Complementation with TgRCC1 restores growth and RanGTP levels in a temperature sensitive mammalian cell line, TsBN2, which contains a point mutation in RCC1. This highlights that even though TgRCC1 is highly divergent, it is still a functional GEF for Ran. We also show that T. gondii allows low-level but stable expression of Ran mutants that are dominant lethal in all other organisms examined. Expression of TgRan T24N in wild type parasites causes reduction in growth in nutrient deprived host cells and in mice, recapitulating the phenotypes of the TgRCC1 mutant. These results illustrate that while alterations to the Ran network do not affect growth of the parasite in standard tissue culture conditions, they are required for pathogenesis.
Results
TgRCC1 associates with chromatin
We had previously identified TgRCC1 as a virulence determinant, yet further characterization of the protein was required due to its abnormally large size and its unusual RCC1 domain organization (Fig. 1A). RCC1 was initially characterized as a nuclear protein that bound to chromatin (Ohtsubo et al., 1989). Knowing that TgRCC1 localizes to the parasite nucleus (Frankel et al., 2007), it was important to ascertain its interaction with chromatin. Proteins bound to chromatin are released into the supernatant under high salt conditions due to the disruption of the ionic interactions (Ohtsubo et al., 1989). The nuclei of T. gondii expressing TgRCC1 with a C-terminal hemagglutinin (HA) tag were treated with varying concentrations of NaCl, separated by centrifugation, and proteins in the pellet and supernatant were analyzed by western immunoblot. While TgRCC1 was found in the pellet in untreated nuclei, NaCl treatment released TgRCC1 from chromatin into the supernatant, similar to human RCC1 (Fig. 1B). TgRCC1 was always observed as two bands by western blot from isolated nuclei, however it was a single band from whole cell lysates (Frankel et al., 2007). This smaller molecular weight band likely represents N-terminal processing of TgRCC1 because only the higher molecular weight band was seen when TgRCC1 was epitope tagged at the N-terminus (data not shown).
Fig. 1. Atypical TgRCC1 associates with chromatin.
A. Depiction of the functional domains of human RCC1 (top) and TgRCC1 (bottom) including the zinc finger (white triangle), the RCC1 repeats (black ovals), and nuclear localization signals (NLSs; grey rectangles).
B. Human RCC1 and TgRCC1 were analyzed for chromatin interaction by treatment of nuclei with NaCl. Nuclei were isolated from BHK cells expressing a FLAG-tagged human RCC1 or T. gondii expressing TgRCC1-HA. Nuclei were treated with varying concentrations of NaCl, centrifuged, and western immunoblots of pellet and supernatant fractions were probed with either FLAG (human RCC1) or HA antibodies (TgRCC1).
TgRCC1 interacts with TgRan
The only known function for RCC1 proteins is to exchange GDP for GTP on Ran. To ascertain whether this function was conserved in TgRCC1, we first identified the T. gondii Ran ortholog. The human Ran protein was used to search the annotated proteins in the T. gondii genome database (www.toxodb.org). While several members of the Ras superfamily were identified by homology, only annotation 50.m00042 had significant identity throughout its entire sequence to human Ran. Protein 50.m00042, named TgRan, was 71 percent identical to human Ran (Fig. S1A). This sequence conservation is shared in other apicomplexan parasites such as P. falciparum and C. parvum. To confirm that protein 50.m00042 is the T. gondii Ran ortholog, we expressed and purified TgRan from E. coli to examine its activity in vitro. E. coli-derived TgRan was compared to human Ran for its ability to bind and hydrolyze GTP. Both human Ran and TgRan bound to radiolabelled GTP and had similar rates of GTPase activity (Fig. 2A). This activity, in addition to its sequence conservation, indicates that protein 50.m00042 is the Ran GTPase ortholog for T. gondii.
Fig. 2. Interaction of TgRCC1 and TgRan.
A. TgRan purified from E. coli was tested for the ability to bind GTP as well as the intrinsic GTPase activity. Samples were taken at the designated times and analyzed by a filter binding assay and scintillation counts. Human Ran (HsRan) was used as a control. Shown is a representative of two experiments.
B. Co-immunoprecipitation of nuclear lysates from parasites expressing a HA-tagged TgRCC1. Anti-HA or anti-Ran antibodies were used for immunoprecipitations and western immunoblots were probed for both TgRCC1 and TgRan. T. gondii not expressing HA-tagged TgRCC1 was used as a negative control.
We next examined whether there was an interaction between TgRCC1 and TgRan in vivo. Co-immunoprecipitations (co-IP) of TgRCC1-HA were performed from parasite nuclei using a polyclonal anti-HA antibody, or by precipitation of TgRan with a monoclonal anti-Ran antibody. Co-IP with either antibody showed the binding of TgRCC1 and TgRan in nuclear lysates (Fig. 2B). We also saw a direct interaction between E. coli-derived TgRan and the putative functional domains of TgRCC1 (amino acids 91-797, from the zinc finger motif to the last RCC1 repeat) purified from E. coli (Fig. S1B). This data strengthens the hypothesis that TgRCC1 is a GEF for TgRan.
TgRCC1 functionally complements a mammalian cell line
Even though TgRCC1 localizes to the nucleus, associates with chromatin, and interacts with TgRan, it was important to examine TgRCC1 GEF activity. We were unable to purify full-length TgRCC1 from E. coli or yeast for in vitro assays. We measured GEF activity in vitro from the E. coli-derived truncated TgRCC1 described above with either TgRan or human Ran. The truncated TgRCC1 was able to bind Ran, but did not have GEF activity (data not shown). We propose that full-length TgRCC1 is required for proper folding and function.
As an alternative, we used a well-established temperature sensitive RCC1 cell line, TsBN2, to test functionality of TgRCC1 by genetic complementation (Uchida et al., 1990; Ohtsubo et al., 1991; Dasso, 1993). TsBN2 is derived from baby hamster kidney (BHK) cells that contain a point mutation in RCC1, which renders it unstable at 40° C, leading to its degradation and subsequent cell death (Uchida et al., 1990). We cloned the complete TgRCC1 open reading frame into a mammalian expression vector and transfected TsBN2 cells at the permissive temperature of 33° C. Twenty-four hours post transfection, the cells were transferred to 40° C and grown in the absence of drug selection. As expected, TsBN2 cells transfected with the vector alone or the mock transfected died within 36 hours of transfer to 40° C. Cells transfected with human RCC1 as a positive control were able to survive and replicate normally at 40° C, becoming confluent within four to five days. TsBN2 cells expressing TgRCC1 were also able to survive at the restrictive temperature (Fig. 3A), although they required approximately two weeks to become confluent. Once they reached confluency, TgRCC1 transfected TsBN2 cells grew at a rate identical to cells transfected with human RCC1 (data not shown). Cells transfected with TgRCC1 and grown at 40° C had abundant TgRCC1 protein, while untransfected TsBN2 cells showed no cross-reactivity (Fig. 3A, first row). As expected, endogenous RCC1 protein was only present at samples grown at 33° C, not at 40° C (Fig. 3A, second row). Although yeast RCC1 mutations may be suppressed by overexpression of Ran (Belhumeur et al., 1993), we did not observe any upregulation of Ran in TgRCC1 complemented cells (Fig. 3A, third row). Actin levels are shown as a loading control (Fig. 3A, fourth row). We examined the protein localization of TgRCC1 in TsBN2 cells by immunofluorescence. TgRCC1 containing a C-terminal HA-epitope tag localized to the nucleus of TsBN2 cells (Fig. 3B). These data confirm that TgRCC1 is able to complement TsBN2 cells in the absence of seven tandem RCC1 repeats and properly localize to the cell nucleus.
Fig. 3. Functional complementation of TsBN2 cells with TgRCC1.
A. The first column is untransfected TsBN2 cells grown at the permissive temperature of 33 °C. The second and third columns were TsBN2 cells transfected with a TgRCC1 expression vector and transferred to the non-permissive growth temperature of 40 °C after 24 hours. Western immunoblots were probed for the presence of TgRCC1 (top panel), mammalian RCC1 (second panel), Ran (third panel) and actin (bottom panel).
B. TsBN2 cells were transiently transfected with an expression vector containing TgRCC1 with a C-terminal HA tag. Differential interference contrast (DIC) is shown on the left, immunofluorescence of anti-HA antibodies to detect TgRCC1 (green), and coverslips were mounted in 4′,6′-diamidino-2-phenylindole (DAPI) to strain the nucleus (red).
C. TsBN2 cells were grown at 33°C and either maintained at that temperature or shifted to 40°C for eight hours. Cells were fixed and stained using anti-Ran antibodies (green) and DAPI to strain the nucleus (red). The top two rows are untransfected TsBN2 cells, the middle two rows have stable expression of TgRCC1, and the bottom two rows have stable expression of human RCC1 as a positive control.
We created mutants of TgRCC1 to determine its functional domains using the TsBN2 complementation assay. Removal of just 125 amino acids from the N-terminus or 115 amino acids from the C-terminus generated a non-functional TgRCC1 in TsBN2 cells (Fig. S2). This data supports the previous finding that truncated TgRCC1 did not have GEF activity. To directly examine the role of the zinc-finger domain, we made specific point mutations that would eliminate zinc coordination. Zinc finger mutant TgRCC1 (Zn-TgRCC1) was examined for its interactions with chromatin and Ran, as well as complementation of TsBN2 cells. Zn-TgRCC1 did not display any defects in these assays (Fig. S3), suggesting that the zinc-finger domain performs a function outside of known RCC1 proteins, or that its function is redundant and was not observed using the techniques used. A crystal structure of TgRCC1 will be necessary to fully characterize this atypical RCC1 protein.
TgRCC1 is able to restore nuclear localization of Ran in TsBN2 cells
The nuclear localization of Ran is dependent on a functional RCC1 to exchange it to the GTP bound form (Ren et al., 1993; Nemergut and Macara, 2000). To confirm functional complementation of TgRCC1 in TsBN2 cells, we analyzed the localization of Ran. TsBN2 cells were incubated at 33° C or switched to 40° C for eight hours, then fixed and stained for localization of Ran. At the permissive growth temperature, all cells displayed a strong nuclear Ran staining (Fig. 3C). At 40° C, RCC1 is degraded and Ran is unable to be exchanged to its GTP bound form, thus it remains bound to GDP and distributed throughout the cell (Nemergut and Macara, 2000; Fig. 3C). In TsBN2 cells expressing human RCC1 or TgRCC1, Ran maintained nuclear localization, even at 40° C (Fig. 3C). These experiments conclude that despite the abnormal protein structure of TgRCC1, it is able to functionally complement TsBN2 cells at 40° C by restoring not only growth but also proper nuclear localization of Ran. This is the first functional characterization of an atypical RCC1 species.
TgRan displays an abnormal parasite-specific localization
In RCC1 depleted cells, Ran changes its localization due to its shift from primarily the GTP to the GDP bound form (Ren et al., 1993; Fig. 3C). We therefore examined the cellular distribution of TgRan in wild type and the original TgRCC1 mutant parasites. We co-stained with either 4′,6′-diamidino-2-phenylindole (DAPI) or T. gondii histone H2B (Dalmasso et al., 2006) to indicate the nucleus, and heat shock protein 90 (Hsp90) as a cytosolic marker (Echeverria et al., 2005). In wild type parasites, TgRan was distributed throughout the cell, in both the nuclear and cytoplasmic compartments (Fig. 4A). The TgRan localization is in contrast to the predominant nuclear localization of Ran in late branching eukaryotes. In the TgRCC1 mutant 73F9, TgRan was localized largely outside of the nucleus, with a prominent signal around the nuclear envelope (Fig. 4A). In 73F9 complemented with TgRCC1, TgRan was localized throughout the cell, similar to wild type parasites (data not shown). To confirm that the unique localization of TgRan was specific to T. gondii, TgRan was expressed in the mammalian cell line, BHK. TgRan was localized to the nucleus within BHK cells, similar to native Ran (Fig. 4B). Overall, TgRan localization in T. gondii is atypical compared to all eukaryotes examined so far. However, similar to other eukaryotes, TgRan is largely excluded from the nucleus in T. gondii depleted of TgRCC1.
Fig. 4. TgRan displays an altered cellular distribution and relocation in 73F9 parasites.
A. Extracellular wild type or TgRCC1 mutant (73F9) parasites were stained for TgRan with anti-Ran antibodies (green) and co-stained (red) with DAPI (nuclear), histone H2B (nuclear), and Hsp90 (cytosolic).
B. BHK cells were transiently transfected with an expression vector encoding TgRan with a C-terminal FLAG epitope tag. Twenty-four hours post transfection, cells were fixed and stained for either native Ran (anti-Ran antibody; upper panel), or TgRan (anti-FLAG antibody; bottom panel), both shown in green. DAPI was added to coverslips to stain the nucleus (red).
T. gondii allows expression of dominant lethal Ran mutants
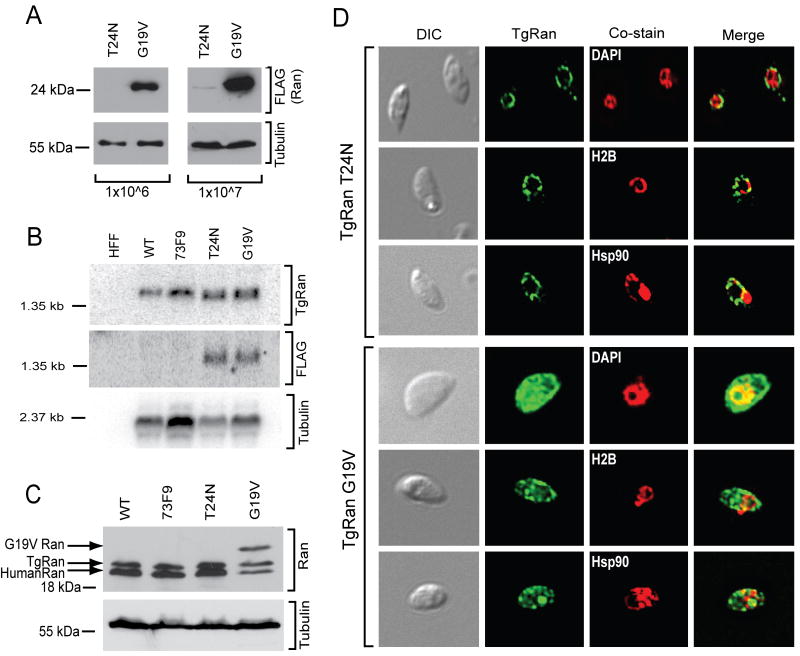
With the altered localization of TgRan, the divergence of TgRCC1, and the apparent absence of a RanGAP protein (Frankel et al., 2007), we speculated that T. gondii possess an atypical Ran network. To examine the Ran network in T. gondii, we used Ran mutants that have been extensively characterized (Dasso et al., 1994; Klebe et al., 1995, Lounsbury et al., 1996, Carey et al., 1996). We created both gain and loss of function Ran mutants in T. gondii (TgRan G19V and TgRan T24N, respectively). While these mutant forms of Ran are lethal in other eukaryotes, T. gondii was able to stably express both TgRan G19V and T24N (Fig. 5). However, TgRan T24N protein was drastically lower than TgRan G19V (Fig. 5A). The difference in mutant TgRan protein was controlled at the post-transcriptional level, as mRNA levels were similar as detected by Northern blot analysis (Fig. 5B). Native TgRan levels were not perturbed in the TgRCC1 mutant (73F9) or in parasites expressing the T24N and G19V TgRan mutations (Fig. 5C).
Fig. 5. T. gondii tolerates expression of Ran mutants.
A. Western immunoblot analysis of T. gondii expressing FLAG-tagged TgRan with either T24N or G19V mutations. Blots were probed with anti-FLAG antibodies to detect the mutated TgRan or β-Tubulin as a loading control. The number of parasites loaded per lane is indicated below.
B. Northern blot analysis of human foreskin fibroblasts (HFF), wild type parasites (WT), TgRCC1 mutant parasites (73F9), or parasites expressing TgRan T24N or G19V were harvested and RNA collected. The membrane was analyzed for TgRan using the TgRan ORF as a probe (top panel), the TgRan T24N and G19V mutants using a FLAG specific probe (middle panel), and α-tubulin as a T. gondii specific loading control (bottom panel).
C. Comparison of Ran protein levels between wild type (WT), TgRCC1 mutant (73F9), and TgRan T24N and G19V expressing parasites by western immunoblot. Blots were probed with anti-Ran antibodies and arrows indicate the three Ran species detected: TgRan G19V containing a FLAG tag, native TgRan, and human Ran from host cells. The blots were reprobed with anti-β-Tubulin as a loading control.
D. Immunofluorescence of extracellular of T. gondii expressing FLAG-tagged TgRan with either T24N (top panels) or G19V (bottom panels) mutations. Mutated TgRan was specifically stained with anti-FLAG antibodies (green). Cells were co-stained (red) with DAPI (nuclear), histone H2B (nuclear), and Hsp90 (cytosolic).
The localization of these mutated Ran proteins was examined. Transient transfection of Ran T24N in BHK cells showed localization within the nucleus as well as around the nuclear envelope (Lounsbury et al., 1996). This Ran T24N location correlates with the dominant negative activity caused by its binding and inactivation of RCC1 (Dasso et al., 1994; Klebe et al., 1995). We observed that TgRan T24N localized largely outside the parasite nucleus, with an undetectable signal in the nucleus (Fig. 5D, top panel), similar to the TgRan localization in the TgRCC1 mutant 73F9 (Fig. 4A). Transient transfection of Ran G19V localized around the nuclear envelope in BHK cells (Lounsbury et al., 1996). In contrast, TgRan G19V was distributed throughout the entire parasite (Fig. 5D, bottom panel). These data are supportive of T. gondii containing an atypical Ran network.
Expression of dominant negative Ran recapitulates TgRCC1 mutant phenotypes
We hypothesized that the decrease of TgRCC1 in 73F9 parasites leads to a reduced ability of the parasite to exchange RanGDP to RanGTP, resulting in an accumulation of RanGDP and an altered TgRan nucleotide gradient. A dominant lethal Ran mutant, T24N, was shown to modify the Ran nucleotide gradient by its inability to bind GTP, resulting in Ran bound to either GDP or no nucleotide at all (Kornbluth et al., 1994). We sought to examine if expression of TgRan T24N in wild type parasites would recapitulate the phenotypes observed in the TgRCC1 mutant 73F9. Due to the random integration of TgRan T24N into T. gondii, three unique clones were examined. We first tested the growth of the three clones in nutrient limiting media, for which host cells were deprived of serum before parasite infection. Wild type parasites expressing TgRan T24N displayed diminished growth in serum starved host cells, without any defect in normal tissue culture conditions (Fig. 6A). This phenotype mimicked the TgRCC1 mutant 73F9. We then examined the phenotype of TgRan T24N expressing parasites in vivo. Mice were infected with either wild type parasites or one of the three unique TgRan T24N clones and the infection was monitored over 22 days. While wild type parasites were highly virulent, with all but one mouse succumbing to infection, expression of TgRan T24N reduced virulence of all three clones (Fig. 6B). These in vivo phenotypes paralleled those for the 73F9 mutant strain, which was severely attenuated (Frankel et al., 2007). Overall, the reduced virulence of TgRan T24N expressing parasites shows that alterations of the Ran nucleotide gradient can affect parasite fitness under nutrient limitation conditions.
Fig. 6. TgRan T24N recapitulates the phenotypes of the TgRCC1 mutant.
A. Wild type (WT) and TgRCC1 mutant (73F9) parasites were grown in either 10% or 0% serum for 36 hours, then the number of parasites per vacuole were counted. T24N 1-3 represent three unique clones of wild type T. gondii expressing TgRan T24N. Error bars indicate standard deviation. Data represents three independent experiments performed in triplicate. Growth differences between 10% to 0% serum for 73F9 and all TgRan T24N mutants are statistically significant with a p value of <0.0001 (Student t-test).
B. Lethality of wild type (WT) or wild type parasites expressing TgRan T24N (T24N 1-3) was examined in a murine model of toxoplasmosis. Infection was monitored over 22 days. Shown is a composite of two individual experiments with four mice per parasite strain (eight mice total). Data are statistically significant with a p value of <0.0001 (Kaplan-Meier curve).
Discussion
RCC1 proteins have been extensively studied in model organisms, but their role in parasite pathogenesis has only recently been characterized (Frankel et al., 2007). In addition to TgRCC1, the annotated genome of T. gondii predicts 12 other proteins with at least two RCC1 domains. These other RCC1 domain-containing proteins are predicted to localize outside of the nucleus or they possess domains that suggest alternative cellular roles. Two of these other RCC1 repeat containing proteins were recently shown to be involved in the cell cycle (Gubbels et al., 2008). While RCC1 proteins are well conserved from yeast to humans, protozoans do not appear to encode a typical ortholog. Despite the dramatic differences in size and organization, TgRCC1 can functionally complement a temperature sensitive mammalian cell line, TsBN2. TgRCC1 complementation of TsBN2 cells was initially delayed, likely due to the cellular adaptations necessary to accommodate such a divergent RCC1. This study reports the first Ran GEF activity of a highly divergent RCC1 ortholog.
Examination of TgRCC1 uncovered the unorthodox properties of the Ran network in T. gondii. TgRan displayed an altered cellular localization. Even though Ran was identified as a Ras-related nuclear protein (Drivas et al., 1990; Bischoff and Postingl, 1991), in T. gondii, TgRan localized throughout the parasite cell with no clear predilection for the nucleus. TgRan localized in the nucleus when expressed in BHK cells, suggestive of a parasite specific factor(s) altering its location. TgRan shared three other critical similarities with eukaryotic Ran orthologs: binding to GTP, GTPase activity, and interaction with RCC1. These data demonstrated that TgRan is indeed the Ran ortholog in T. gondii. The Ran network in T. gondii allowed expression of dominant lethal forms of TgRan, G19V and T24N. To our knowledge, this is the first report of the stable expression of these Ran mutants. These observations suggest that the TgRan network is unlike late branching eukaryotes. While expression of TgRan T24N in wild type parasites did not result in a growth defect in normal tissue culture conditions, TgRan G19V expression caused a reduction in parasite proliferation when expressed in wild type parasites (Fig. S4). This growth defect was not observed when TgRan G19V was expressed in the TgRCC1 mutant 73F9. However, 73F9 parasites expressing TgRan G19V were not restored in virulence (data not shown). The deleterious cellular effects of TgRan G19V expression likely mask any positive effects in 73F9 parasites.
T. gondii allowed sustained, albeit low-level expression of TgRan T24N. In BHK cells, Ran T24N is found within and around the nucleus (Lounsbury et al., 1996). Both in vitro and in Xenopus extracts, Ran T24N binds to RCC1 and inactivates it (Dasso et al., 1994; Klebe et al., 1995). In T. gondii, TgRan T24N is largely located in a ring surrounding the nucleus. Interaction between TgRan T24N and TgRCC1 was not detected by immunoprecipitation (data not shown), but this may be due to the low expression of TgRan T24N. We did not observe any up regulation of TgRCC1 in parasites expressing TgRan T24N as a means to compensate for the dominant negative effects (data not shown). Perhaps T. gondii is able to tolerate low-level expression of TgRan T24N because it is not located in the nucleus to inactivate TgRCC1, or due to the divergence of TgRCC1. It is interesting how such a low-level of TgRan T24N can attenuate parasites in serum-starved host cells and mice. It is possible that TgRan T24N is having its deleterious effects on parasite pathogenesis by blocking the Ran-specific transporter, NTF2, in the nuclear envelope (Ribbeck et al., 1998). This inhibition would likely decrease the overall rate of nuclear trafficking, which we speculate only has harmful effects on the parasite during nutrient limitation in vitro and in vivo.
With the differences in the Ran network between T. gondii and other eukaryotes, it is a mystery how T. gondii maintains a Ran gradient. The spatial separation of RCC1 in the nucleus and RanGAP in the cytosol argued for a steep gradient of RanGTP in the nucleus and RanGDP in the cytosol. RanGAP does not appear to be encoded in any sequenced apicomplexan, either because it is highly divergent or the protein does not exist. If RanGAP activity were not present, GTP to GDP hydrolysis would rely on the slow intrinsic GTPase activity of TgRan. Alternatively, to compensate for the absence of a RanGAP, TgRan could have a higher intrinsic GTPase activity, although we have shown that the rate of TgRan GTP hydrolysis is similar to human Ran (Fig. 2A). Preliminary work has identified two protein partners of TgRan and neither has sequence identity to RanGAP (M.B. Frankel and L.J. Knoll, unpublished data). Future work will determine if these protein partners increase the slow intrinsic GTPase activity of TgRan. Dissection of these alternative nuclear transport components may shed light on the evolution of these processes. The divergence of the Ran network in parasites highlights that this pathway could represent a target for new therapeutic agents.
Experimental procedures
Cell culture and transfections
Propagation of parasites was done in human foreskin fibroblast (HFF) monolayers at 37° with 5% CO2 under standard conditions (Ware and Kasper, 1987). All parasites were derived from PruΔ (hypoxanthine-xanthine-guanosine phosphoribosyl transferase deletion) strain. T. gondii electroporations were performed with 50 μg linear plasmid. Pyrimethamine selections were performed in 1μM final concentration. Growth rates of T. gondii in serum starved host cells were measured as described previously (Frankel et al., 2007).
TsBN2 cells were maintained at 33°C, 5% CO2 in DMEM supplemented with 10% fetal bovine serum. Cells were transfected using the TransIT®-LT1 transfection reagent (Mirus, Madison, WI) according to manufacture's protocol. 24 hours post-transfection, cells were switched to 40°C and growth was monitored visually over two weeks. Confluent cells were passaged and examined by western immunoblotting. Complemented TsBN2 cells were maintained at 40°C unless otherwise stated.
Western immunoblotting and Northern blots
SDS-PAGE gels were transferred to either PVDF (Immobilon P, Millipore) or nitrocellulose (Hybond, Amersham) for subsequent analysis. Anti-HA antibodies (Zymed) were used at a 1:1000 dilution, anti-TgRCC1 at 1:500 (Frankel et al., 2007), anti-RCC1 at 1:500 (BioLegend), anti-FLAG at 1:1000 (Sigma), anti-Ran at 1:2000 (BD Biosciences), anti-actin at 1:5000 (Calbiochem).
For Northern blots, total RNA was isolated using ULTRASPEC (BIOTECX) following manufacturer's protocol. Parasites were force lysed from the host cell by syringing through a 27-gauge needle twice. RNA was run on a 0.8% formaldehyde gel. The gel was transferred to a Zeta-probe® blotting membrane (BioRad) followed by prehybridization (50% formamide, 0.12M Na2HPO4, 0.25M NaCl, 7% w/v SDS, 1mM EDTA) for 4-6 hours. Radiolabeled DNA was hybridized overnight at 42°C. The blot was then washed in 3X SSC, 0.1% SDS; 2X SSC, 0.1% SDS; 1X SSC, 0.1% SDS; 0.1X SSC, 0.1% SDS; all washes were performed at 42°C for 30 minutes each. For FLAG specific probing, the primer 5′-CTTGTCGTCGTCGTCCTTGTAGTC-3′ was labeled with α-P32 ATP using T4 PNK (NEB) according to manufacturer's protocol. Hybridization was performed at 50°C overnight, followed by 4 five minute washes using the same buffers as noted above.
Immunofluorescence assays
For intracellular parasite immunofluorescence assays, parasites were allowed to invade HFF cells on coverslips for 24 hours before they were fixed in 3% formaldehyde for 20 minutes. Parasites and TsBN2 cells were stained as described previously (Frankel et al., 2007) except anti-Ran was used at a 1:1000 dilution (BD Biosciences). To avoid excessive signal from host cell Ran, we analyzed TgRan in extracellular parasites. For extracellular parasite immunofluorescence assays, parasites were force lysed through a 27-gauge needle twice and allowed to settle by gravity for 20-30 minutes onto a coverslip in a 24-well plate. The parasites were naturally adherent to the coverslips. Media was removed and parasites were gently washed once with PBS, then fixed with 3% formaldehyde for 20 minutes. Rabbit anti-FLAG antibodies were used at a 1:1000 dilution, mouse anti-FLAG at 1:200, rabbit anti-H2B (1:500), and rabbit anti-Hsp90 (1:1000). TgRan was detected with Alexafluor 488 secondary antibodies, while H2B and Hsp90 was detected by Alexafluor 633 secondary antibodies (Invitrogen). All T. gondii images were captured at 100X and TsBN2 cells at 40X as described previously (Frankel et al., 2007).
Nuclear isolation, NaCl treatment, and immunoprecipitation
For NaCl treatment, nuclei from parasites expressing a HA-epitope tagged version of TgRCC1 (Frankel et al., 2007) were isolated and treated according to Cushman et al. (Cushman et al., 2004). Briefly, cells were gently lysed on ice and spun to isolate intact nuclei. The nuclei were then treated with varying concentrations of NaCl on ice for 15 minutes, followed by centrifugation to isolate the supernatant and pellet fractions. Nuclei were used for all immunoprecipitations. Nuclei were isolated from approximately 5 × 108 parasites as previously described (Kibe et al., 2005). Anti-HA (Zymed) or anti-Ran (BD Biosciences) antibodies were used in accordance with the Catch and Release immunoprecipitation kit (Upstate). Lysates were separated by SDS-PAGE and subsequent western blot analysis as described above.
For in vitro IPs, similar protein concentrations of TgRan and TgRCC1 were incubated in standard buffer (64mM Tris pH 7.4, 5mM MgCl2, 100mM NaCl) for two hours at 4°C rotating. Anti-Ran antibodies were added and incubated an additional two hours, followed by addition of protein A sepharose, and incubated for two hours. Tubes were then centrifuged and washed three times. Bound material was then separated by SDS-PAGE, transferred to nitrocellulose and probed with anti-HIS antibodies to detect both purified TgRan and TgRCC1.
TgRCC1 and TgRan expression plasmids
Site directed mutations were introduced using QuikChange mutagenesis (Stratagene) and PCR products were cloned into the pCR2.1-TOPO (Invitrogen) before subcloning using endogenous restriction enzyme sites. All plasmids are described in Table S1, and primers used are listed in Table S2.
Truncated TgRCC1 and TgRan purification, GEF and GTPase activity assays
The truncated TgRCC1, pMF157, was electroporated into the Rosetta E. coli strain. Cells were grown overnight, then diluted into 500mL of LB broth and grown for an additional 3 hours before protein induction with 1mM IPTG for 3 hours at 37°C. Purification of TgRCC1 was performed from the insoluble fraction in 6M Urea in 64mM Tris pH 7.4, 5mM MgCl2, 100mM NaCl Novagen using His-Bind® Quick 900 Nickel columns according to manufacturer's protocol. Protein was eluted in this buffer containing 100mM immidazole, dialyzed to remove the immidazole and Urea, then flash frozen in liquid nitrogen. RanGEF assays were performed as previously described (Klebe et al., 1993).
For TgRan purification, plasmid pMF141 was electroporated into the Rosetta E. coli strain. Cells were grown overnight, diluted 1:100, grown for 3 hours at 37°C, and then induced by addition of 1mM IPTG for 3 hours at 37°C. Purification of recombinant protein was performed using Novagen His-Bind® Quick 900 Nickel columns according to manufacturer's protocol. Proteins were eluted in 64mM Tris pH 7.4, 1mM MgCl2, 100mM NaCl, and 250mM immidazole. Fractions were dialyzed to remove the immidazole and flash frozen in liquid nitrogen.
For intrinsic Ran GTPase assays, approximately 1 μM of TgRan or HsRan (Human, positive control) was incubated with 10 μM [γ-32P] GTP in the presence of 20mM EDTA. Excess radioactivity was removed by applying samples to a sephadex G-50 prepacked column equilibrated with incubation buffer (64mM Tris pH 7.4, 1mM MgCl2, 100mM NaCl). Samples were brought up to 300 μl, incubated at 37°C and 50 μl aliquots were vacuum filtered through nitrocellulose. Filters were washed in ice-cold reaction buffer and analyzed by a scintillation counter for the amount of 32P present on Ran.
Mouse infections
Female CBA/J mice 7-8 weeks old (JAX) were intraperitoneal (i.p.) injected with 2 × 104 parasites and time of death was monitored over 22 days. Mice that were moribund were euthanized. Plaque counts were performed immediately after infection to insure correct and equal dosages. Four mice each were used per strain per experiment, and was repeated at least twice.
Acknowledgments
We sincerely thank Ian Macara for the TsBN2 cell line, and the PK-FLAG and human RCC1 expression plasmids. We are grateful to Fred Porter and Ann Palmenberg for providing purified recombinant human Ran, Robert Striker for providing transfection reagents, and Spencer Hoover for technical assistance with the TsBN2 transfections. T. gondii-specific histone H2B antiserum was kindly provided by Carolina Dalmasso, and anti Hsp90 antisera was a generous gift from Sergio Angel. Thanks to Mary Dasso and Christiane Wiese for insightful discussions and helpful suggestions, as well as Angela Pollard for critical evaluation of this manuscript. This research was supported by NIH Award A1054603.
References
- Azuma Y, Renault L, Garcia-Ranea JA, Valencia A, Nishimoto T, Wittinghofer A. Model of the ran-RCC1 interaction using biochemical and docking experiments. J Mol Biol. 1999;289:1119–1130. doi: 10.1006/jmbi.1999.2820. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Seino H, Seki T, Uzawa S, Klebe C, Ohba T, Wittinghofer A, Hayashi N, Nishimoto T. Conserved histidine residues of RCC1 are essential for nucleotide exchange on Ran. J Biochem (Tokyo) 1996;120:82–91. doi: 10.1093/oxfordjournals.jbchem.a021397. [DOI] [PubMed] [Google Scholar]
- Belhumeur P, Lee A, Tam R, DiPaolo T, Fortin N, Clark MW. GSP1 and GSP2, genetic suppressors of the prp20-1 mutant in Saccharomyces cerevisiae: GTP-binding proteins involved in the maintenance of nuclear organization. Mol Cell Biol. 1993;13:2152–2161. doi: 10.1128/mcb.13.4.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991;354:80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991;88:10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KL, Richards SA, Lounsbury KM, Macara IG. Evidence using a green fluorescent protein-glucocorticoid receptor chimera that the Ran/TC4 GTPase mediates an essential function independent of nuclear protein import. J Cell Biol. 1996;133:985–996. doi: 10.1083/jcb.133.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman I, Stenoien D, Moore MS. The dynamic association of RCC1 with chromatin is modulated by Ran-dependent nuclear transport. Mol Biol Cell. 2004;15:245–255. doi: 10.1091/mbc.E03-06-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso MC, Echeverria PC, Zappia MP, Hellman U, Dubremetz JF, Angel SO. Toxoplasma gondii has two lineages of histones 2b (H2B) with different expression profiles. Mol Biochem Parasitol. 2006;148:103–107. doi: 10.1016/j.molbiopara.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993;18:96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Dasso M, Seki T, Azuma Y, Ohba T, Nishimoto T. A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. Embo J. 1994;13:5732–5744. doi: 10.1002/j.1460-2075.1994.tb06911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore B, Ciciarello M, Lavia P. Mitotic functions of the Ran GTPase network: the importance of being in the right place at the right time. Cell Cycle. 2004;3:305–313. [PubMed] [Google Scholar]
- Drivas GT, Shih A, Coutavas E, Rush MG, D'Eustachio P. Characterization of four novel ras-like genes expressed in a human tetracarcinoma cell line. Mol Cell Biol. 1990;10:1793–1798. doi: 10.1128/mcb.10.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria PC, Matrajt M, Harb OS, Zappia MP, Costas MA, Roos DS, Dubremetz JF, Angel SO. Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. J Mol Biol. 2005;350:723–734. doi: 10.1016/j.jmb.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Frankel MB, Mordue DG, Knoll LJ. Discovery of parasite virulence genes reveals a unique regulator of chromosome condensation 1 ortholog critical for efficient nuclear trafficking. Proc Natl Acad Sci U S A. 2007;104:10181–10186. doi: 10.1073/pnas.0701893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, Szatanek T, Flynn J, Parrot B, Radke J, Striepen B, White MW. Forward Genetic Analysis of the Apicomplexan Cell Division Cycle in Toxoplasma gondii. PLoS Pathog. 2008;4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibe MK, Coppin A, Dendouga N, Oria G, Meurice E, Mortuaire M, Madec E, Tomavo S. Transcriptional regulation of two stage-specifically expressed genes in the protozoan parasite Toxoplasma gondii. Nucleic Acids Res. 2005;33:1722–1736. doi: 10.1093/nar/gki314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe C, Nishimoto T, Wittinghofer F. Functional expression in Escherichia coli of the mitotic regulator proteins p24ran and p45rcc1 and fluorescence measurements of their interaction. Biochemistry. 1993;32:11923–11928. doi: 10.1021/bi00095a023. [DOI] [PubMed] [Google Scholar]
- Klebe C, Bischoff FR, Ponstingl H, Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- Kornbluth S, Dasso M, Newport J. Evidence for a dual role for TC4 protein in regulating nuclear structure and cell cycle progression. J Cell Biol. 1994;125:705–719. doi: 10.1083/jcb.125.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury KM, Richards SA, Carey KL, Macara IG. Mutations within the Ran/TC4 GTPase. Effects on regulatory factor interactions and subcellular localization. J Biol Chem. 1996;271:32834–32841. doi: 10.1074/jbc.271.51.32834. [DOI] [PubMed] [Google Scholar]
- Macara IG. Transport into and out of the nucleus. Microbiol Mol Biol Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS. Ran and nuclear transport. J Biol Chem. 1998;273:22857–22860. doi: 10.1074/jbc.273.36.22857. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Scott-Weathers CF, Tobin CM, Knoll LJ. A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages. Mol Microbiol. 2007;63:482–496. doi: 10.1111/j.1365-2958.2006.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemergut ME, Macara IG. Nuclear import of the ran exchange factor, RCC1, is mediated by at least two distinct mechanisms. J Cell Biol. 2000;149:835–850. doi: 10.1083/jcb.149.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG. Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B. Science. 2001;292:1540–1543. doi: 10.1126/science.292.5521.1540. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Okazaki H, Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989;109:1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Yoshida T, Seino H, Nishitani H, Clark KL, Sprague GF, Jr, Frasch M, Nishimoto T. Mutation of the hamster cell cycle gene RCC1 is complemented by the homologous genes of Drosophila and S.cerevisiae. Embo J. 1991;10:1265–1273. doi: 10.1002/j.1460-2075.1991.tb08068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Drivas G, D'Eustachio P, Rush MG. Ran/TC4: a small nuclear GTP-binding protein that regulates DNA synthesis. J Cell Biol. 1993;120:313–323. doi: 10.1083/jcb.120.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault L, Kuhlmann J, Henkel A, Wittinghofer A. Structural basis for guanine nucleotide exchange on Ran by the regulator of chromosome condensation (RCC1) Cell. 2001;105:245–255. doi: 10.1016/s0092-8674(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Renault L, Nassar N, Vetter I, Becker J, Klebe C, Roth M, Wittinghofer A. The 1.7 A crystal structure of the regulator of chromosome condensation (RCC1) reveals a seven-bladed propeller. Nature. 1998;392:97–101. doi: 10.1038/32204. [DOI] [PubMed] [Google Scholar]
- Ribbeck K, Lipowsky G, Kent HM, Stewart M, Gorlich D. NTF2 mediates nuclear import of Ran. Embo J. 1998;17:6587–6598. doi: 10.1093/emboj/17.22.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Sekiguchi T, Nishitani H, Miyauchi K, Ohtsubo M, Nishimoto T. Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol Cell Biol. 1990;10:577–584. doi: 10.1128/mcb.10.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware PL, Kasper LH. Strain-specific antigens of Toxoplasma gondii. Infect Immun. 1987;55:778–783. doi: 10.1128/iai.55.3.778-783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen NR, Blobel G. Two distinct classes of Ran-binding sites on the nucleoporin Nup-358. Proc Natl Acad Sci U S A. 1999;96:5516–5521. doi: 10.1073/pnas.96.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]