Abstract
Temporal summation of “second pain” (TSSP) is considered to be the result of C-fiber-evoked responses of dorsal horn neurons, termed ‘windup’. TSSP is dependent on stimulus frequency (≥ 0.33 Hz) and relevant for central sensitization and chronic pain. We have previously shown that compared to normal controls (NC), fibromyalgia (FM) subjects show abnormal TSSP, requiring lower stimulus intensities/frequencies to achieve similar TSSP. However, it is unknown whether abnormal TSSP in FM is influenced by peripheral sensitization of C-fiber nociceptors and/or bias in pain ratings. Thus we evaluated 14 FM subjects and 19 NC with pain threshold tests to selective C-fiber stimulation, 30 sec heat stimuli, and repetitive brief (1.5 sec) heat pulses at 0.33 Hz using a contact heat stimulator (CHEPS). The intensity of heat pulses was varied to achieve maximal TSSP ratings of 45 ± 10 (numerical pain scale 0–100) in both FM and NC groups. We found that NC and FM subjects had similar pain thresholds to C-fiber stimulation and yet FM subjects required lower heat pulse temperatures to generate the same magnitudes of TSSP (p < .05). This combination of findings does not support peripheral sensitization and suggests central TSSP abnormalities in FM subjects. In a second experiment, all aspects of individually adjusted TSSP heat pulses were kept the same except the baseline temperature (BT) between heat pulses was surreptitiously alternated between 35°C and 40°C. These changes of BT resulted in significantly greater TSSP ratings of FM subjects compared to NC subjects, both at 35°C or 40°C, but did not change their response to the first heat pulse of a stimulus train. These findings provide strong support for alterations of central pain sensitivity and not peripheral sensitization or rating bias as responsible for TSSP differences between NC and FM subjects.
Keywords: Temporal summation, Hyperalgesia, Fibromyalgia, Dermatome, Chronic pain
1. Introduction
An important mechanism for alterations of pain sensitivity is activation-dependent neural plasticity, manifested by slow temporal summation of C fiber-evoked responses of dorsal horn neurons, termed windup. C-fiber impulse frequencies of ≥ 0.3 Hz result in the release of glutamate and other neuromodulators [7] producing slow post-synaptic potentials lasting fractions of seconds [40]. These slow potentials summate to produce windup [31]. Windup is dependent on N-methyl-D-aspartate (NMDA) receptor currents following removal of the Mg blockade of these ion channels [18]. These events result in central sensitization as evidenced by lowered pain thresholds and expanded receptive fields [5; 30; 44]. Importantly, windup can be used to test central sensitivity in pain patients and normal controls. When tested with repetitive heat pulses or mechanical stimuli fibromyalgia (FM) patients have abnormally enhanced temporal summation of second pain (TSSP), the psychophysical counterpart of windup [28; 32; 33; 35; 36; 39]. Although such findings are consistent with central sensitization, other possible mechanisms have yet to be excluded, in particular peripheral sensitization of C-fiber nociceptors and rating bias. Indirect evidence for the former was suggested by studies that showed heightened microvascular reactivity in FM patients [8; 15; 29]. Signal detection has been used to analyze normative data concerning discriminability across noxious intensities and thus bias [16], but methods more specific for central sensitization or windup are needed. Thus we tested both the contribution of C-fiber sensitization and response bias of NC and FM subjects using three specific tests: 1) pain threshold to selective C-fiber stimulation, 2) long duration (30 sec) heat stimuli (35°C, 38°C, and 40°C), one of which is just below the nociceptive range (i.e., 40°C) and 3), TSSP trains of brief (1.5 sec), individually adjusted heat pulses at 0.33 Hz that normalize TSSP of NC and FM subjects. We hypothesized that FM subjects would have pain thresholds, ratings of long duration heat stimuli, and ratings of repetitive heat pulses similar to NC subjects. FM subjects, however, would require lower peak temperatures to evoke the same magnitude of TSSP. Such results would argue against peripheral C-fiber sensitization and systematic response bias in FM subjects. To provide additional evidence for abnormal central sensitivity of FM subjects we systematically varied the baseline temperature (BT) between pulses (38°C) within the non-painful range while keeping the peak temperature of the repetitive heat pulses constant. Based on preliminary data, we hypothesized that changing BT to 35°C or 40°C would result in higher ratings in FM subjects as compared to NC, but only after the first heat pulse.
2. Materials and Methods
We recruited 19 middle-aged healthy pain-free female subjects [mean age (SD): 41.2 (11.0) years] using advertisements posted throughout the University of Florida, Gainesville and 14 female FM patients [43.4 (8.5) years] from the local community and FM support groups. All FM subjects fulfilled the 1990 American College of Rheumatology Criteria [43]. Informed consent was obtained from all subjects and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The University of Florida Institutional Review Board approved the procedures and protocol for this study. All participants were right handed and included 30 Caucasians, one African-American and two Asian subjects. Prior to testing, all subjects underwent a clinical examination and were excluded from the study if they had abnormal findings unrelated to FM. Use of analgesics, including non-steroidal anti-inflammatory drugs (NSAID) and acetaminophen, was not allowed during the study. All subjects were asked to discontinue analgesics for the duration of five drug half-lives before testing, except narcotics which had to be stopped at least two weeks prior to study entry. Low dose muscle relaxants and/or amitriptyline (≤ 10 mg/day) were permissible during the study for treatment of FM-related insomnia.
2.1 Experimental Design
TSSP was elicited by repetitive heat pulses (see below) to the palmar surfaces of the hands of study subjects (for more details, see [35]). Using heat stimuli between 48°C and 51°C, subjects usually report moderate pain and TSSP during repetitive pulses at frequencies of ≥ 0.33 Hz [35; 39]. The painful sensations evoked by such repetitive stimuli display latencies consistent with C-fiber transmission [34; 39]. During repetitive heat stimuli to the hands latent pain sensations are well separated from early sensations such as 1st pain and can be easily detected by most study subjects. Importantly, repetitive heat pulses applied to the glabrous skin of the hands can evoke brief latency sensations of weak pain or warmth, but do so only during the first two or three pulses of a heat pulse train [25; 26; 41]. Thus, pain ratings related to repetitive heat pulses almost exclusively result from C-fiber input and TSSP.
To examine the contribution of C-fiber sensitization to TSSP as well as the response bias of NC and FM subjects, three specific tests were used: 1) heat pain threshold testing, using a slow ramp; 2) long duration (30 sec) heat stimuli to test the contribution of three different BT (35°C, 38°C, and 40°C) to pain from heat pulse trains; and 3) repetitive heat pulses at 0.33 Hz adjusted to the individual heat pain sensitivity of NC and FM subjects, resulting in normalized TSSP.
2.2 Ratings of Pain
2.2.1 Ratings of Experimental Pain
A standardized numerical pain scale (NPS) was utilized for rating the magnitude of painful sensations produced by thermal stimulation as described previously [34]. The scale ranged from 0 to 100, in increments of 5, with verbal descriptors at intervals of 10. Previous experience with the scale has shown that increments of 5 provide appropriate resolution for discriminable levels of late sensation intensity from threshold to nearly intolerable levels [39; 41]. This numerical scale has been found to be particularly advantageous for pain ratings during a series of repetitive stimuli [41].
2.2.2 Ratings of Somatic Pain
Visual analogue scales (0 – 100) were used for ratings of somatic pain before and after the experimental protocol [22]. Although the NC subjects were required to be pain free at enrollment somatic pain ratings were obtained before and after the testing session to capture possible new onset pains like back pain, headache, etc. The scales were anchored on the left with “no pain at all” and on the right with “the most intense pain imaginable”.
2.3 Thermal probe
Thermal pulses were generated by a “Contact Heat Evoked Potential Stimulator” (CHEPS) (Medoc Advanced Medical Systems, Ramat Yishai, Israel). This apparatus is comprised of a heatfoil/Peltier thermode (HP-thermode) that provides extremely fast heating rates of up to 70°C/sec and cooling rates of up to 40°C/sec. The HP-thermode can stimulate a circular skin area of 27 mm diameter (5.73 cm2), resulting in similar spatial recruitment of heat receptors as previously used intermittent contact TSSP stimulators [17; 39; 41]. The fast heating capability of the HP-thermode is the result of advanced heat foil technology in combination with a Peltier element. The HP-thermode is composed of two layers: 1) an external layer which is comprised of a very thin fast heating foil with two thermocouples (electronic thermal sensors) that can provide an estimate of the skin temperature at the thermode surface; and 2) a second layer consisting of a Peltier element with heating and cooling capabilities and two thermistors (electronic thermal sensors). The extremely rapid heating rate is provided by the external heat foil, while the cooling rate is generated by the internal Peltier element. Special hardware and software controls allow temperature adjustments at a rate of 150 times per second. Thus during each heat pulse the skin temperature is obtained every 7 msec (see legend Figure 1). The thermal sensors of the HP-thermode were calibrated before the experiments using a Testo 720 Thermistor (Testo Electronics, Lenzkirch, Germany).
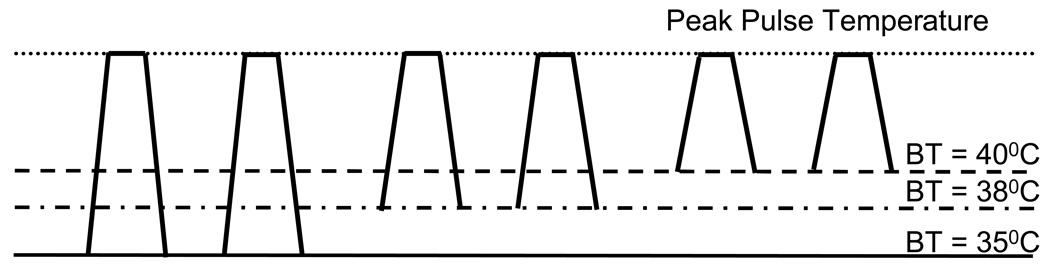
Figure 1.
Heat pulse design used for TSSP experiments. Each heat pulse increased from baseline to peak temperature in 0.5 sec, remained at peak temperature for 0.5 sec, and returned to baseline in 0.5 sec. Repetitive heat pulse trains were adjusted to each subject’s heat pain sensitivity at BT 38°C. During previous experiments the stimulus temperature for each individual subject was identified that resulted in maximal TSSP ratings of 45 ± 10 NPS using BT 38°C. During subsequent TSSP pulse trains, the BT was systematically varied between 35°C, 38°C, and 40°C, whereas the temperature of sensitivity adjusted heat stimuli was kept constant for each subject. TSSP = temporal summation of second pain; BT = baseline temperature; NPS = numerical pain scale
2.3.1 Warm Threshold and Pain Threshold Testing
Thresholds for warmth and heat pain were tested at the hands of study participants using the ascending Method of Limits. The CHEPS thermode was applied to one of four predetermined areas on the palmar surface in counterbalanced fashion. The probe was programmed to increase in temperature from ambient (32°C) to 46°C in 10 sec, thereby producing a rise time of 1.4°C/sec. It has been demonstrated in several studies that stimulus temperature rates below 2.0° C/sec selectively activate C-nociceptors [45; 46]. We compared thresholds of C-nociceptor pain in NC and FM subjects in order to determine whether C-nociceptor sensitization could partially account for differences in TSSP across these two populations. The NC and FM subjects were instructed to immediately indicate when a sensation of warmth or pain was felt for the first time by pressing a button. Each threshold test was repeated four times.
2.3.2 TSSP Training Sessions
All subjects were trained to attend to and rate late sensations evoked by repetitive thermal stimulation of the palmar surface of the hands during at least one separate training session. Details of similar TSSP procedures have been previously reported in detail [34; 36; 39]. Prior to the testing sessions, a technician who was unfamiliar with the hypotheses of the experiment instructed each subject to provide ratings of the intensity of any delayed pain sensations produced by repeated heat pulses to the palmar surface. Study subjects were told that they may or may not feel sensations of heat pain during each pulse and that a late sensation of heat or heat pain would likely be perceived 1 to 2 sec after each heat pulse. They were also asked to pay attention to and provide numerical ratings of the magnitude of the late sensation, which could increase or decrease with stimulus repetition.
The ability of FM and NC to distinguish first and second pain has been established in previously published experiments [37]. Heat pulses applied to the glabrous skin of the hand evoke early sensations of weak pain or warmth and these early sensations diminish somewhat and do not temporally summate during trains of heat pulses [37; 39]. Thus, TSSP and associated neural activity results from repeated C-fiber input [25; 37].
2.3.3 Heat Pulses Used for TSSP Experiments
During the TSSP experiments, each subject received heat pulse trains to the thenar or hypothenar eminence of the hands (Figure 1). The rise and return times of heat pulses to and from BT were individually adjusted to provide identical pulse configurations (rise time 0.5 sec, plateau time 0.5 sec, return time 0.5 sec). Repetitive heat pulses at 0.33 Hz were used to test the dependence of TSSP on stimulus number, i.e. TSSP was expected to increase dependent on the number of stimuli. Previous work by our group has shown that stimulus frequencies of ≥ 0.33 Hz are sufficient to elicit TSSP in most NC [21] and FM participants [35–37; 39]. All heat pulse trains were repeated three times and counterbalanced to control for order effects. The interval between stimulus trains was always 2 min.
2.3.4 Adjustment of TSSP Stimuli to Each Subject’s Pain Sensitivity
Pain sensations from heat stimuli vary as a function of each subject’s peripheral and central sensitivity, which influences the rate of TSSP and its decay. Because this variability is likely to affect comparisons of TSSP between NC and FM subjects, we intended to provide measures of TSSP sensitivity across all subjects. Thus, to measure TSSP sensitivity, we determined the unique stimulus temperature for each subject during preliminary experiments that resulted in final heat pulse ratings of 45 ± 10 NPS units at 0.33 Hz. Stimulus intensities resulting in such TSSP ratings (45 ± 10 NPS units) were chosen to avoid alterations of peripheral and central pain sensitivity of study subjects during repeated trials. To identify individual stimulus intensities associated with moderate TSSP ratings (45 ± 10 NPS units), each subject underwent several TSSP trials at 0.33 Hz. Stimulus trains initially comprised 47°C peak pulses at baseline temperature (BT) of 38°C. If necessary, the peak pulse temperatures were raised or lowered during subsequent trials until subjects achieved maximal TSSP ratings of 45 ± 10 NPS. The number of heat pulses was allowed to slightly vary between subjects (range: 8 ± 2 pulses). Thus, these initial TSSP trials at BT 38°C provided a standard condition which was designed to be very similar within and between groups.
2.3.5 Changes of BT
In psychophysical experiments TSSP is usually modified by changes in stimulus intensity and/or frequency. However, during our TSSP experiments, each individual’s heat pulse characteristics were kept constant (see 2.3.3) but BT was either increased to 40°C or decreased to 35°C to evaluate the effects of BT changes on TSSP. Three different BT (35°C, 38°C, and 40°C) (Figure 1) were used because they were rated as painless by most NC and FM subjects outside of TSSP trials (see 3.5). These manipulations were utilized to characterize central sensitization within and between groups.
To evaluate the effects of BT without heat pulses on NC and FM subjects, all participants were tested with two trains of 35°C, 38°C, or 40°C heat stimuli to the hands for 30 sec in counterbalanced order. The duration of each heat stimulus was chosen to reflect the typical duration of a TSSP stimulus train (30 sec). Experimental heat/pain ratings were obtained at 1 sec, 15 sec, and 30 sec.
2.4 Statistical Analysis
Statistical comparisons of the psychophysical data were conducted using SPSS 16.0 software (SPSS, Inc., Chicago, IL). A series of mixed model ANOVAs for repeated measures was utilized to test experimental pain ratings for differences within and between groups (Alpha level = .05). A priori hypotheses were evaluated by simple contrasts. Threshold testing of warmth and heat pain was evaluated by independent t-tests.
3.0 Results
3.1 Ratings of Somatic Pain in NC and FM Subjects
The healthy subjects reported no somatic pain before and during the experiments. In contrast the FM subjects’ overall pain VAS scores were 2.9 (1.2) before and 3.7 (1.4) after the testing procedures (t(31) = 1.24; p > .05).
3.2 Warmth and Heat Pain Threshold Testing
Independent t-tests of warmth and heat pain thresholds showed no significant temperature differences for either hand (p > .05). Thus the ratings of warmth and heat pain threshold testing were combined for both hands, respectively. The average (SD temperature for warmth and heat pain thresholds were 34.3 (1.1) and 34.2 (.9) as well as 41.4 (2.1) and 41.2 (2.2) for NC and FM subjects, respectively. Independent t-tests (two-tailed) showed no significant difference between NC and FM subjects in either warmth (t(31) = .18; p > .05) or heat pain thresholds (t(31) = .04; p > .05).
3.3 Ratings of BT
First, we analyzed the ratings of NC and FM subjects related to 30 sec heat pulses at BT 35°C, 38°C, and 40°C which were subsequently used during TSSP experiments (Figure 2). These ratings were obtained at 1 sec, 15 sec, and 30 sec. A mixed model ANOVA of heat stimulus ratings with diagnostic group (2), time (3), and BT (3) as independent variables revealed significant main effects for diagnostic group (F(1,31) = 8.9; p = .005), time (F(2,62) = 9.1; p < .001), and temperature (F(2,62) = 23.8; p < .001). However, there were no significant interaction effects noted for temperature × diagnosis (F(2,62) = .31; p > .05) or temperature × time (F(4,124) = 1.7; p > .05). These findings indicate that heat sensations increased with rising temperatures and time. The average ratings of the heat sensations at three different BTs, however, were neither painful nor statistically different between NC or FM subjects.
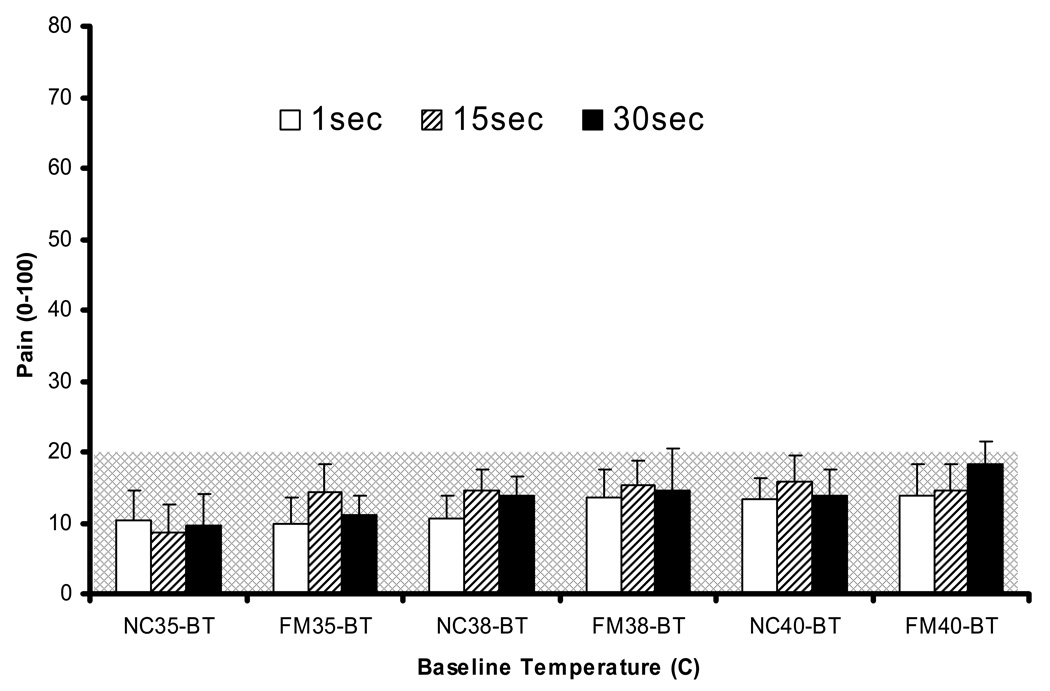
Figure 2.
Ratings of 30 sec heat pulses at three BT (35°C, 38°C, 40°C). BT pulses were applied to each hand for 30 sec similar to the duration of heat pulse trains. Ratings were obtained after 1 sec, 15 sec, and 30 sec. Mean ratings for the 30 sec BT heat pulses increased with increasing temperatures for NC and FM subjects (p < .05), but did not reach pain threshold for any of the BTs. Pain threshold = 20 NPS. BT = baseline temperature; NPS = numerical pain scale; NC = normal controls; FM = fibromyalgia subjects
3.4 Heat Pulse Intensities Used for TSSP Stimuli
As described above (2.3.4), TSSP stimuli were adjusted to each subject’s heat pain sensitivity. The average peak stimulus temperature (SD) necessary to achieve 45 ± 10 NPS ratings during repetitive heat pulse trains at BT 38°C (baseline condition) was 48.3 (2.2) °C and 46.3 (2.6) °C for NC and FM subjects, respectively. An independent t-test showed that the mean peak heat pulse temperature used for TSSP testing was significantly lower in FM than NC subjects (t(31) = 2.1; p = .007).
3.5 TSSP of Sensitivity Adjusted Heat Stimuli at Baseline (BT 38°C)
TSSP ratings of NC and FM subjects using sensitivity adjusted heat stimulus trains at 0.33 Hz and BT 38°C are shown in Figure 3 & 4. This condition was used to compare the changes in TSSP associated with changes in BT to either 35°C or 40°C. The average (SD) number of heat pulses necessary to achieve TSSP of 45 ± 10 NPS was 8.6 (1.8) which was not different for NC and FM subjects (p > .05). To test whether our method of sensitivity adjusted heat pulses was successful in normalizing TSSP across individuals and diagnostic groups, we performed a mixed model ANOVA of TSSP ratings with diagnostic group (2) and stimulus number (3) as independent variables. The ANOVA showed a significant main effect for stimulus number (F(2,62) = 119.3; p < .001) but not for diagnostic group (F(1,31) = .17; p > .05). In addition, the interaction effect of stimulus number × diagnostic group was not significant (F(2,62) = .53; p > .05), indicating that the same rate of TSSP was achieved for NC and FM subjects.
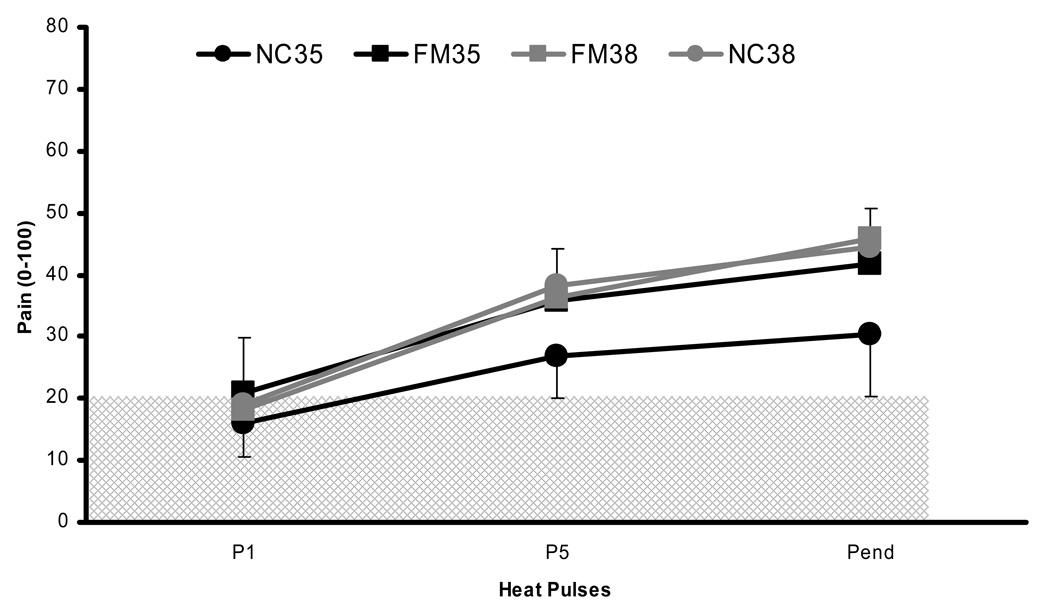
Figure 3.
Experimental pain ratings of NC (black circles) and FM subjects (black squares) during 0.33 Hz TSSP stimuli at the BT of 35°C. All subjects underwent heat pulses of 1.5 sec duration. In addition, the subjects’ ratings of sensitivity adjusted TSSP stimuli at BT 38°C are shown as a reference (gray circles and squares). TSSP of FM subjects was statistically greater than NC at BT 35°C (p < .03). In contrast, the ratings of TSSP stimuli at the BT of 38°C were not different between groups (p > .05). The hatched area represents painless sensations. BT = baseline temperature; P = heat pulse; Pend = end heat pulse; TSSP = temporal summation of second pain
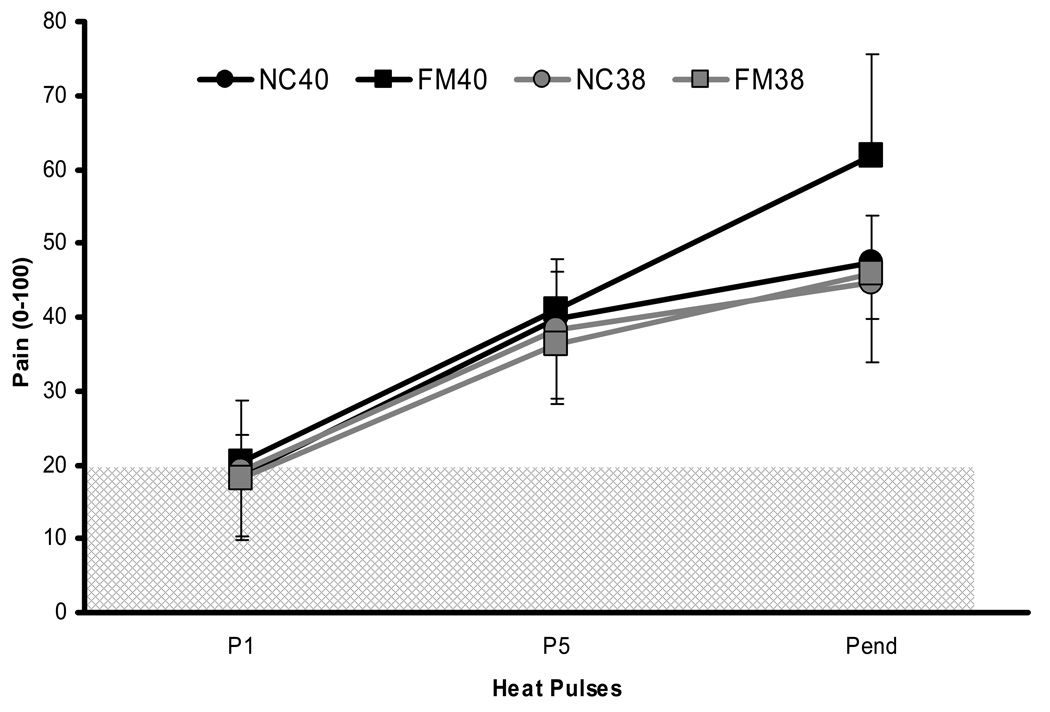
Figure 4.
Experimental pain ratings of NC (black circles) and FM subjects (black squares) during 0.33 Hz TSSP stimuli at the BT of 40°C. All subjects underwent heat pulses of 1.5 sec duration. In addition, the subjects’ ratings of sensitivity adjusted TSSP stimuli at BT 38°C are shown as a reference (gray circles and squares). TSSP of FM subjects was statistically greater than NC at 40°C (p < .03). The hatched area represents painless sensations. BT = baseline temperature; P = heat pulse; Pend = last heat pulse; TSSP = temporal summation of second pain
3.6 Effects of Different BT on TSSP Ratings
NC and FM subjects’ ratings of sensitivity adjusted heat pulses at 0.33 Hz using BT 38°C and the effects of BT changes (to 35°C or 40°C) on TSSP are shown in Figure 3 – 4. Whereas BTs were varied during these experiments, peak temperatures remained unchanged. A mixed model ANOVA of heat pulse ratings with diagnostic group (2), stimulus number (3), and BT (3) as independent variables showed a significant main effect for diagnostic group (F(1,31) = 9.9; p = .004), heat pulse (F(2,62) = 245.6; p < .001), and BT (F(2,62) = 41.7; p < .001). There was a significant interaction effect of BT × diagnosis (F(2,62) = 6.6; p = .002), BT × heat pulse (F(4,124) = 24.6; p < .001), and BT × heat pulse × diagnostic group (F(4,124) = 4.1; p = .004) noted. These findings indicate that TSSP was elicited in NC and FM subjects and that its magnitude was dependent on BT. Simple contrasts were used to further analyze the interactions effects. They showed that BT changes had different effects on TSSP in NC and FM subjects, i.e. TSSP was less in NC compared to FM subjects at 35°C BT (F(1,31) = 16.9; p < .001) (Figure 3) and greater in FM subjects than NC at 40°C BT (F(1,31) = 4.9; p = .04) (Figure 4). The ratings of heat pulse trains by both diagnostic groups were not statistically different after the first pulse (p > .05) but were statistically lower for NC at BT 35°C from the 5th pulse on compared to FM subjects (p < .035). Ratings of the first heat pulse of all TSSP trains, however, were not statistically different between diagnostic groups at any BT (p > .05).
4. Discussion
The notion that widespread peripheral sensitization of heat sensitive C-nociceptors contributes to abnormal TSSP in FM patients is not supported by our study. Specifically, FM patients’ pain thresholds to selective C-fiber stimulation, ratings of 30 sec BT stimuli at 40°C, and the first pulse of repetitive heat pulse trains were not different from NC. Our results add to the growing evidence that abnormalities of cutaneous C-fiber pain are related to specific aspects of central sensitization/ temporal summation in FM patients [6; 32; 36]. In addition, such specificity is inconsistent with the concept that widespread hyperalgesia of FM patients is the result of indiscriminate pain rating bias. Our results, however, should not be generalized for all body areas or tissues and do not exclude other primary afferent abnormalities related to FM, particularly those associated with impulse input from muscle and other deep tissues.
4.1 Lack of Evidence for Widespread Peripheral Sensitization of C-Nociceptors in FM
Several groups of investigators have proposed that peripheral sensitization of cutaneous C-fiber nociceptors is a factor that may influence pain in FM. Indirect evidence for this possibility was provided by studies that showed heightened microvascular reactivity in FM patients [8; 15; 29]. However, not all studies found significant differences in cutaneous microvascular reactivity between FM patients and control subjects [1]. The results of our present study are inconsistent with widespread peripheral sensitization of cutaneous C-nociceptors in FM patients. This conclusion is supported by our findings that responses of FM patients and NC subjects did not differ on: 1) pain thresholds to selective C-fiber stimulation; 2) 30 sec BT stimuli at 35°C, 38°C, 40°C, and 3) the first pulse of repetitive heat pulse trains. When applied to glabrous skin, all three tests predominantly activate heat sensitive C-nociceptors and warm receptors. When sensitized, the former demonstrate lowered thresholds (e.g. 39°C – 40°C) and show enhanced responses to 39°C – 40°C stimuli [3; 4; 13]. Thus, the lack of group differences in ratings of the long duration 40°C stimulus is especially indicative of a lack of peripheral sensitization. In addition, peripheral sensitization of C-nociceptors would also result in enhanced responses to the first pulse of heat pulse trains. Since NC subjects and FM patients responded similarly on all three tests, it is highly unlikely that widespread peripheral sensitization of cutaneous C-nociceptors accounts for abnormal TSSP of FM patients. It is also unlikely that peripheral sensitization is a significant contributor to TSSP in either NC or FM patients. Using heat pulses that are very similar to those used in the present experiment, C-nociceptive afferent recordings of monkeys show reductions not increases in magnitude as more heat pulses occur [25]. Moreover, TSSP still occurs even when the location of the thermode changes between heat pulses [25].
Deep tissues, however, may represent an important source of impulse input for FM patients. Frequently described threshold abnormalities (i.e. tender points) and elevated ratings of nociceptive muscle stimulation (hyperalgesia) of FM subjects possibly are a consequence of abnormal input from sensitized peripheral nociceptors and resultant central sensitization [2; 11; 19].
4.2 Abnormal Heat Evoked Hyperalgesia in FM Patients is Related to Specific Aspects of TSSP
Previous comparisons between NC and FM patients provide multiple lines of evidence for specific abnormalities of TSSP in FM. In comparison to NC subjects, FM patients show enhanced TSSP but no differences in response to the first sensitivity adjusted or unadjusted heat pulse, as also shown in the present study [27; 36]. FM patients demonstrate TSSP at lower stimulus frequencies in comparison to NC subjects. Once TSSP is achieved in FM patients, enhanced second pain sensations can be maintained by very low stimulus frequencies that by themselves are unable to initiate this phenomenon [36; 37]. This phenomenon occurs to a much lesser degree in NC subjects. When NC and FM patients are subjectively equated for TSSP, FM patients demonstrate the same level of pain-related neuronal activation within multiple brain areas despite requiring lower stimulus intensities than NC subjects [33]. In comparison to NC, FM patients also have much longer duration painful “TSSP aftersensations” and the magnitudes of “TSSP aftersensations” strongly correlate with clinical pain [27; 38]. Finally, when BT temperatures were surreptitiously lowered or raised from 38°C in the present study, FM ratings of TSSP were higher than those of NC subjects after the fifth pulse, while no significant changes occurred for the first pulse in the heat pulse trains. These findings suggest that the magnitude of TSSP is resistant to lowering of overall input (i.e., BT reduced from 38 C to 35°C) and sensitive to raising overall input (i.e., BT raised from 38 C to 40°C) in FM patients but not in NC subjects. Thus, FM patients seem to have abnormalities of specific aspects of TSSP. Some of these TSSP abnormalities appear to closely parallel the plasticity of sensitized dorsal horn neurons during windup experiments [9; 14]. In particular, once windup has occurred, sensitized wide-dynamic range neurons in the dorsal horn show larger magnitudes of windup, longer “after-discharges”, and lower frequencies of C-fiber stimulation needed to maintain enhanced responses to C-fiber stimulation once windup occurs [23].
The differences between NC and FM patients with respect to specific aspects of TSSP are unlikely to reflect a general response bias in pain responding. Such response bias should not only result in increased TSSP of FM patients but also in decreased pain thresholds and enhanced ratings of the first heat pulse in a train of heat pulses. A generalized response bias of FM patients is also difficult to reconcile with highly specific differences in TSSP, such as the effects of BT change and the prolonged after-sensations [39], which persist even after equating TSSP magnitudes across NC and FM patients (Figure 3 and Figure 4).
4.3 Lack of Rating Bias Associated with Repeated Stimulation or Ascending Pain Intensity
It is conceivable that abnormal TSSP in FM patients reflects a highly specific bias related to the number of stimuli in a train of heat pulses or to the fact that pain intensity increases throughout this train. Several lines of evidence, however, rule against this possibility. A bias that is specifically a function of the number of heat pulses given should result in higher ratings as more stimuli are applied. While this true for heat pulse trains with 10 or fewer pulses, TSSP shows a plateau in FM patients when more than 10 pulses are applied [39; 42] similar to windup in spinal dorsal horn neurons [9; 14]. This response plateau is inconsistent with a response bias mechanism. If the bias is related to the fact that pain increases with repeated stimulation, then FM should be more sensitive to an ascending series of nociceptive temperatures than NC subjects. Instead, [20]) found that NC subjects and FM patients had the same rate of increase in pain ratings during ascending series of noxious temperature and mechanical stimuli. Finally, when FM and NC groups are equated for TSSP, they show statistically equal levels of neural activation in brain areas at all levels of pain processing, including early levels (i.e., thalamus, S-1) [33]. All of these lines of evidence rule against response bias as an explanation for TSSP abnormalities in FM.
4.4 Why rating bias is not likely a major contributor to TSSP in general
There are multiple lines of evidence that argue against rating bias as a major contributor to TSSP in general. The psychophysical TSSP curve largely parallels the WU curve of spinothalamic tract neurons of unconscious monkeys [24]. Brain activations strongly associated with TSSP occur at all brain levels associated with pain processing, including the earliest stages at thalamic and S-1 levels, a result inconsistent with report bias [33; 34]. However, if expected increases in pain exert an influence at the earliest level of central processing, the dorsal horn, then WU and TSSP might be enhanced by expectation. If so, then expectations are an integral part of WU and TSSP, consistent with demonstrations that expectations help shape afferent processing of pain [10; 12]. However, expectation is not the same construct as report bias, which is providing proportionately higher ratings to lower magnitudes of pain sensations. Therefore, although TSSP may be influenced by expectation, it is unlikely to have been strongly and systematically influenced by report bias in most experiments conducted on this phenomenon so far.
ACKNOWLEDGMENTS
Supported by NIH grants NS38767, AR053541 and in part by Clinical Research Center grant RR00082.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al Allaf AW, Khan F, Moreland J, Belch JJF, Pullar T. Investigation of cutaneous microvascular activity and flare response in patients with fibromyalgia syndrome. Rheumatology. 2001;40:1097–1101. doi: 10.1093/rheumatology/40.10.1097. [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen L, Norregaard J, Jensen R, Olesen J. Evidence of qualitatively altered nociception in patients with fibromyalgia. Arthritis Rheum. 1997;40:98–102. doi: 10.1002/art.1780400114. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JN, Khan AA, Meyer RA, Raja SN. Responses to heat of C-fiber nociceptors in monkey are altered by injury in the receptive field but not by adjacent injury. Pain. 1988;32:327–332. doi: 10.1016/0304-3959(88)90044-9. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- 5.Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 6.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 7.Duggan AW, Hope PJ, Jarrott B, Schaible HG, Fleetwood WS. Release, spread and persistence of immunoreactive neurokinin A in the dorsal horn of the cat following noxious cutaneous stimulation. Studies with antibody microprobes. Neuroscience. 1990;35:195–202. doi: 10.1016/0306-4522(90)90134-p. [DOI] [PubMed] [Google Scholar]
- 8.Granot M, Buskila D, Granovsky Y, Sprecher E, Neumann L, Yarnitsky D. Simultaneous recording of late and ultra-late pain evoked potentials in fibromyalgia. Clinical Neurophysiology. 2001;112:1881–1887. doi: 10.1016/s1388-2457(01)00646-0. [DOI] [PubMed] [Google Scholar]
- 9.Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 10.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. %19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosek E, Ekholm J, Hansson P. Increased pressure pain sensibility in fibromyalgia patients is located deep to the skin but not restricted to muscle tissue. Pain. 1995;63:335–339. doi: 10.1016/0304-3959(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 12.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: Where expectations become reality. PNAS. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 15.Littlejohn GO, Weinstein C, Helme RD. Increased neurogenic inflammation in fibrositis syndrome. J Rheumatol. 1987;14:1022–1025. [PubMed] [Google Scholar]
- 16.Lloyd MA, Appel JB. Signal detection theory and the psychophysics of pain: an introduction and review. Psychosom Med. 1976;38:79–94. doi: 10.1097/00006842-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Mauderli AP, Vierck CJ, Cannon RL, Rodrigues A, Shen CY. Relationships between skin temperature and temporal summation of heat and cold pain. J Neurophysiol. 2003;90:100–109. doi: 10.1152/jn.01066.2002. [DOI] [PubMed] [Google Scholar]
- 18.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 19.Mikkelsson M, Latikka P, Kautiainen H, Isomeri R, Isomaki H. Muscle and bone pressure pain threshold and pain tolerance in fibromyalgia patients and controls. Arch Phys Med Rehabil. 1992;73:814–818. [PubMed] [Google Scholar]
- 20.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 21.Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol. 1972;37:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- 22.Price DD, Harkins SW. The combined use of visual analogue scales and experimental pain in providing standardized measurement of clinical pain. Clin J Pain. 1987;3:1–8. [Google Scholar]
- 23.Price DD, Hayes RL, Ruda M, Dubner R. Neural representation of cutaneous aftersensations by spinothalamic tract neurons. Fed Proc. 1978;37:2237–2239. [PubMed] [Google Scholar]
- 24.Price DD, Hayes RL, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol. 1978;41:933–947. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 26.Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 27.Price DD, Staud R. The neurobiology of fibromyalgia syndrome. J Rheumatol. 2005;32:22–28. [PubMed] [Google Scholar]
- 28.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon RL, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 29.Sann H, Pierau FK. Efferent functions of C-fiber nociceptors. Z Rheumatol. 1998;57:8–13. doi: 10.1007/s003930050226. [DOI] [PubMed] [Google Scholar]
- 30.Simone DA, Baumann TK, Collins JG, LaMotte RH. Sensitization of cat dorsal horn neurons to innocuous mechanical stimulation after intradermal injection of capsaicin. Brain Res. 1989;486:185–189. doi: 10.1016/0006-8993(89)91293-6. [DOI] [PubMed] [Google Scholar]
- 31.Sivilotti LG, Thompson SW, Woolf CJ. Rate of rise of the cumulative depolarization evoked by repetitive stimulation of small-caliber afferents is a predictor of action potential windup in rat spinal neurons in vitro. J Neurophysiol. 1993;69:1621–1631. doi: 10.1152/jn.1993.69.5.1621. [DOI] [PubMed] [Google Scholar]
- 32.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 33.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–142. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (wind-up) J Pain. 2006;7:575–582. doi: 10.1016/j.jpain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staud R, Robinson ME, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 39.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 40.Thompson SW, King AE, Woolf CJ. Activity-Dependent Changes in Rat Ventral Horn Neurons in vitro; Summation of Prolonged Afferent Evoked Postsynaptic Depolarizations Produce a d-2-Amino-5-Phosphonovaleric Acid Sensitive Windup. Eur J Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 41.Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 42.Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 44.Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarnitsky D, Ochoa JL. Studies of heat pain sensation in man: perception thresholds, rate of stimulus rise and reaction time. Pain. 1990;40:85–91. doi: 10.1016/0304-3959(90)91055-N. [DOI] [PubMed] [Google Scholar]
- 46.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]