Abstract
The canonical transient receptor potential (TRPC) family of ion channels is implicated in many neuronal processes including calcium homeostasis, membrane excitability, synaptic transmission and axon guidance. TRPC channels are postulated to be important in the functional neurobiology of the enteric nervous system (ENS); nevertheless, details for expression in the ENS are lacking. RT-PCR, Western blotting, and immunohistochemistry were used to study the expression and localization of TRPC channels. We found mRNA transcripts, protein on Western blots and immunoreactivity (IR) for TRPC1/3/4/6 expressed in the small intestinal ENS of adult guinea pigs. TRPC1/3/4/6-IR was localized to distinct subpopulations of enteric neurons and was differentially distributed between the myenteric and submucosal divisions of the ENS. TRPC1-IR was widely distributed and localized to neurons with cholinergic, calretinin, and nitrergic neuronal immunochemical codes in the myenteric plexus. It was localized to both cholinergic and non-cholinergic secretomotor neurons in the submucosal plexus. TRPC3-IR was found only in the submucosal plexus and was expressed exclusively by neuropeptide Y-IR neurons. TRPC4/6-IR was expressed in only a small population of myenteric neurons, but was abundantly expressed in the submucosal plexus. TRPC4/6-IR was coexpressed with both cholinergic and nitrergic neurochemical codes in the myenteric plexus. In the submucosal plexus, TRPC4/6-IR was expressed exclusively in non-cholinergic secretomotor neurons. No TRPC1/3/4/6-IR was found in calbindin-IR neurons. TRPC3/4/6-IR was widely expressed along varicose nerve fibers and colocalized with synaptophysin-IR at putative neurotransmitter release sites. Our results suggest important roles for TRPC channels in ENS physiology and neuronal regulation of gut function.
Keywords: neurogastroenterology, TRP channels, gastrointestinal tract, myenteric plexus, submucosal plexus
The transient receptor potential (TRP) channel superfamily comprises a group of non-selective cation channels that is widely expressed in vertebrates. Transmembrane movement of cations down their electrochemical gradients through these channels elevates intracellular Ca2+ and Na+ concentrations, which depolarizes the resting membrane potential. Of the six mammalian TRP subfamilies (TRPA1, TRPC, TRPM, TRPML, TRPP and TRPV), TRPC channels share the most sequence homologies with the first TRP channel originally cloned from Drosophila photoreceptors (Clapham et al., 2005). To date, seven TRPC subunits have been cloned. Based on their sequence homology as well as functional similarities, the seven members of the TRPC channel subfamily can be divided into four subgroups: TRPC1; TRPC4/5; TRPC3/6/7; and TRPC2 (Clapham et al., 2005). TRPC proteins function as both store-operated and receptor-operated non-selective cation channels. Activation of TRPC channels occurs down a transduction cascade following stimulation of phospholipase C (PLC), either through Gq/11-coupled receptors or receptor tyrosine kinases (Putney, 2005). PLC catalyzes the hydrolysis of phosphatidylinositol 4, 5-bisphosphate to membrane-bound diacylglycerol (DAG) and soluble inositol trisphosphate (IP3). Three possible mechanisms have been proposed for downstream opening of TRPC channels: 1) activation by IP3 either directly or indirectly by Ca2+ released from intracellular stores (Zhu et al., 1996; Zitt et al., 1996; Kiselyov et al., 1998); 2) activation by DAG independent of Ca2+ (Hofmann et al., 1999; Okada et al., 1999); 3) translocation of the TRPC channel proteins from intracellular vesicles to plasma membrane (Bezzerides et al., 2004; Cayouette et al., 2004).
TRPC channels have diverse functional roles. Most of the TRPC subunits are expressed prominently in the brain. TRPC5 is implicated as a regulator of neurite outgrowth and axonal pathfinding for maturing hippocampal neurons (Greka et al., 2003). TRPC3 and TRPC6 are suggested to be involved in brain-derived neurotrophic factor-induced chemo-attraction of axonal growth cones (Li et al., 2005) and to promote neuronal survival (Jia et al., 2007). TRPC4 is involved in regulation of release of gamma amino butyric acid from the dendrites of thalamic interneurons (Munsch et al., 2003). TRPC1 or TRPC1-containing heteromultimers underlie metabotropic glutamate receptor-mediated excitatory synaptic transmission in cerebellar Purkinje neurons (Kim et al., 2003), in dopamine neurons in the substantia nigra (Bengston et al., 2004), and in pyramidal neurons in the lateral amygdala (Faber et al., 2006). TRPC2 is distinct from other TRPC subfamily members. It is a pseudogene in human (Vannier et al., 1998). In rodents, TRPC2 participates in pheromone sensory signaling and sperm fertilization (Jungnickel et al., 2001; Keverne, 2002). In the cardiovascular system, TRPC6 is responsible for α1-adrenoceptor-activated Ca2+ entry (Inoue et al., 2001) and TRPC4 is involved in the regulation of vascular tone and vascular permeability (Freichel et al., 2001; Tiruppathi et al., 2002). Several TRPC subunits are found in smooth muscle of the gastrointestinal tract and the interstitial cells of Cajal (Walker et al., 2001; 2002). TRPC4, TRPC6 and TRPC7 are predominant in smooth muscle fibers in the small and large intestine of dogs and mice (Walker et al., 2001), and are suggested to carry the nonselective cation currents activated by muscarinic receptors (Zholos et al., 2004). Finally, TRPC4 is implicated in the generation of pacemaker current in interstitial cells of Cajal (Walker et al., 2002).
The enteric nervous system (ENS) is the third division of the autonomic innervation of the digestive tract, which behaves like an independent integrative nervous system. It is comprised of two major ganglionated plexuses: the myenteric plexus, which contains the motor neurons that innervate muscularis externa, and the submucosal plexus, which contains the motor neurons that innervate the secretory glands and blood vasculature. A role for TRP channels in ENS signaling is a reasonable hypothesis. Many Gq protein-coupled receptors are expressed by ENS neurons and are known to active phospholipase C signal transduction pathways. Among these are the bradykinin B2 receptor (Hu et al., 2004), group 1 metabotropic glutamate receptors (Hu et al., 1999; Liu & Kirchegessner, 2000), muscarinic M1 receptor (North et al., 1985; Galligan et al., 1989), protease activated receptors (PARs) (Gao et al., 2002), purinergic P2Y1 receptor (Hu et al., 2003; Monro et al., 2004), and tachykinin receptors (Johnson and Bornstein, 2004). Given the functional connection between the Gq-PLC pathway and the activation of TRPC channels, it seems likely that, if TRPC channels are expressed in enteric neurons, they might play important roles in ENS cellular neurophysiology. Little is known at present about the expression and function of TRPC channels in the ENS. In the present study, we used RT-PCR, Western blot, and immunohistochemistry to examine the expression of TRPC1−7 in the ENS of the guinea pig ileum. The rational for an initial focus on guinea pig ileum is that neurochemical coding and functional identification of neurons are best established for this part of the gut in this species (Furness, 2006). A study of TRPC expression in the different neurochemically identified subclasses of enteric neurons in the myenteric and submucosal plexuses can be a starting point for associating one or the other of the 7 subtypes of TRPCs with functional classes of enteric neurons, which include musculomotor neurons, secretomotor neurons, vasculomotor neurons and interneurons. Expression of the channels by enteric neurons with specific functions might prove to be important for understanding certain digestive disorders as is suggested by findings for other systems in which mutation-related channelopathies in at least four TRP channels underlie disease (Trebak, 2006; Mukerji et al., 2007; Venkatachalam & Montell, 2007).
MATERIALS AND METHODS
Animal and Tissue Preparation
Male albino Hartley guinea pigs (300 − 400 g; Charles River, Wilmington, MA) were euthanized by stunning and exsanguination from the cervical vessels. The procedures were approved by The Ohio State University Laboratory Animal Care and Use Committee and United State Department of Agriculture inspectors. The small intestine was removed 10 cm proximal to the ileocecal junction and placed in chilled Krebs’ solution containing (in mM): NaCl, 120.9; KCl, 5.9; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 14.4; CaCl2, 2.5; and glucose, 11.5. Longitudinal muscle-myenteric plexus (LMMP) and submucosal plexus (SMP) preparations were microdissected by methods described in detail elsewhere (Wood & Mayer, 1978; Zafirov et al., 1991). Myenteric ganglia were isolated by enzymatic digestion as described by Xia et al (1991). About 80−100 myenteric ganglia were used for RT-PCR and Western blotting analysis. The brain was carefully removed, washed with artificial cerebrospinal fluid and placed in a 50 ml tube. All preparations were snap-frozen in liquid nitrogen and stored in −70°C for later analysis.
For immunohistochemical studies, segments of ileum were removed and placed in chilled Krebs’ solution containing 1 μM scopolamine and nifedipine to minimize muscle contraction and permit maximal stretching of the preparations. Small intestinal specimens were opened along the mesenteric border, stretched tautly, pinned out flat with mucosa side up to Sylgard® at the bottom of the dish. Preparations that were not to be treated with colchicine were immediately fixed in Zamboni's fixative (4% formaldehyde plus 0.2% picric acid in 0.1 M sodium phosphate buffer, pH 7.0) for 3 hours at room temperature. To enhance somal immunostaining for vasoactive intestinal peptide (VIP), some preparations were incubated in Dulbecco's Modification of Eagle's Medium (DMEM) containing 40 μM colchicine at 37°C for 4 hours prior to fixation. After fixation, tissues were subsequently washed (3 × 10 minutes) in phosphate-buffered saline (PBS; 0.9% NaCl in 0.1 M sodium phosphate buffer, pH 7.0). Whole-mounts of the LMMP and SMP were dissected from these segments.
RNA Isolation and RT-PCR
Frozen specimens were thoroughly pulverized with a mortar and pestle on dry ice. The powder was transferred to a 1.5 ml tube and Trizol Reagent (Life Technology, Gaithersburg, MD) was added to extract and precipitate RNA according to the protocol of the manufacturer. All samples were treated with DNase (Invitrogen, Carlsbad, CA) for 2 h at 37°C, extracted, and precipitated again. First-strand cDNA was synthesized from 1 μg of total RNA using random hexamer primer and AMV reverse transcriptase (Roche Diagnostics Corp., Indianapolis, IN). The cDNA was amplified by PCR using the TRPC isoform-specific primers published previously (Ong et al., 2003). Amplification was carried out using Taq DNA polymerase and an iCycler™ (Bio-Rad Laboratories, Hercules, CA). 5.0 μl of cDNA was added to 25 μl of PCR master mix (Roche Diagnostics Corp., Indianapolis, IN), 2.0 μl of TRPC isoform-specific primers and 18 μl of PCR grade sterile water. PCR cycles consisted of denaturation for 1 minute at 94°C, annealing at 56°C for 2 minutes, and extension at 72°C for 3 minutes, respectively, and the reactions were repeated for 35 cycles, followed by final extension at 72°C for 7 minutes. Amplification products were separated on a 2% agarose gel, stained with ethidium bromide, and viewed under ultra-violet light. RT-PCR was also performed on aliquots of RNA for each sample in which reverse transcriptase was not added during the cDNA synthesis step to confirm that there was no genomic DNA contamination. About 80 myenteric ganglia were used for RNA isolation and 1 μg total RNA was used for RT-PCR.
RT-PCR controls were done to check the ganglion samples for contamination with smooth muscle RNA. Primers specific for guinea pig smooth muscle myosin beta heavy chain were used to assess the purity of the isolated myenteric ganglia. We did not find mRNA for smooth muscle myosin beta heavy chain in the isolated myenteric ganglia, which ruled out the possibility of contamination by smooth muscle cells. The same pair of primers detected a band in RNA isolated from circular muscles (Suppl. Fig. 1).
Western Blotting Analysis
Membrane proteins were extracted from brain, small intestinal LMMP and SMP preparations, and isolated myenteric ganglia. Frozen specimens were crushed to powder using a liquid nitrogen-cooled biopulverizer unit (Research Products International, Philadelphia, PA) and were homogenized in 350 μl lysing buffer [20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% NP-40, 0.25% deoxycholate, 1 mM sodium orthovanadate, 1 mM PMSF, 1 mM NaF, with Complete Mini EDTA-free protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN)]. Homogenates were continually incubated in lysing buffer on ice for 1 h and subsequently centrifuged at 10,000 g for 20 min at 4°C to obtain the cell membrane fraction in the supernatant. Protein levels were determined with the use of the detergent-compatible protein assay system (Bio-Rad Laboratories, Hercules, CA), and 40 μg of protein per lane was resolved by gel electrophoresis followed by their transfer to the nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked with 5% nonfat milk in Tris·HCl-buffered saline (TBS) for 1 h at room temperature. After being washed with TBS, the membranes were incubated overnight at 4°C with primary antibodies for TRPC1/3/4/5/6 (1:200; Alomone Labs), or TRPC7 (1:200; Chemicon). After washing, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000; Amersham Biosciences, Piscataway, NJ). The immunoblots were detected with enhanced chemiluminescence reagents (Amersham Biosciences).
Immunofluorescence
Sources of all primary and secondary antibodies, as well as the optimized dilutions used in this study, are listed in Tables 1 and 2. To minimize nonspecific binding and to permeabilize the tissue, preparations were placed in PBS containing 10% normal donkey serum and 0.3% Triton X-100 for 30 minutes at room temperature. The tissue was incubated in primary antibodies for TRPC1/3/4/5/6/7 overnight at 4°C. After washing in PBS, the tissues were incubated in indocarbocyanin (Cy3)-labeled donkey anti-rabbit IgG at room temperature for 1 hour. The tissue was washed in PBS and coverslipped with VECTASHIELD Mounting Medium (Vector lab, Burlingame, CA), which inhibits photobleaching. Fluorescence labeling was examined under a Nikon Eclipse 90i fluorescence microscope (Fryer Company, Cincinnati, OH).
TABLE 1.
Primary Antibodies Used in the Study
| Antibody | Immunizing antigen | Host species | Dilution | Source/Cat. No./Lot No. |
|---|---|---|---|---|
| HuC/D | 12 amino acid synthetic peptide representing amino acids 240−251 from human HuD (monoclonal 16A11) | Mouse | 1:50 | Mol. Probes/A21271/53877A |
| Calbindin | Recombinant rat calbindin D-28K | Rabbit | 1:3,000 | Swant/CB38/9.03 |
| Calretinin | Purified guinea pig calretinin protein | Goat | 1:2,500 | Chemicon/AB1550/23100764 |
| ChAT | 22 amino acid synthetic peptide from porcine ChAT, coupled to KLH. The peptide sequence is H-GLFSSYRL PGHTQDTL VAQKSS-NH2 | Goat | 1:100 | Chemicon/AB144P/LV35940 |
| NeuN | Purified cell nuclei from mouse brain | Mouse | 1:500 | Chemicon/MAB377/22040372 |
| NOS | 21 amino acid peptide (1409−1429) of the neuronal form of NOS from rat cerebellum, coupled to KLH. The sequence is H-RSESIAFIEESKKD ADEVFSS-NH2 | Sheep | 1:1,000 | Chemicon/AB1529/0703053959 |
| NPY | Synthetic NPY peptide conjugated to bovine thyroglobulin | Sheep | 1:5,000 | Chemicon/AB1583/0703053959 |
| Synaptophysin | Protein p386 from crude fractions of coated vesicles from bovine brain | Mouse | 1:200 | Dako/M0776/00017473 |
| TRPC1 | Peptide QLYDK GYTSK EQKDC, corresponding to amino acid residues 557−571 of human TRPC1 (Accession P48995) | Rabbit | 1:100 | Alomone/ACC-010/AN-04 |
| TRPC3 | Peptide HKLSE KLNPS VLRC, corresponding to residues 822−835 of mouse TRPC3 (Accession Q9QZC1) | Rabbit | 1:100 | Alomone/ACC-016/AN-03 |
| TRPC4 | Peptide (C)KEKH AHEED SSIDY DL, corresponding to residues 943−958 of mouse TRPC4 (Accession Q9QUQ5) | Rabbit | 1:100 | Alomone/ACC-018/AN-01 |
| TRPC5 | Peptide (C)HKWGDGQEEQVTTRL corresponding to residues 959−973 of human TRPC5 (Accession Q9UL62) | Rabbit | 1:100 | Alomone/ACC-020/AN-07 |
| TRPC6 | Peptide (C)RRNE SQDYL LMDELG, corresponding to residues 24−38 of mouse TRPC6 (Accession Q61143) | Rabbit | 1:100 | Alomone/ACC-017/AN-02 |
| TRPC7 | Peptide ILKEL SKEEE DTDSS EEMLA, corresponding to residues 658−677 of human TRPC7 (Accession NM_020389) | Rabbit | 1:100 | Chemicon/AB9326/VR1377347 |
| VIP | Synthetic peptide (1−28) coupled to KLH with glutaraldehyde | Sheep | 1:200 | Chemicon/AB1581/0611044826 |
Hu, anti-human neuronal protein; ChAT, choline-acetyltransferase; NeuN; neuronal nuclear protein; NOS; nitric oxide synthase; NPY, neuropeptide Y; TRPC, canonical transient receptor potential channel; VIP, vasoactive inhibitory peptide.
TABLE 2.
Secondary Antibodies Used in the Study
| Secondary antibody | Conjugated fluorophore | Company and catalog number | Dilution |
|---|---|---|---|
| Donkey anti-goat IgG | FITC | Jackson, 705−095−147 | 1:100 |
| Donkey anti-mouse IgG | FITC | Jackson, 715−095−150 | 1:100 |
| Donkey anti-rabbit IgG | Cy3 | Jackson, 711−165−152 | 1:500 |
| Donkey anti-sheep IgG | FITC | Jackson, 713−095−147 | 1:100 |
IgG, immunoglobulin G; FITC, fluorescine isothiocyanate; Cy3, indocarbocyanin.
Double labeling of TRPC with other specific neurochemical markers was used to identify the cell types that express TRPC subunits and performed in a sequential manner. The tissues were first incubated with the primary and secondary antibodies for the TRPC subunits as indicated above. Samples were examined under the fluorescence microscope to ensure the quality of the labeling. The tissues were then washed in PBS and subsequently incubated with the appropriate primary and secondary antibodies for the neurochemical markers. After a thorough rinse, the tissues were coverslipped with VECTASHIELD mounting medium and examined under a Nikon Eclipse 90i fluorescence microscope. In some cases, immunostaining was conducted with two different primary antisera that were both raised in rabbit (e.g. TRPC and calbindin). In these situations, tissues were first incubated overnight at room temperature in the primary antiserum for one of the TRPC subunits, and then placed in donkey anti-rabbit IgG conjugated to Cy3. The tissues were then rinsed and incubated with normal rabbit serum for 1 h to saturate any open antigen binding sites on the first, secondary antibody. After washing in PBS, the tissues were incubated with an excess of unconjugated Fab fragment of the donkey anti-rabbit IgG antibody for 1 h to block any remaining binding sites on the first rabbit primary antiserum. After another set of rinses, the tissues were incubated overnight in the second, primary antibody (e.g. calbindin), followed by a donkey anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC). As with other immunostained preparations, the tissues were then mounted on slides, coverslipped with VECTASHIELD mounting medium, and examined under a Nikon Eclipse 90i fluorescence microscope.
Antibody Characterization
Information on primary antibodies used in this study is summarized in Table 1. Further details regarding the specificity of each of the primary antibodies are given below.
Anti-TRPC1 antibody recognized a single, clear band at ∼ 100 kDa by Western blotting in HEK-293 cells, hTRPC1-overexpressing HEK-293 cells (Wu et al., 2002), and CHO cells transfected with hTRPC1 (Kim et al., 2003). The same antibody recognized a protein of ∼120 kDa by Western blotting in guinea pig liver and airway smooth muscle cells (Ong et al., 2002), guinea pig gallbladder smooth muscles (Morales et al., 2007), rat myometrium (Babich et al., 2004), rat brain and pulmonary arterial smooth muscle cells (Lin et al., 2004). Preadsorption of the antibody with its immunizing peptide abolished the immunoblots (Kim et al., 2003) and immunostaining (Castellano et al., 2003).
Anti-TRPC3 antibody identified a single band of ∼100 kDa by Western blotting in rat heart and brain membranes (Strübing et al., 2003; manufacturer's technical information), mice skeletal muscles (Rosenberg et al., 2004), human myometrial tissue (Dalrymple et al., 2002), and TRPC3-transfected HEK-293 cells (Wu et al., 2002). Preadsorption of the antibody with its immunizing peptide abolished the immunoblots (Dalrymple et al., 2002; Strübing et al., 2003; Rosenberg et al., 2004) and immunostaining ( Castellano et al., 2003; Fusco et al., 2004).
Anti-TRPC4 antibody stained a single band of ∼97 kDa on Western blotting of rat brain membranes (manufacturer's technical information), rat aorta, glomeruli, and preglomerular resistance vessels (Facemire et al., 2004), human myometrial tissue (Dalrymple et al., 2002), and TRPC4-transfected HEK-293 cells (Schaefer et al., 2002; Wu et al., 2002). Preadsorption of the antibody with its immunizing peptide abolished the immunoblots (Dalrymple et al., 2002; Facemire et al., 2004) and immunostaining (Castellano et al., 2003).
Anti-TRPC5 antibody stained a single band of ∼100 kDa on Western blotting of rat and mice brain membranes (Fowler et al., 2007; manufacturer's technical information), rat aorta, glomeruli, and preglomerular resistance vessels (Facemire et al., 2004), and TRPC5-transfected HEK-293 cells (Fowler et al., 2007). Preadsorption of the antibody with its immunizing peptide abolished the immunoblots (Facemire et al., 2004; Fowler et al., 2007) and immunostaining (De March et al., 2006).
Anti-TRPC6 antibody identified a single band of ∼100 kDa by Western blotting in rat brain membranes (Strübing et al., 2003), rat aorta, glomeruli, and preglomerular resistance vessels (Facemire et al., 2004), human neutrophil membranes (Itagaki et al., 2004), human myometrial tissue (Dalrymple et al., 2002), and TRPC6-overexpressing cells (Tseng et al., 2004). Preadsorption of the antibody with its immunizing peptide abolished the immunoblots (Dalrymple et al., 2002; Strübing et al., 2003; Facemire et al., 2004) and immunostaining (Calupca et al., 2002).
Anti-TRPC7 antibody identified a single band of ∼100 kDa by Western blotting in TRPC7-overexpressing cells (Zhang et al., 2003). Preadsorption of the antibody with its immunizing peptide abolished the immunoblots (Zhang et al., 2003) and immunostaining (Chung et al., 2007).
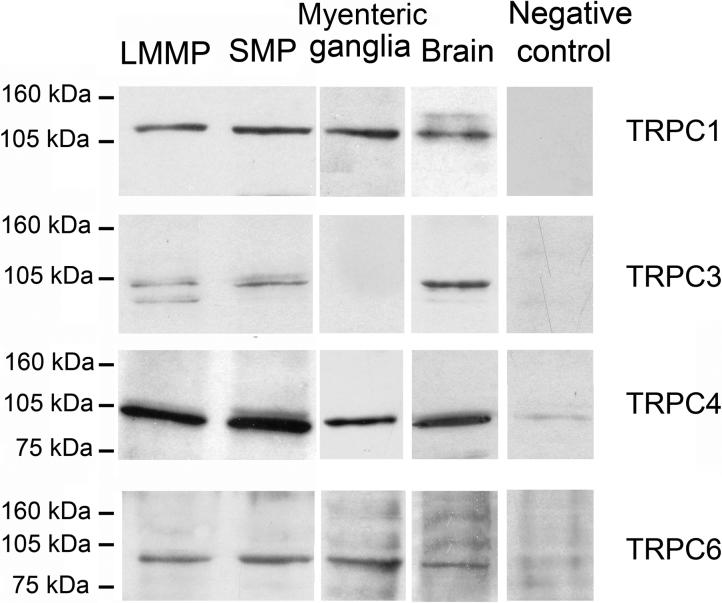
The specificity of the TRPC1/3/4/5/6/7 antibodies was also reconfirmed in guinea pig tissues by Western blotting (See Western Blotting Analysis section in MATERIALS AND METHODS). The TRPC antibodies recognized a single band of the expected molecular weights in guinea pig brain membrane protein extracts (Fig. 2). Preadsorption of the TRPC antibodies with the corresponding blocking peptides provided by the manufacturers abolished the immunoblots (Fig. 2). The TRPC antibodies were also tested by antibody preadsorption in immunofluorescence experiments (See Immunofluorescence section in MATERIALS AND METHODS). Preadsorption of the TRPC antibodies with the corresponding blocking peptides abolished immunostaining in the ENS (Fig. 3I-L).
Fig. 2.
TRPC protein expression in the guinea pig ENS. Western blot confirmed the expression of TRPC1 (120kDa), TRPC3 (∼95−100kDa), TRPC4 (∼95−100kDa), and TRPC6 (∼95−100kDa) proteins in LMMP and SMP of the guinea pig ileum, and TRPC1/4/6 proteins in dissociated myenteric ganglia. The immunoblots were at the same level as their corresponding positive controls (i.e., brain lysate). Immunoblots were either completely blocked or significantly reduced by preadsorbing the antibodies with their corresponding blocking peptides.
Fig. 3.
Immunohistochemical demonstration of TRPC subunits in the ENS. TRPC subunits were labeled with indocarbocyanine (Cy3, red). The pan neuronal marker HuC/D (Hu) was labeled with fluorescein isothiocyanate (FITC, green). TRPC1 immunoreactivity (IR) was expressed in a subpopulation of neurons in the myenteric (A) and submucosal (B) plexuses. Double-labeling with an antibody for Hu confirmed that all TRPC1-IR cells are immunoreactive for Hu (A’, B’). TRPC3-IR was not found in the myenteric plexus (C, C’), but was expressed in a small neuronal population in submucosal ganglia (D) and was colocalized with Hu-IR (D’). TRPC4-IR was found in neuronal cell bodies and nerve fibers in both the myenteric (E) and submucosal (F) plexuses. TRPC4-immunoreactive nerve cell bodies were colocalized with Hu (E’, F’). TRPC6- IR was found in neuronal cell bodies and nerve fibers in both the myenteric (G) and submucosal (H) plexuses. TRPC6-IR nerve cell bodies were colocalized with Hu-IR (G’, H’). Preadsorption with the corresponding blocking peptide decreased or abolished immunostaining as shown in the example for the submucosal plexus (I-L). BP, blocking peptide. Scale bar = 20 μm for all panels. Magenta-green copy of this figure is available as supplementary figure 2.
Anti-calbindin CB38 polyclonal antibody identified a single band of 28 kDa by Western blotting in the brain and kidney of wildtype (WT) mice; however, no signals were detected in calbindin knockout mice, whereas reduced staining was observed in heterozygous mice (Airaksinen et al., 1997). This antibody revealed the same population of neurons in the guinea pig stomach and ileum as stained by another anti-calbindin antibody (Swant 300, batch 17-F) raised in mouse against chicken intestinal calbindin (Reiche et al., 1999).
Anti-calretinin antibody recognized a protein of the expected molecular weight (∼31 kDa) by Western blotting in rat posterior pituitary (Miyata et al., 2000).
Anti-choline acetyltransferase (ChAT) antibody stained a single band corresponding to the predicted molecular weight of ChAT (68 kDa) on Western blot (Corcoran et al., 2004; Lee et al., 2007). Staining pattern of cellular morphology and distribution in the present work was the same as previously described for the ENS (Hu et al., 2002; Chiocchetti et al., 2003; Wang et al., 2005; Liu et al., 2006).
Anti-human neuronal protein C/D (HuC/D) monoclonal (clone 16A11) antibody labeled neuronal cell nuclei and perikarya (Marusich et al., 1994). Staining pattern of cellular morphology and distribution in the present work was the same as previously described for the ENS (Lin et al., 2002).
The monoclonal mouse anti-neuronal nuclear protein (NeuN) antibody (clone A60) recognized the neuron-specific protein NeuN, present in most CNS and peripheral nervous system neuronal cell types, of all vertebrates tested (see manufacturer's technical data sheet for list of species; Mullen et al., 1992). This antibody recognized four bands on Western blot (Unal-Cevik et al., 2004), and was thought to reflect multiple phosphorylation states of NeuN (Lind et al., 2005).
The nitric oxide synthase (NOS) antibody was raised against the C-terminal (amino acid 1409−1429) of the rat neuronal NOS (nNOS). No Western blotting data are available for this antibody. Nevertheless, as detailed in RESULTS, the nNOS antibody displayed the same pattern of cellular morphology and distribution as previously published for the ENS (Hu et al., 2002; Lin et al., 2005; Liu et al., 2005, 2006).
The specificity of the neuropeptide Y (NPY) antibody was determined by radioimmunoassay (RIA). The antibody cross-reacts 100% with NPY with less than 0.005% cross reactivity for somatostatin-14, leu-enkephalin, met-enkephalin, oxytocin, Agr8-vasopressin, VIP, angiotensin II and less than 0.02% cross reactivity for SP. Peptide YY and bovine pancreatic polypeptide showed approximately 36% and 0.05% cross reactivity respectively (manufacturer's technical information). Preadsorption with excess amount of NPY produced no staining in the ENS (data not illustrated).
Anti-synaptophysin antibody labeled a band of 38 kDa in Western blotting of a fraction of synaptic vesicles of bovine brain (manufacturer's technical information). Similar results were obtained with corresponding fractions from human, mouse and rat brain (Wiedenmann and Franke, 1985). Electron microscopy showed that the antibody almost exclusively labeled synaptic terminals in brain and spinal cord, and specifically decorated the cytoplasmic surfaces of the presynaptic 40−90 nm vesicles (Wiedenmann and Franke, 1985).
The sheep VIP antibody was raised against a synthetic peptide VIP (1−28). No Western blotting data are available for this antibody. Nevertheless, as detailed in RESULTS, the VIP antibody displayed the same pattern of cellular morphology and distribution as previously published for the ENS (Rühl et al., 2005; Wang et al., 2005; Liu et al., 2006).
Analysis of Immunohistochemically Labeled Preparations
Photomicrographs were acquired with a CoolSnap HQ2 monochrome digital camera mounted on a Nikon Eclipse 90i fluorescence microscope using the MetaMorph software (Molecular Devices, Sunnyvale, CA), stored on disk and analyzed with MetaMorph. Images were minimally adjusted for brightness and contrast using MetaMorph. All pictures for publication were imported into Adobe Photoshop CS2 (San Jose, CA) for alignment and labeling and saved as tiff files at 600 dpi.
Immunoreactive neurons for the TRPC subunits and the marker populations, as well as the number of double-labeled cells, were assessed in randomly chosen ganglia situated through out the preparations. Counts of double-labeled cells were assessed in three to six animals for each chemical marker. At least 30 ganglia in the myenteric plexus and 100 ganglia in the submucosal plexus were counted. Results are expressed as means ± SEM with n-values representing the numbers of animals studied.
RESULTS
Expression of TRPC mRNA Transcripts
The expression of TRPC mRNA transcripts was determined by performing RT-PCR on total RNA isolated from the SMP, LMMP, and dissociated myenteric ganglia. Total RNA extracted from the guinea pig brain was used as a positive control for the various TRPC primers to test their ability to produce the correct amplicon. Transcripts encoding TRPC1−7 were detected in the guinea pig brain (Fig. 1). The sizes of the bands correctly matched the expected lengths of the amplified fragments of each TRPC subunit (TRPC1, 400 bp; TRPC2, 370 bp; TRPC3, 340 bp; TRPC4, 370 bp; TRPC5, 484 bp; TRPC6 327 bp; TRPC7 429 bp) and were consistent with previous studies on guinea pig tissues (Ong et al., 2003). Transcripts encoding TRPC1/3/4/6/7 were found in the SMP preparations (Fig. 1). Bands representing TRPC2/5 were not detected in the SMP preparations. Transcripts encoding TRPC1−7 were all present in the LMMP preparations (Fig. 1), while transcripts encoding TRPC1−6 were also detected in dissociated myenteric ganglia (Fig. 1). RT-PCR performed on aliquots of RNA in which reverse transcriptase was omitted during the cDNA synthesis step resulted in no amplicons (results not shown).
Fig. 1.
Canonical transient receptor potential channel (TRPC) mRNA expression in the guinea pig enteric nervous system (ENS). RT-PCR analysis with primers specific for each of the TRPC subunits identified mRNA transcripts for TRPC1 through TRPC7 in the guinea pig brain (i.e., positive controls). Transcripts encoding TRPC1/3/4/6/7 were expressed in the submucosal plexus (SMP). Bands representing TRPC2/5 were not detected in the SMP preparations. Transcripts encoding TRPC1 to TRPC7 were all observed in the longitudinal muscle-myenteric plexus (LMMP) preparations, while transcripts encoding TRPC1 to TRPC6 were detected also in dissociated myenteric ganglia. Sizes of the bands matched the expected lengths of the amplified fragments of each TRPC subunit (TRPC1, 400 bp; TRPC2, 370 bp; TRPC3, 340 bp; TRPC4, 370 bp; TRPC5, 484 bp; TRPC6 327 bp; TRPC7 429 bp).
Expression of TRPC Proteins
Western blotting was done to investigate subtypes of TRPC proteins that might be expressed in the guinea pig ENS. We probed lysates from SMP and LMMP with specific anti-TRPC1/3/4/6 and were able to identify immunoblots for each of the proteins (Fig. 2). The immunoblots were located at the same level as their corresponding positive controls (i.e., lysates from the guinea pig brain). TRPC1/4/6, but not TRPC3 were also detected in lysates from the dissociated myenteric ganglia (Fig. 2). The immunoblot detected with TRPC1 antibody was ∼120 kDa, which is in the range previously reported for TRPC1 protein with this commercial antibody in guinea pig (Ong et al., 2002; Morales et al., 2007) and rat (Babich et al., 2004; Kunichika et al., 2004; Lin et al., 2004), but not human tissue (Dalrymple et al., 2002; Yang et al., 2002). Differences in the mobility of the protein between humans and other animal species may reflect species differences in the expression of TRPC1 such as glycosylation. For TRPC3/4/6, the molecular masses were ∼95−100 kDa. These sizes are in agreement with previous reports for TRPC3/4/6 (Dalrymple et al., 2002; Ong et al., 2003; Babich et al., 2004; Lin et al., 2004; Morales et al., 2007). The immunoblots for TRPC1/3/4/6 were either completely blocked or significantly reduced by antigen competition (Fig. 2), which confirmed signal specificity. TRPC5/7 were not detectable in the SMP or LMMP preparations (not shown). Expression of TRPC2 protein was not investigated due to lack of availability of a specific antibody.
Immunohistochemical Localization of TRPC Subunits
Localization of TRPC subunits was determined by indirect immunohistochemical staining in whole-mount preparations of the myenteric and submucosal plexuses. Immunoreactivity specific for TRPC1/4/6 was observed in the myenteric plexus (Fig. 3A, 3E, 3G), while immunoreactivity specific for TRPC1/3/4/6 was observed in the submucosal plexus (Fig. 3B, 3D, 3F, 3H). This was in agreement with the Western blot result (Fig. 2). Immunofluorescence was expressed clearly at the surfaces of the neuronal cell bodies and sometimes in the cytoplasm. Western blotting detected TRPC3 protein in the LMMP preparations (Fig. 2), with no TRPC3-IR being found in myenteric ganglia (Fig. 3C), which suggested that the signal detected in the LMMP preparations might have been protein from attached smooth muscle. Western blotting for isolated circular muscle strips confirmed expression of TRPC3 protein by the muscle (not shown). Immunoreactivity to TRPC1/3/4/6 either decreased or disappeared when the antiserum was preadsorbed with the corresponding immunizing peptide provided by the manufacturer (Fig. 3I-L). Preadsorption of each TRPC antibody with non-corresponding immunizing peptides was done to determine if this would abolish the immunostaining, which would be an indication of cross-reactivity among the TRPC antibodies. No cross-reactivity among the TRPC antibodies was found (results not illustrated). Immunostaining was also abolished by omitting either the primary or the secondary antibody (results not shown). These controls support confidence that the results presented below are specific.
Double labeling with an antibody specific for the pan neuronal marker HuC/D protein (Lin et al., 2002) confirmed that TRPC1/3/4/6-IR was localized to enteric neurons and did not show up on neighboring glial cells (Fig.3). TRPC1 was the most abundant subunit expressed in the myenteric plexus. TRPC1-IR nerve cell bodies accounted for 63.2 ± 7.8% of the total myenteric neuronal population, as assessed with Hu-IR. Few TRPC4/6-IR nerve cell bodies were observed in the myenteric plexus, which represented only 1.7 ± 0.1% and 1.5 ± 0.1% of the total myenteric neuronal population, respectively. In the submucosal plexus, TRPC1-IR neurons accounted for 67.7 ± 11.6% of the total submucosal neuronal population. TRPC3-IR nerve cell bodies accounted for 13.4 ± 3.6% of the total submucosal neuronal population. TRPC4/6-IR nerve cell bodies were more abundant in the submucosal plexus than in the myenteric plexus and represented 40.3 ± 3.3% and 36.2 ± 1.1%, respectively, of the total submucosal neuronal population. All of the TRPC3/4/6-IR neurons had uniaxonal Dogiel type I morphology.
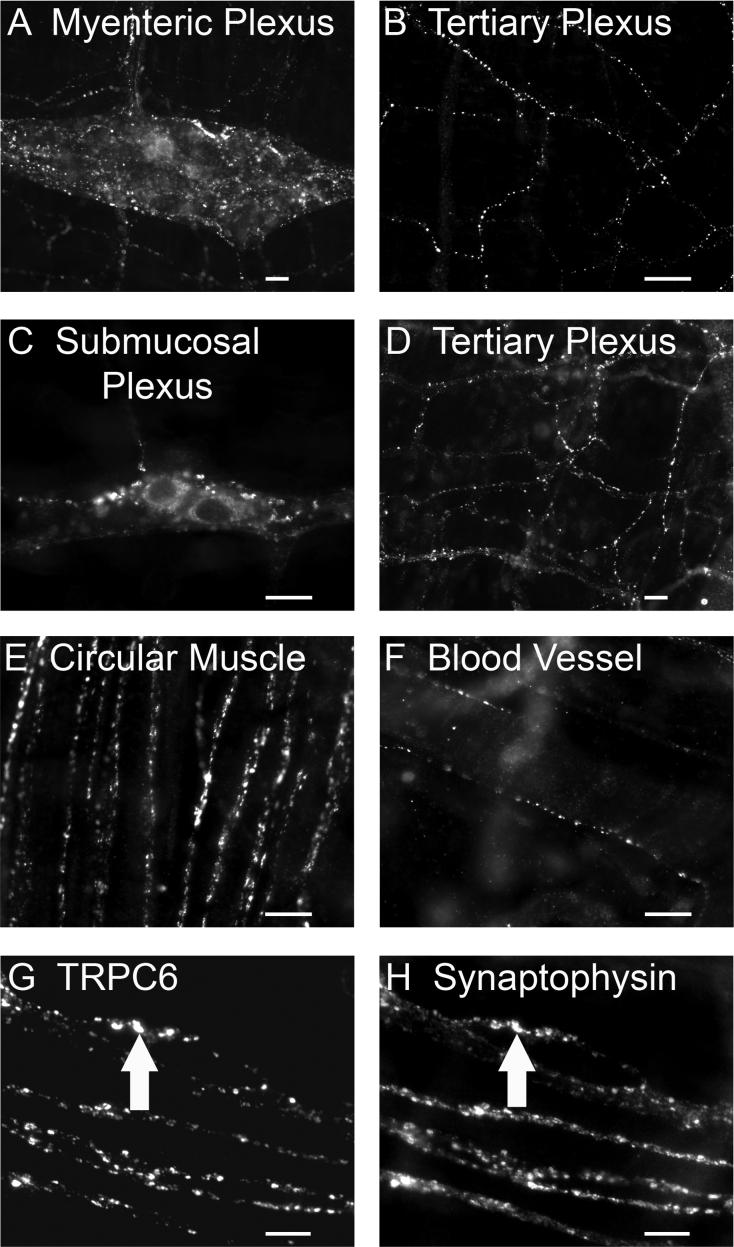
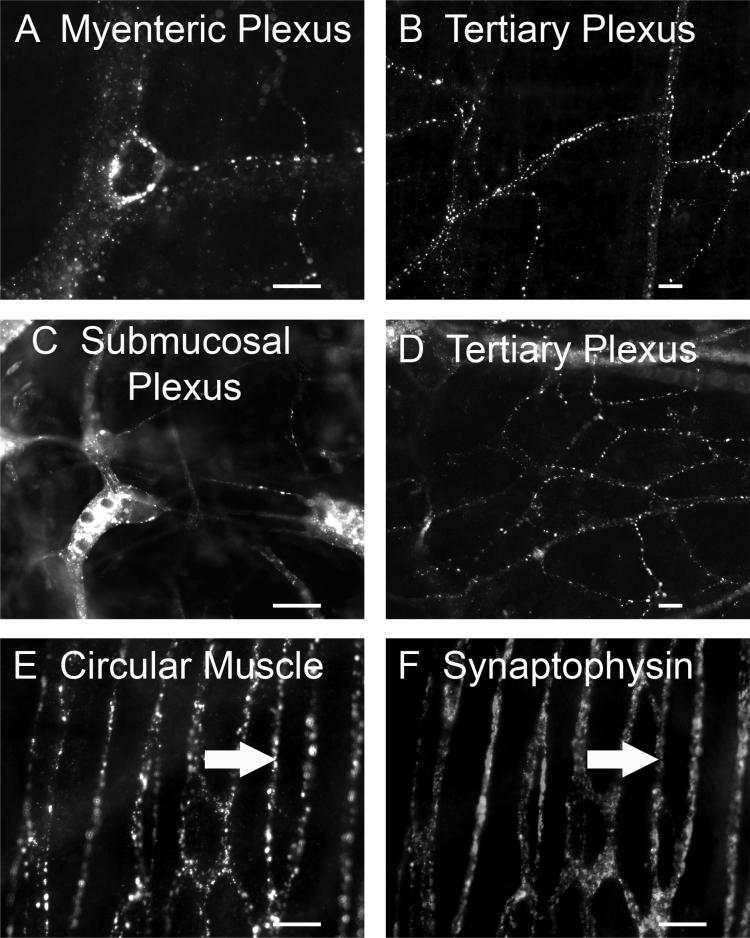
TRPC1-IR was restricted to nerve cell bodies and proximal processes and not found in varicose nerve fibers. TRPC3/4/6-IR was associated with varicose nerve fibers (Fig. 4-6). TRPC3-IR varicose nerve fibers occurred in the tertiary components of the submucosal plexus (Fig. 4A) and around the submucosal blood vessels (Fig. 4B), but not in myenteric (Fig. 3C) and submucosal ganglia (Fig. 3D). TRPC4-IR varicose nerve fibers were abundant in myenteric and submucosal ganglia and usually encircled ganglionic cells (Fig. 5A, 5C). TRPC4-IR nerve fibers also appeared in interganglionic connectives (Fig. 5A, 5C), in the tertiary components of the myenteric and submucosal plexuses (Fig. 5B, 5D), and in the circular muscle plexus (Fig. 5E). TRPC6-IR varicose nerve fibers were found in the myenteric and submucosal ganglia (Fig. 6A, 6C), in interganglionic connectives (Fig. 6A, 6C), in the tertiary components of the myenteric and submucosal plexuses (Fig. 6B, 6D), in the circular muscle plexus (Fig. 6E), and around submucosal blood vessels (Fig. 6F).
Fig. 4.
TRPC3-IR nerve fibers in the submucosal plexus. A: TRPC3-IR varicose nerve fibers were observed in the tertiary division of the submucosal plexus. B: TRPC3-IR varicose nerve fibers were seen surrounding submucosal blood vessels. C-D: Paired images demonstrating colocalization of TRPC3-IR with IR for the presynaptic vesicular protein, synaptophysin. The arrows in C and D point to nerve fibers that express TRPC3-IR and synaptophysin-IR. TRPC3-IR was expressed at some, but not all, varicosities. Scale bar = 20 μm for all panels.
Fig. 6.
TRPC6-IR nerve fibers in myenteric and submucosal plexuses. A: TRPC6-IR varicose nerve fibers in myenteric ganglia and in interganglionic fiber bundles. Within some myenteric ganglia, TRPC6-IR fibers form complexes that encircle individual ganglionic cell somas. B: TRPC6-IR nerve fibers were present in the tertiary division of the myenteric plexus. C: TRPC6-IR nerve cell bodies and varicose nerve fibers in submucosal ganglia and in interganglionic connectives. D: TRPC6-IR nerve fibers in the tertiary division of the submucosal plexus. E: TRPC6-IR nerve fibers in the circular muscle plexus. F: TRPC6-IR nerve fibers around submucosal blood vessels. G-H: Paired images showing colocalization of TRPC6-IR with synaptophysin-IR in the circular muscle plexus. The arrows point to nerve fibers that coexpress TRPC6- and synaptophysin-IR. Scale bar = 20 μm for all panels.
Fig. 5.
TRPC4-IR nerve fibers in myenteric and submucosal plexuses. A: TRPC4-IR varicose nerve fibers were found in myenteric ganglia and in interganglionic fiber bundles. Within some myenteric ganglia, TRPC4-IR fibers formed complexes that encircled individual ganglionic cells. B: Nerve fibers that expressed TRPC4-IR appeared in the tertiary division of the myenteric plexus. C: TRPC4-IR varicose nerve fibers were found in submucosal ganglia and in interganglionic connectives. D: TRPC4-IR nerve fibers in the tertiary division of the submucosal plexus. E: TRPC4-IR nerve fibers in the circular muscle plexus. F: The same tissue preparation as in E stained with anti-synaptophysin. The arrows in E and F point to nerve fibers that coexpress TRPC4- and synaptophysin-IR. Scale bar = 20 μm for all panels.
Double labeling was done with an antibody to the presynaptic vesicular protein, synaptophysin, to explore the possibility that TRPC channels might be expressed at presynaptic transmitter release sites. The synaptophysin antibody labeled a dense network of punctate fibers in the myenteric and submucosal plexuses. TRPC3/4/6-IR varicose nerve fibers were closely associated with synaptophysin-IR (Fig. 4C, 4D, 5E, 5F, 6G, 6H), which suggests that TRPC3/4/6 are expressed at some, but not all, putative transmitter release sites.
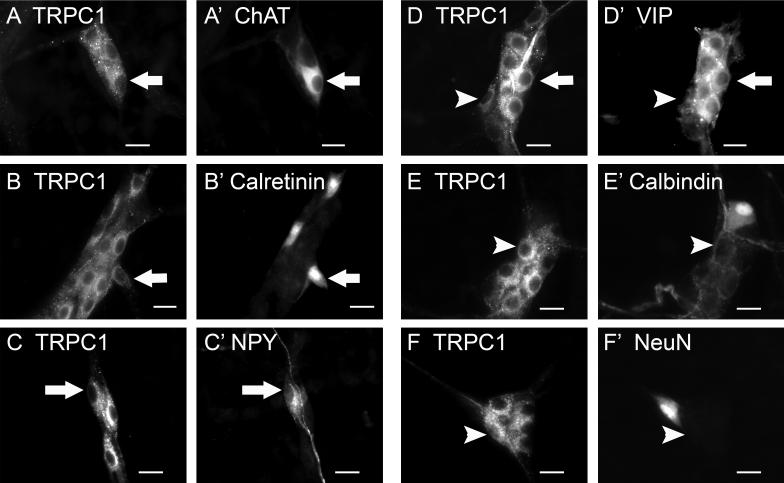
Neurochemical Coding of TRPC1-IR Neurons
Double label immunohistochemistry for various neurochemical markers was done to identify major classes of ganglion cells that might express TRPC1-IR in the myenteric and submucosal plexuses. In the myenteric plexus, a majority of the ganglion cells that expressed TRPC1-IR (79.4 ± 9.3%) also expressed ChAT-IR (Fig.7A). Conversely, 57.8 ± 11.7% of ChAT-IR neurons expressed TRPC1-IR. TRPC1-IR was expressed in all calretinin-IR myenteric neurons (Fig. 7B), and this accounted for 22.6 ± 5.4% of the TRPC1-IR. 26.5 ± 7.5% of the TRPC1-IR myenteric neurons coexpressed NOS-IR (Fig. 7C). Conversely, 82.1 ± 17.9% of all NOS-IR neurons expressed TRPC1-IR. As shown in Fig. 7D, a large majority of the TRPC1-IR neurons was not IR for calbindin (among 3885 TRPC1-IR neurons counted in 4 guinea pigs, only 28 were calbindin-IR). In fact, only 2.3 ± 0.7% of calbindin-IR neurons were TRPC1-IR, and this accounted for 0.6 ± 0.3% of all TRPC1-IR neurons.
Fig. 7.
Neurochemical coding of TRPC1-IR neurons in the myenteric plexus. TRPC1-IR was colocalized with choline acetyltransferase (ChAT; A, A’), calretinin (B, B’), nitric oxide synthase (NOS; C, C’), but not calbindin (D, D’). Arrows indicate neurons that are positive for TRPC1 as well as the neurochemical markers. Arrowheads indicate neurons that are positive for TRPC1 only. Scale bar = 20 μm for all panels.
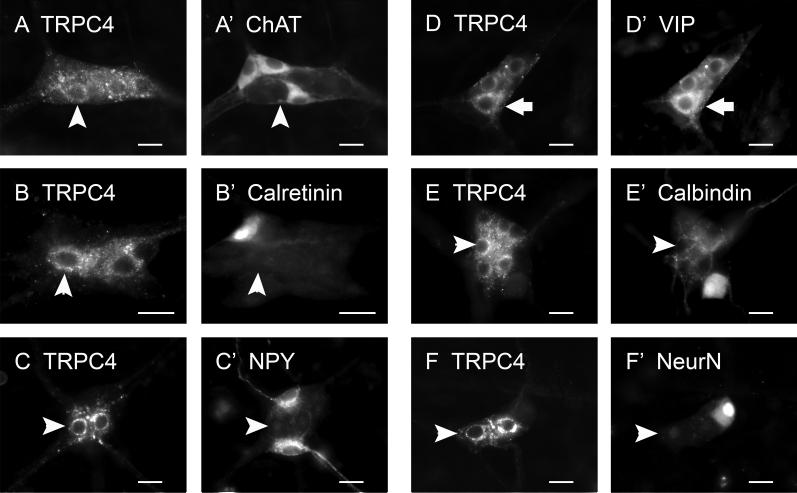
In the submucosal plexus, TRPC1-IR was expressed in 55.1 ± 8.4% of the ChAT-IR neurons (Fig. 8A), in 100% of calretinin-IR neurons (Fig. 8B), in 67.6 ± 12.9% of NPY-IR neurons (Fig. 8C), and in 88.7 ± 10.6% of neurons with IR for VIP (Fig. 8D). Conversely, of all the TRPC1-IR submucosal neurons, 37.0 ± 6.4% were found to express ChAT-IR, 16.3 ± 5.3% expressed calretinin-IR, 20.5 ± 4.0% expressed NPY-IR and 62.7 ± 3.2% expressed VIP-IR. TRPC1-IR was not present in submucosal neurons that expressed calbindin-IR or NeuN-IR (Fig. 8E, 8F).
Fig. 8.
Neurochemical coding of TRPC1-IR in the submucosal plexus. TRPC1-IR was colocalized with ChAT (A, A’), calretinin (B, B’), neuropeptide Y (NPY; C, C’), and vasoactive intestinal peptide (VIP; D, D’), but was not colocalized with calbindin (E, E’) nor neuronal nuclear protein (NeuN, F, F’). Arrows indicate neurons that are positive for TRPC1 as well as neurochemical markers. Arrowheads indicate neurons that are positive for TRPC1 only. Scale bar = 20 μm for all panels.
Neurochemical Coding of TRPC3-IR Neurons
No TRPC3-IR cell bodies or nerve fibers were observed in myenteric ganglia; whereas in the submucosal plexus, TRPC3-IR was localized to neurons as well as to nerve fibers (Fig. 4, 9). Immunoreactivity was prominent at the cell membrane and in the cytoplasm of submucosal neurons, but was weak in the nucleus. All of the TRPC3-IR neurons expressed ChAT- and NPY-IR (Fig. 9A, 9B). This accounted for 25.0 ± 3.0% of ChAT-IR neurons and 95.3 ± 1.0% of NPY-IR neurons. None of the TRPC3-IR neurons expressed calretinin-, VIP-, calbindin-, or NeuN-IR (Fig. 9C-9F).
Fig. 9.
Neurochemical coding of TRPC3-IR in the submucosal plexus. TRPC3- IR was colocalized with ChAT (A, A’) and NPY (B, B’). No colocalization of TRPC3 with calretinin (C, C’), VIP (D, D’), calbindin (E, E’), nor NeuN (F, F’) was found. Arrows indicate neurons that are positive for TRPC3 as well as neurochemical markers. Arrowheads indicate neurons that are positive for TRPC3 only. Scale bar = 20 μm for all panels.
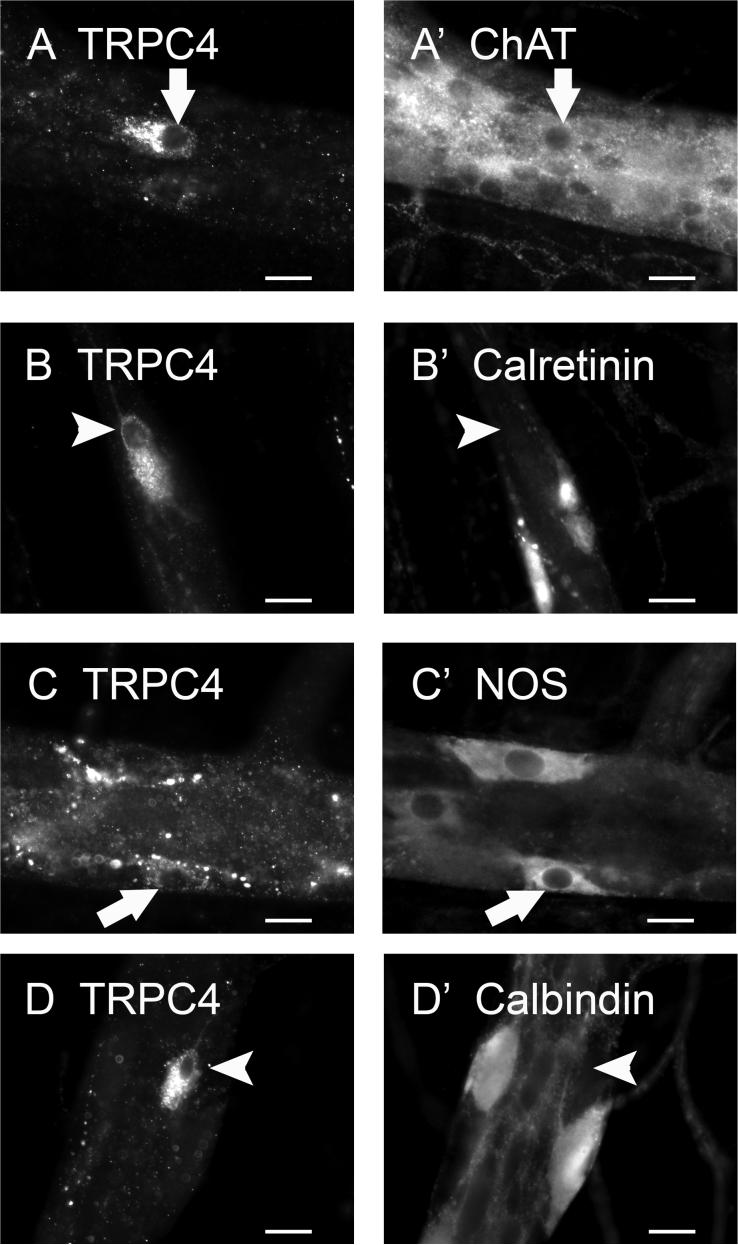
Neurochemical Coding of TRPC4-IR Neurons
In the myenteric plexus, TRPC4-IR nerve cell bodies accounted for a minority of the total myenteric ganglion neurons (1.7 ± 0.1%), and was detected in 1.5 ± 0.1% of ChAT-IR neurons (Fig. 10A) and 1.0 ± 0.5% of NOS-IR neurons (Fig. 10C). Of all the TRPC4-IR neurons, 76.0 ± 2.6% were cholinergic and 18.6 ± 5.0% were nitrergic. No colocalization of TRPC4 with calretinin- or calbindin-IR was found in the myenteric plexus (Fig. 10B, 10D).
Fig. 10.
Neurochemical coding of TRPC4-IR in the myenteric plexus. TRPC4-IR was colocalized with ChAT (A, A’) and NOS (C, C’), but was not colocalized with calretinin (B, B’) nor calbindin (D, D’). Arrows indicate neurons that are positive for TRPC4 as well as neurochemical markers. Arrowheads indicate neurons that are positive for TRPC4 only. Scale bar = 20 μm for all panels.
In the submucosal plexus, all TRPC4-IR nerve cell bodies expressed VIP-IR (Fig. 11D), which was 80.0 ± 9.1% of total VIP-IR neurons. Colocalization of TRPC4 with ChAT-, calretinin-, NPY-, calbindin-, or NeuN-IR was not observed in the submucosal plexus (Fig. 11A-11C, 11E-11F).
Fig. 11.
Neurochemical coding of TRPC4-IR in the submucosal plexus. TRPC4-IR was exclusively colocalized with VIP (D, D’). No colocalization of TRPC4 with ChAT (A, A’), calretinin (B, B’), NPY (C, C’), calbindin (E, E’) nor NeuN (F, F’) was found. Arrows indicate neurons that are positive for TRPC4 as well as neurochemical markers. Arrowheads indicate neurons that are positive for TRPC4 only. Scale bar = 20 μm for all panels.
Neurochemical Coding of TRPC6-IR Neurons
In the myenteric plexus, TRPC6-IR nerve cell bodies were present in only 1.5 ± 0.1% of the total ganglion cell population and was detected in 0.4 ± 0.1% of ChAT-IR neurons (Fig. 12A) and 3.0 ± 0.5% of NOS-IR neurons (Fig. 12C). Of all the TRPC6-IR neurons, 30.0 ± 6.1% were cholinergic and 58.4 ± 5.0% were nitrergic. Similar to the situation for TRPC4, no colocalization of TRPC6 with calretinin- or calbindin-IR was found in the myenteric plexus (Fig. 12B, 12D).
Fig. 12.
Neurochemical coding of TRPC6-immunoreactive neurons in the myenteric plexus of the guinea pig ileum. TRPC6 IR was colocalized with ChAT (A, A’) and NOS (C, C’), but was not colocalized with calretinin (B, B’) nor calbindin (D, D’). Arrows indicate neurons that are positive for TRPC6 as well as neurochemical markers. Arrowheads indicate neurons that are positive for TRPC6 only. Scale bar = 20 μm for all panels.
In the submucosal plexus, all TRPC6-IR nerve cell bodies also expressed VIP-IR (Fig. 13D). TRPC6-IR neurons accounted for 67.8 ± 11.9% of total VIP-IR neurons. Akin to TRPC4-IR, no TRPC6-IR neurons were double labeled with ChAT-, calretinin-, NPY-, calbindin-, or NeuN-IR in the submucosal plexus (Fig. 13A-13C, 13E-13F).
Fig. 13.
Neurochemical coding of TRPC6-IR in the submucosal plexus. TRPC6-IR was exclusively colocalized with VIP (D, D’). No colocalization of TRPC6 with ChAT (A, A’), calretinin (B, B’), NPY (C, C’), calbindin (E, E’) nor NeuN (F, F’) was found. Arrows indicate neurons that are positive for TRPC6 as well as neurochemical markers. Arrowheads indicate neurons that are positive for TRPC6 only. Scale bar = 20 μm for all panels.
DISCUSSION
The ENS is referred to as the “brain-in-the-gut” due to its multiple neurobiological parallels with the brain and spinal cord (Wood, 1981). Our results suggest that expression of TRPC channels in the ENS is another property that is shared with the brain. TRPC1/4/5 and the diacylglycerol-activated TRPC3/6 subunits are widely distributed in the mammalian brain (Strubing et al., 2003; Giampa et al., 2007) and we found this also for the ENS. These are channels which, in a majority of cases, are directly linked to PLC activity. Three members of this family, TRPC3/6/7, are activated by DAG. Two others, TRPC4/5, are activated as a consequence of PLC activity; however, details for the steps in the postreceptor signal transduction cascade for TRPC4/5 in the brain are not yet fully understood (Putney, 2007).
The connection of TRPC channels in the brain with G protein-coupling of metabotropic receptors to PLC initiated transduction pathways in brain neurons is reminiscent of slow excitatory synaptic events in the ENS where PLC is a key early event preceding the opening of cation channels that underlie membrane depolarization and elevated neuronal excitability (Wood and Kirchgessner, 2004). This is the case for elevation of excitability in enteric S-type neurons evoked by stimulation of bradykinin B2, prostaglandin EP, purinergic P2Y1 and serine protease receptors (PAR-1, -2 and -4 receptors) (Gao et al., 2002; Hu et al., 2003; 2004). The widespread and differential distribution of TRPC channels and previously known involvement of PLC in metabotropic signaling in the ENS are characteristics in common with TRPC neurobiology in the brain.
In addition to their wide brain-like distribution in the ENS, we found that the TRPC subunits were distributed differentially between myenteric and submucosal plexuses and had distinct colocalization with neurochemical codes that identify functionally distinct classes of ENS neurons. Messenger RNA transcripts for all members of the TRPC family were expressed in the LMMP preparations, which contained both muscle and myenteric ganglia. On the other hand, only TRPC1−6 mRNA was detected in dissociated myenteric ganglia, suggesting that TRPC7 expression was localized to the muscle. In fact, mRNA encoding TRPC4/6/7 has been identified in gastrointestinal smooth muscle (Walker et al., 2001). RNA transcripts for TRPC1/3/4/6/7 were expressed in the submucosal plexus. Technical difficulty encountered in attempts to isolate submucosal ganglia from the surrounding connective tissue precluded RT-PCR analysis for dissociated submucosal ganglia.
Our TRPC antibodies were produced for either human or mouse epitope sequences. Specificity of TRPC1/3/4/6 antibodies for the corresponding guinea pig TRPC channel has been confirmed by others (Calupca et al., 2002; Morales et al., 2007; Raybould et al., 2007). Nevertheless, we confirmed specificity of the antibodies with Western blotting of guinea pig brain, which is known to express TRPC1/3/4/6. Western blots revealed expression of TRPC1/3/ 4/6 in LMMP preparations. Only TRPC1/4/6 proteins were detected in dissociated myenteric ganglia, which was consistent with supplementary Western blotting results (data not illustrated) that TRPC3 was expressed by circular muscle component of the muscularis externa. TRPC1/3/4/6 proteins were also expressed in the submucosal plexus preparations. Because of the difficulty encountered in successfully isolating submucosal ganglia, Western blots for dissociated submucosal ganglia were not done and we cannot exclude the possibility that TRPC1/3/4/6 in the submucosal preparations reflected expression in cells other than neurons. The results from Western blot analysis were consistent with immunohistochemical data from whole-mount preparations of the myenteric and submucosal plexuses. Consistent with the Western blot results, immunofluorescence for TRPC1/4/ 6 proteins was detected in the myenteric plexus, while immunoreactivity for TRPC1/3/4/6 was detected in the submucosal plexus.
Expression of TRPC1/4/6 in the Myenteric Plexus
TRPC1 was the predominant isoform in myenteric ganglia. The majority (63%) of TRPC1-IR neurons expressed ChAT-IR, which coded them as being cholinergic neurons. A smaller population (23%) expressed calretinin-IR. Both codes identify the neurons as either excitatory musculomotor neurons to the longitudinal muscle coat or cholinergic interneurons (Furness, 2006). Firing of cholinergic longitudinal musculomotor neurons underlies descending shortening of the intestine during peristatic propulsion (Wood, 2008). A distinct subpopulation of TRPC1-IR neurons (26%) in the myenteric plexus expressed NOS-IR. NOS-IR is a code for inhibitory musculomotor neurons to the longitudinal and circular muscle coats, which have a single axon projecting down the intestine in the anal direction (Furness, 2006). These neurons are responsible for descending inhibition of the circular muscle coat during peristaltic propulsive motility (Wood, 2008).
Both ChAT-IR and NOS-IR uniaxonal enteric neurons express S- Type (synaptic) electrophysiological behavior (Wood & Kirchgessner, 2004). Only a very small population of TRPC1-IR neurons (0.6%) expressed calbindin-IR. Calbindin is the neurochemical code for enteric neurons with multipolar Dogiel Type II morphology and AH (after-polarization) type electrophysiological behavior (Iyer et al., 1988; Wood & Kirchgessner, 2004; Furness, 2006). AH neurons are interneurons in which metabotropic excitatory neurotransmission involves post-receptor activation of adenylate cyclase, closure of Ca2+-gated K+ channels and elevated input resistance (Wood & Kirchgessner, 2004; Wood, 2008). On the other hand, metabotropic neurotransmission in myenteric musculomotor neurons involves post-receptor activation of PLC and depolarizing responses associated with decreased input resistance, which are mediated by opening of non-selective cation channels (Gao et al., 2002; Hu et al., 2003, 2004).
Unlike TRPC1-IR, TRPC4/6-IR was expressed in only a small minority of the total myenteric neuronal population (1.7% and 1.5%, respectively). Like the TRPC1-IR neurons, all of the TRPC4/6-IR neurons were ChAT- or NOS-IR with unipolar Dogiel Type I morphology. TRPC4/6-IR was never co-expressed with calretinin-IR or calbindin-IR, which is the marker for AH-Type neurons with Dogiel II morphology.
Expression of TRPC1/3/4/6 in the Submucosal Plexus
Neurons in submucosal ganglia are subdivided into four classes based on morphology, axonal projections and neurochemical codes as follows: 1) Dogiel Type I non-cholinergic secretomotor/vasodilator motor neurons with VIP-IR; (2) Dogiel Type I cholinergic secretomotor/vasodilator motor neurons with ChAT/calretinin-IR; (3) Dogiel Type I cholinergic secretomotor/non-vasodilator motor neurons with ChAT/NPY-IR; (4) Dogiel Type II interneurons with ChAT/calbindin/NeuN-IR (Furness, 2006). TRPC1/3/4/6-IR was absent from Dogiel Type II interneurons in the submucosal plexus. This was in contrast with VIP-immunoreactive non-cholinergic secretomotor neurons, which expressed TRPC1/4/6-IR. Cholinergic secretomotor/non-vasodilator motor neurons with ChAT/NPY-IR expressed TRPC1/3-IR. Cholinergic secretomotor/vasodilator motor neurons with ChAT/calretinin-IR expressed only TRPC1-IR. The overlap of TRPC1/4/6-IR in non-cholinergic secretomotor neurons and TRPC1/3-IR in cholinergic secretomotor neurons might be a reflection of formation of heteromultimeric complexes by combinations of TRPC proteins. If so, these could be the non-selective cation channels that underlie the metabotropic-mediated excitatory responses, which are associated with decreased input resistance and a reversal potential near zero in submucosal secretomotor neurons (Hu et al., 2003; Monro et al., 2004).
Presynaptic Distribution of TRPC3/4/6
Varicose nerve fibers expressed TRPC3/4/6-IR, which was suggestive of localization of TRPC channels at presynaptic neurotransmitter release sites. TRPC3-IR nerve fibers were restricted to the submucosal plexus, while TRPC4/6-IR nerve fibers appeared in both the myenteric and submucosal plexuses. TRPC4/6-IR nerve fibers formed pericellular complexes encircling ganglion cell bodies in both myenteric and submucosal plexuses, which suggested innervation of the cell bodies by TRPC4/6-IR axons.
The putative axonal TRPC3/4/6-IR was associated with the presynaptic vesicular protein, synaptophysin, which would be consistent with localization and functional involvement of TRPC cationic channels in neurotransmitter release from axons in the ENS. This is reminiscent of the many parallels between the ENS and the CNS where TRPC1/3/7 proteins are found in synaptosomes of the rat brain (Goel et al., 2002).
Acknowledgments
Grant sponsors: National Institutes of Health R01 DK 37238 (J.D. Wood), R01 DK 068258 (J. D. Wood), Pharmaceutical Manufactures of American Foundation Research Starter Award (S. Liu), and University of North Carolina Center for Functional GI & Motility Disorders Seed Grant Award (S. Liu).
LITERATURE CITED
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci U S A. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. J Physiol. 2004;555:323–330. doi: 10.1113/jphysiol.2003.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Locknar SA, Parsons RL. TRPC6 immunoreactivity is colocalized with neuronal nitric oxide synthase in extrinsic fibers innervating guinea pig intrinsic cardiac ganglia. J Comp Neurol. 2002;450:283–291. doi: 10.1002/cne.10322. [DOI] [PubMed] [Google Scholar]
- Castellano LE, Treviño CL, Rodríguez D, Serrano CJ, Pacheco J, Tsutsumi V, Felix R, Darszon A. Transient receptor potential (TRPC) channels in human sperm: expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Lett. 2003;541:69–74. doi: 10.1016/s0014-5793(03)00305-3. [DOI] [PubMed] [Google Scholar]
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–7246. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- Chiocchetti R, Poole DP, Kimura H, Aimi Y, Robbins HL, Castelucci P, Furness JB. Evidence that two forms of choline acetyltransferase are differentially expressed in subclasses of enteric neurons. Cell Tissue Res. 2003;311:11–22. doi: 10.1007/s00441-002-0652-6. [DOI] [PubMed] [Google Scholar]
- Chung YH, Kim D, Moon NJ, Oh CS, Lee E, Shin DH, Kim SS, Lee WB, Lee JY, Cha CI. Immunohistochemical study on the distribution of canonical transient receptor potential channels in rat basal ganglia. Neurosci Lett. 2007;422:18–23. doi: 10.1016/j.neulet.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Corcoran JPT, So PL, Maden M. Disruption of the retinoid signaling pathway causes a deposition of amyloid in the adult rat brain. Eur J Neurosci. 2004;20:896–902. doi: 10.1111/j.1460-9568.2004.03563.x. [DOI] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Beech D, Poston L, Tribe RM. Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod. 2002;8:946–951. doi: 10.1093/molehr/8.10.946. [DOI] [PubMed] [Google Scholar]
- De March Z, Giampà C, Patassini S, Bernardi G, Fusco FR. Cellular localization of TRPC5 in the substantia nigra of rat. Neurosci Lett. 2006;402:35–39. doi: 10.1016/j.neulet.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience. 2006;137:781–794. doi: 10.1016/j.neuroscience.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286:F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS ONE. 2007;2:e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2002;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system. Blackwell; Oxford: 2006. pp. 29–102. [Google Scholar]
- Fusco FR, Martorana A, Giampà C, De March Z, Vacca F, Tozzi A, Longone P, Piccirilli S, Paolucci S, Sancesario G, Mercuri NB, Bernardi G. Cellular localization of TRPC3 channel in rat brain: preferential distribution to oligodendrocytes. Neurosci Lett. 2004;365:137–142. doi: 10.1016/j.neulet.2004.04.070. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, North RA, Tokimasa T. Muscarinic agonists and potassium currents in guinea-pig myenteric neurons. Br J Pharmacol. 1989;96:193–203. doi: 10.1111/j.1476-5381.1989.tb11800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Liu S, Hu HZ, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- Giampa C, DeMarch Z, Patassini S, Bernardi G, Fusco FR. Immunohistochemical localization of TRPC6 in the rat substantia nigra. Neurosci Lett. 2007;424:170–174. doi: 10.1016/j.neulet.2007.07.049. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hu H-Z, Ren J, Liu S, Gao C, Xia Y, Wood JD. Functional group I metabotropic glutamate receptors in submucous plexus of guinea-pig ileum. Br J Pharmacol. 1999;128:1631–1635. doi: 10.1038/sj.bjp.0702980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD. Chemical coding and electrophysiology of enteric neurons expressing neurofilament 145 in guinea pig gastrointestinal tract. J Comp Neurol. 2002;442:189–203. [PubMed] [Google Scholar]
- Hu H-Z, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol. 2003;550:493–504. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H-Z, Gao N, Liu S, Ren J, Xia Y, Wood JD. Metabotropic signal transduction for bradykinin in submucosal neurons of guinea-pig small intestine. J Pharmacol Exp Ther. 2004;309:310–319. doi: 10.1124/jpet.103.059204. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha (1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Kannan KB, Singh BB, Hauser CJ. Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol. 2004;172:601–607. doi: 10.4049/jimmunol.172.1.601. [DOI] [PubMed] [Google Scholar]
- Iyer V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T. Electrophysiology of guinea pig myenteric neurons correlated with immunoreactivity for calcium binding proteins. J Auto Nerv Sys. 1988;22:141–150. doi: 10.1016/0165-1838(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Bornstein JC. Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience. 2004;126:137–147. doi: 10.1016/j.neuroscience.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Pheromones, vomeronasal function, and gender-specific behavior. Cell. 2002;108:735–738. doi: 10.1016/s0092-8674(02)00687-6. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2004;287:L962–L969. doi: 10.1152/ajplung.00452.2003. [DOI] [PubMed] [Google Scholar]
- Lee E-J, Merwine DK, Padilla M, Grzywacz NM. Choline acetyltransferaseimmunoreactive neurons in the retina of normal and dark-reared turtle. J Comp Neurol. 2007;503:768–778. doi: 10.1002/cne.21416. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Lin A, Lourenssen S, Stanzel RD, Blennerhassett MG. Nerve growth factor sensitivity is broadly distributed among myenteric neurons of the rat colon. J Comp Neurol. 2005;490:194–206. doi: 10.1002/cne.20654. [DOI] [PubMed] [Google Scholar]
- Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Lin Z, Gao N, Hu HZ, Liu S, Gao C, Kim G, Ren J, Xia Y, Peck OC, Wood JD. Immunoreactivity of Hu proteins facilitates identification of myenteric neurons in guinea-pig small intestine. Neurogastroenterol Motil. 2002;14:197–204. doi: 10.1046/j.1365-2982.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Liu M, Kirchgessner AL. Agonist- and reflex-evoked internalization of metabotropic glutamate receptor 5 in enteric neurons. J Neurosci. 2000;20:3200–3205. doi: 10.1523/JNEUROSCI.20-09-03200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang GD, Xia Y, Wood JD. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J Comp Neurol. 2005;481:284–298. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol. 2006;494:63–74. doi: 10.1002/cne.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich MF, Furneauy HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Miyata S, Nakai S, Kiyohara T, Hatton GI. Calbindin-D28k and calretinin in the rat posterior pituitary; light and electron microscopic localization and upregulation with dehydration. J Neurocytol. 2000;29:5–17. doi: 10.1023/a:1007180328597. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S, Diez A, Puyet A, Camello PJ, Camello-Almaraz C, Bautista JM, Pozo MJ. Calcium controls smooth muscle TRPC gene transcription via the CaMK/calcineurin-dependent pathways. Am J Physiol Cell Physiol. 2007;292:C553–C563. doi: 10.1152/ajpcell.00096.2006. [DOI] [PubMed] [Google Scholar]
- Mukerji N, Damodaran TV, Winn MP. TRPC6 and FSGS: the latest TRP channelopathy. Biochim Biophys Acta. 2007;1772:859–868. doi: 10.1016/j.bbadis.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Munsch T, Freichel M, Flockerzi V, Pape HC. Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proc Natl Acad Sci USA. 2003;100:16065–16070. doi: 10.1073/pnas.2535311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Slack BE, Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J Physiol. 1985;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Ong HL, Brereton HM, Harland ML, Barritt GJ. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology. 2003;8:23–32. doi: 10.1046/j.1440-1843.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Ong HL, Chen J, Chataway T, Brereton H, Zhang L, Downs T, Tsiokas L, Barritt G. Specific detection of the endogenous transient receptor potential (TRP)-1 protein in liver and airway smooth muscle cells using immunoprecipitation and Western-blot analysis. Biochem J. 2002;364:641–648. doi: 10.1042/BJ20020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr. Physiological mechanisms of TRPC activation. Pflugers Arch. 2005;451:29–34. doi: 10.1007/s00424-005-1416-4. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr. Inositol lipids and TRPC channel activation. Biochem Soc Symp. 2007:37–45. doi: 10.1042/BSS0740037. [DOI] [PubMed] [Google Scholar]
- Raybould NP, Jagger DJ, Kanjhan R, Greenwood D, Laslo P, Hoya N, Soeller C, Cannell MB, Housley GD. TRPC-like conductance mediates restoration of intracellular Ca2+ in cochlear outer hair cells in the guinea pig and rat. J Physiol. 2007;579:101–113. doi: 10.1113/jphysiol.2006.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche D, Pfannkuche H, Michel K, Hoppe S, Schemann M. Immunohistochemical evidence for the presence of calbindin containing neurones in the myenteric plexus of the guinea-pig stomach. Neurosci Lett. 1999;270:71–74. doi: 10.1016/s0304-3940(99)00471-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc Natl Acad Sci U S A. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl A, Hoppe S, Frey I, Daniel H, Schemann M. Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J Comp Neurol. 2005;490:1–11. doi: 10.1002/cne.20617. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Stresow N, Albrecht N, Schultz G. Functional differences between TRPC4 splice variants. J Biol Chem. 2002;277:3752–3759. doi: 10.1074/jbc.M109850200. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Trebak M. Canonical transient receptor potential channels in disease: targets for novel drug therapy? Drug Discov Today. 2006;11:924–930. doi: 10.1016/j.drudis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004;43:11701–11708. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Vannier B, Zhu X, Brown D, Birnbaumer L. The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry. J Biol Chem. 1998;273:8675–8679. doi: 10.1074/jbc.273.15.8675. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol Cell Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Fang XC, Liu S, Gao N, Xia Y, Wood JD. Angiotensin receptors and actions in guinea pig enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G614–G626. doi: 10.1152/ajpgi.00119.2005. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wood JD. Intrinsic neural control of intestinal motility. Annu Rev Physiol. 1981;43:33–51. doi: 10.1146/annurev.ph.43.030181.000341. [DOI] [PubMed] [Google Scholar]
- Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149–158. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]
- Wood JD, Kirchgessner A. Slow excitatory metabotropic signal transmission in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 1):71–80. doi: 10.1111/j.1743-3150.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- Wood JD, Mayer CJ. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflugers Arch. 1978;374:265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]
- Wu X, Babnigg G, Zagranichnaya T, Villereal ML. The role of endogenous human Trp4 in regulating carbachol-induced calcium oscillations in HEK-293 cells. J Biol Chem. 2002;277:13597–13608. doi: 10.1074/jbc.M110881200. [DOI] [PubMed] [Google Scholar]
- Xia Y, Baidan LV, Fertel RH, Wood JD. Determination of levels of cyclic AMP in the myenteric plexus of guinea-pig small intestine. Eur J Pharmacol. 1991;206:231–236. doi: 10.1016/s0922-4106(05)80023-9. [DOI] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod. 2002;67:988–994. doi: 10.1095/biolreprod.102.004119. [DOI] [PubMed] [Google Scholar]
- Zafirov DH, Cooke HJ, Wood JD. Thyrotropin-releasing hormone excites submucous neurons in guinea-pig ileum. Eur J Pharmacol. 1991;204:109–112. doi: 10.1016/0014-2999(91)90843-f. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–16229. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]
- Zholos AV, Zholos AA, Bolton TB. G-protein-gated TRP-like cationic channel activated by muscarinic receptors: effect of potential on single-channel gating. J Gen Physiol. 2004;123:581–598. doi: 10.1085/jgp.200309002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. Trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]