Abstract
A circadian rhythm is documented in duodenal melatonin in rats, peaking 16.8 hours after light onset. This component is more readily detected after logl0-transformation of the data. It differs between male and female rats, females having a larger circadian amplitude and an earlier acrophase. The circadian rhythm in duodenal melatonin is also found to lead that of pineal melatonin. The results are qualified by the presence at the start of mapping of the second extremum of a double magnetic storm.
Keywords: Chronomics, Duodenum, Rats, Circadian, Melatonin
1. Aim
To examine in rats, as a widely used model, whether the mammalian gut is characterized by a variation for which the zero circadian amplitude assumption can be rejected. If by this objective time-microscopic cosinor approach, a rhythm can be validated, we can also examine the timing of a duodenal rhythm in the light of circadian acrophases in other tissues obtained concomitantly. Data from the same rats on other tissues were also analyzed to assess their time structure by chronomics, in international cooperative studies along the scale of the day and the week.
2. Background
As a rule, in health [1–5] in many species, a prominent time-macroscopic human, murine and avian circadian melatonin rhythm characterizes not only the circulation but also the pineal. Melatonin is found to peak during the daily dark span, with but few exceptions in humans [6] and other organisms [7–9], when time-microscopy is used. The variation is obvious to the naked eye and quantifiable with its parameters by time- microscopy [7]. Chronomics, the study of chronomes (time structures) in data of the stroke-prone spontaneously hypertensive Okamoto rat, has revealed phase differences among circadian melatonin rhythms in the hypothalamus and the pituitary vs. that in the pineal [8]. This study was designed to explore any circadian variation of melatonin in the duodenum, in the context of that in other different tissues of the same group of rats, sampled around the clock for 7 days, even though each rat could only be sampled once. Duodenal tissue was only available from a fraction of the animals used for measurement of melatonin in plasma, pineal gland and hypothalamus [9]. An additional meta-analysis on data from Bratislava on the quail [10] is beyond our scope herein, even if, as originally stated, it represents an important qualification of any generalization.
3. Materials and methods
One hundred and three Wistar (Amsterdam) rats (52 males and 51 females) had been randomly assigned to two rooms kept for a standardization span of 1 month on opposite LD12:12 regimens, to be sampled for 7 days only during working hours at 4-h intervals at six circadian stages should the adjustment to the antiphase regimen be complete. This adjustment was validated by the agreement of data on plasma and pineal with those reported earlier. After bleeding, the animals were killed by cervical dislocation; the tissues, including the duodenum, were removed and placed on dry ice. Blood and tissues were kept frozen at −18 °C until required for analysis [9]. Melatonin was determined by radioimmunoassay [11] directly in plasma and after methanol and chloroform extraction in pineal glands and duodenum, respectively. The assay and extraction procedures were previously validated in our lab for rats [12]. Efficiency of extraction was tested by adding 3H-MEL into each sample and was about 80%. Sheep MEL antiserum (G/S/704–8485, Stockgrand Ltd., Guildford, UK) and 3H-labeled MEL (specific activity of 0.925–1.85 TBq/mmol; NEN Life Science Products, Bad Homburg, Germany) were used in the radioimmunoassay. Duodenal melatonin could be determined in only 31 samples. The data were log10-transformed to normalize their distributions, but only for didactic purposes the analyses of the original data are also shown. All data were analyzed by cosinor [13,14]. Parameter tests were used to test the equality of circadian acrophases [15].
4. Results
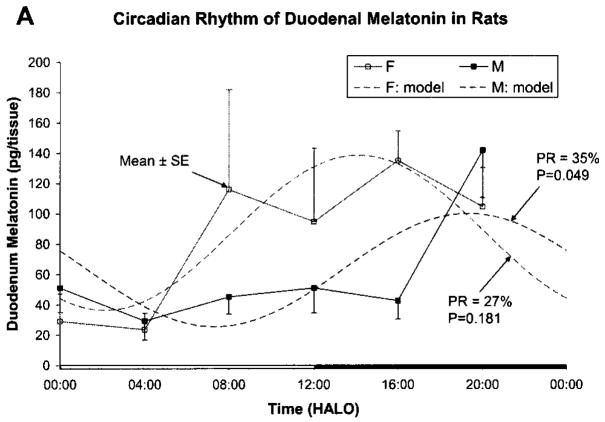
Fig. 1 shows results as original mean values to indicate that the naked eye may find a peak both in the light and in the dark span, in keeping with the original statement in an early investigation, a quarter-century ago [16]. By the unaided eye, with the large standard errors, a rhythm may well be questioned. A log10-transformation (Fig. 1b) more closely approximates a circadian pattern with the peak at 08:00 h after light onset (HALO) seen with the original data being much less prominent after log10-transformation. Fig. 2a, b shows the data separately for male and female rats, again as original values and after log10-transformation, respectively. In Fig. 2a, a rhythm cannot be detected for the males, whereas in Fig. 2b, after log10-transformation, the P-value from the zero-amplitude test for males is 0.057, whereas the P-value derived from the data on females remains below the 5% level of statistical significance. When data from both sexes are combined, statistical significance is reached. The percentage rhythm, PR, representing the proportion of the variance accounted for by the fitted 24-h cosine model is 26% for the duodenum and the corresponding P-value from the zero-amplitude (norhythm) test is 0.016.
Figure 1.
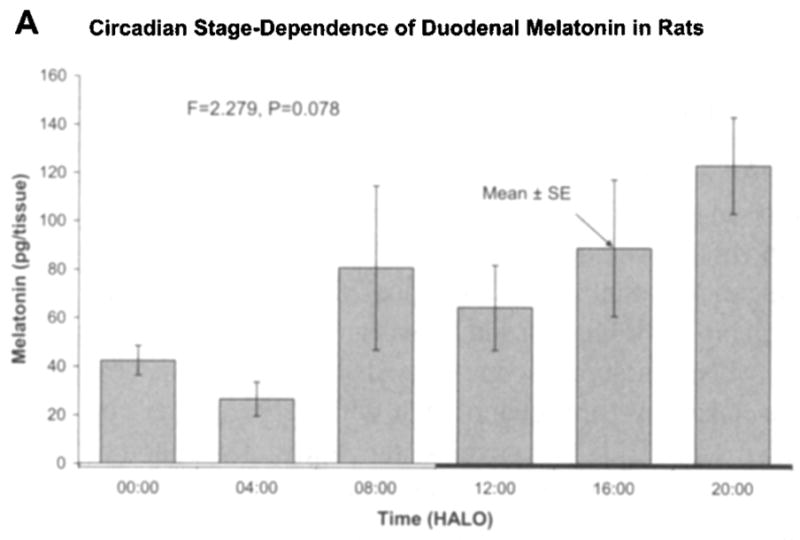
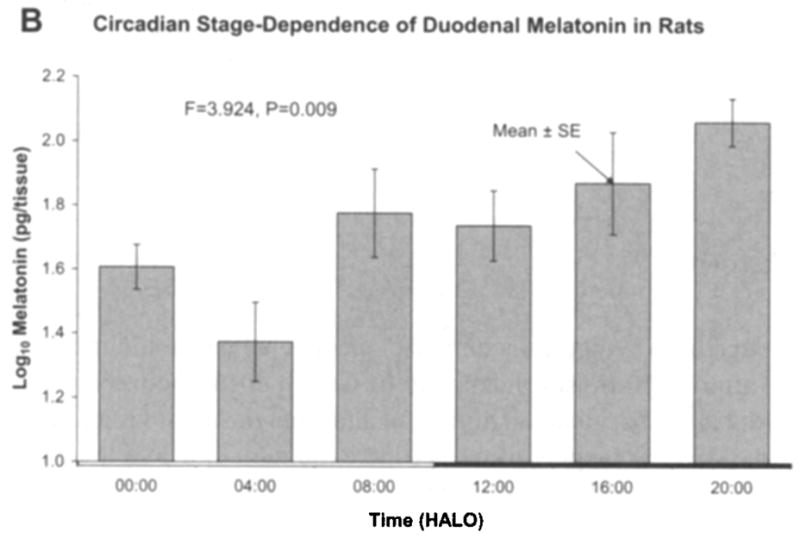
Fig. 1a. Original data of Michal Zeman on duodenal melatonin concentration of Wistar (Amsterdam) rats of both sexes, standardized in antiphasic lighting regimens for 1 month prior to blood collection, summarized as mean and standard error of the mean seemingly reveal (albeit with great dispersion indices; see SEs) two peaks to the naked eye, one in darkness and perhaps another in light. An analysis of variance does not allow the detection of a significant time effect at the 5% level (P = 0.078). Time scale is given in HALO. © Halberg.
Fig. 1 b. After log10-transformation of the data in Fig. 1a, a peak at 08:00 h after light onset (HALO) is less prominent and an analysis of variance allows demonstration of a time effect below the 1% level. © Halberg.
Figure 2.
Fig. 2a. Original data of Michal Zeman separately assessed for males and females allow cosinor demonstration below the 5% level for the data on males ■ but not on those for females □. © Halberg.
Fig. 2b. After log10-transformation of the data in a, the P-values from the zero-amplitude test are 0.057 for the males and 0.026 for the females. © Halberg.
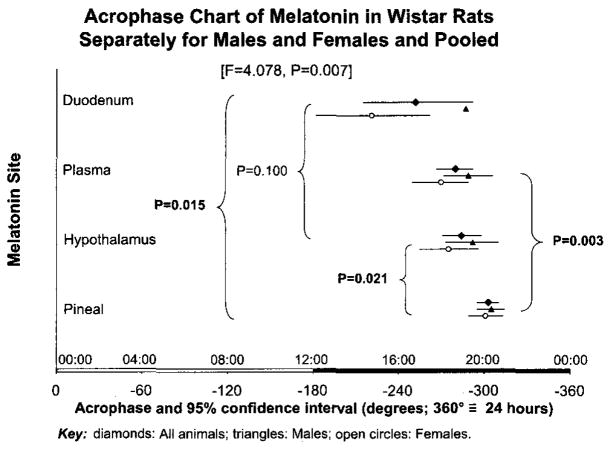
Acrophases, expressed in negative degrees, with 360 degrees equated to 24 h and light onset used as reference, are estimated to be −252°, −280° and −303° for duodenum, plasma and pineal, respectively. They are found to differ ( F = 4.078, P = 0.007), Fig. 3. Duodenal melatonin leads pineal melatonin. The findings of this study are in keeping with a meta-analysis of data collected earlier in Germany from rats and chickens [5,17,18], but differ from very close acrophases found in quail [10].
Fig. 3.
Parameter tests yield P-values that characterize differences among the acrophases found in this study on the duodenum vs. acrophases found in Pecs by Rita Jozsa in other tissues [9], documenting the extrapineal lead in phase vs. the pineal, also specifically the lead in phase of the hypothalamus vs. the pineal. © Halberg.
5. Discussion
Credit for the first demonstration of dramatic day-night differences in the melatonin concentration of the intestine, as well as the pineal, eye, plasma, hypothalamus and Harderian gland goes unquestionably to Vakkuri et al. [19], who studied the pigeon, Columba livia. A two-timepoint approach was a lucky strike, encountering remarkably different peaks and troughs in all tissues examined [19]. The conclusion, in 1985 [19] is pertinent two decades later: “In all tissues [melatonin] had a clear diurnal variation with low levels at midday and high levels at midnight. The highest amounts of melatonin were found in the eyes, duodenum, and the pineal so that extrapineal melatonin exceeded pineal melatonin. Further studies are needed to evaluate the significance of extrapineal melatonin” [19,20].
Species differences in this case are an as yet unsolved challenge. Denser sampling is indicated, even if the phase differences were found with 4-h sampling in rats but not found with about 3-h sampling in the quail [10]. The peak of melatonin in mouse pineal was missed because of sparse sampling that may suffice on some but not all occasions [7].
A gut-brain connection via melatonin would be in keeping with a lead of the gut versus the hypothalamus. A phase difference between hypothalamus and pineal was much larger in the spontaneously hypertensive Okamoto rat (SHR) [7,8] than in this investigation. A modulation of central neural pathways by melatonin has earlier been suggested by one of us in the context of the effect of melatonin on growth and growth hormone [4]. But time relations, although suggestive, are never necessarily causal. They can serve as hints and would be much more conclusive in our case on starving organisms.
Melatonin is often regarded as a solely pineal product, even though it is detected in many parts of the body and has been called a vitamin. Whereas alternatives, such as lags of pineal melatonin to reach other sites, remain to be examined [5,18], the results herein and elsewhere [9] indicate that pineal melatonin has a later phase as compared to the duodenum and other extrapineal sites in the rat. It is also important to note that the results herein are confirmed for the rat and extended to the chicken albeit on an asymmetrical LD15:9 regimen [17,18]. Generalizations to other species are unwarranted [10,21]. While the peaks are close and all in the scotophase, it must not be forgotten that in human disease the circulating melatonin peak can occur during the daily light span [6], as can the hypothalamic and pituitary peaks in the SHR rat [8].
6. Conclusion
A cosinor-documented circadian rhythm characterizes the duodenum of rats kept on an antiphasic LD12:12 regimen for 1 month before study in Pecs, Hungary, as it does on an asymmetrical lighting regimen in Goettingen, Germany, where this finding has been extended to chickens.
Acknowledgments
VEGA1/1294/04 and APVT-20-022704 (M.Z.); ETT 82/2003 (R.J.) GM-13981 (F.H.) and Dr. h.c. Dr. h.c. Earl Bakken Fund (F.H., G.C.).
References
- 1.Wurtman RJ, Axelrod J, Kelly DE. The pineal. New York: Academic Press; 1968. p. 108. [Google Scholar]
- 2.Poeggeler B. PhD Thesis. Georg-August-Universitaet; Goettingen, Germany: 1992. Vergleichende Untersuchungen ueber Melatonin und strukturverwandte Tryptophanmetabolite; p. 63. [Google Scholar]
- 3.Huether G, Poeggeler B, Reimer A, George A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992;51:945–53. doi: 10.1016/0024-3205(92)90402-b. [DOI] [PubMed] [Google Scholar]
- 4.Zeman M, Buyse J, Larnosova D, Herichova I, Decuypere E. Role of melatonin in the control of growth, and growth hormone secretion in poultry. Domestic Anim Endocrinol. 1999;17:199–207. doi: 10.1016/s0739-7240(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 5.Hardeland R, Pöggeler B, Huether G, Cornélissen G, Jozsa R, Zeman M, et al. Chronomics supports lead in phase of duodenal vs. pineal circadian rhythms of melatonin. In: Matsubayasi K, editor. Abstract (Read by Title) 19, Proceedings, 5th International Workshop on Chronoastrobiology and Chronotherapy. Division of Human-Nature Dynamics, Center for Southeast Asian Studies; Nov 6, 2004. pp. 84–8. [Google Scholar]
- 6.Cornélissen G, Halberg F, Tarquini R, Perfetto F, Salti R, Laffi G, et al. Point and interval estimations of circadian melatonin ecphasia in Smith-Magenis syndrome. Biomed Pharmacother. 2003;57(Suppl 1):31s–4s. doi: 10.1016/j.biopha.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Halberg F, Cornélissen G, Conti A, Maestroni G, Maggioni C, Perfetto F, et al. The pineal gland and chronobiologic history: mind and spirit as feedsidewards in time structures for prehabilitation. In: Bartsch C, Bartsch H, Blask DE, Cardinali DP, Hrushesky WJM, Mecke W, editors. The pineal gland and cancer: neuroimmunoendocrine mechanisms in malignancy. Heidelberg: Springer; 2001. pp. 66–116. [Google Scholar]
- 8.Wetterberg L, Sanchez de la Peña S, Halberg F. Circadian rhythm of melatonin release from pineal, hypothalamus and pituitary in hypertensive rats. Prog Clin Biol Res. 1990;341B:145–53. [PubMed] [Google Scholar]
- 9.Zeman M, Józsa R, Cornélissen G, Stebelova K, Bubenik G, Olah A, et al. Biomed Pharmacother. Supp 1. Vol. 59. 2005. Chronomics: circadian lead of murine extrapineal vs. pineal melatonin rhythms with an infradian hypothalamic exploration; pp. $213–$219. [DOI] [PubMed] [Google Scholar]
- 10.Zeman M, Cornélissen G, Balazova K, Herichova I, Jozsa R, Olah A, et al. Circadian acrophase differences in plasma, pineal, hypothalamic and duodenal melatonin in rats -- qualified in quail. In: Matsubayasi K, editor. Abstract (Read by Title) 18, Proceedings, 5th International Workshop on Chronoastrobiology and Chronotherapy. Division of Human-Nature Dynamics, Center for Southeast Asian Studies; Nov 6, 2004. pp. 81–4. [Google Scholar]
- 11.Fraser S, Cowen P, Franklin M, Franey C, Arendt J. Direct radioimmunoassay for melatonin in plasma. Clin Chem. 1983;29:396–7. [PubMed] [Google Scholar]
- 12.Zeman M, Nosalova V, Bobek P, Zakalova M, Cerna S. Changes of endogenous melatonin and protective effect of diet containing pleuran and extract of black elder in colonic inflammation in rats. Biologia. 2001;56:695–701. [Google Scholar]
- 13.Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- 14.Cornélissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. Vol. 1. Chichester, UK: John Wiley & Sons Ltd.; 1998. pp. 642–9. [Google Scholar]
- 15.Bingham C, Arbogast B, Cornélissen Guillaume G, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 16.Bubenik GA. Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Hormone Res. 1980;12:313–23. doi: 10.1159/000179137. [DOI] [PubMed] [Google Scholar]
- 17.Poeggeler B. Vergleichende Untersuchungen über Melatonin und strukturverwandte Tryptophanmetabolite. Zur Rolle von Melatonin und 5-Methoxytryptamin bei einem Dinoflagellaten, Gonyaulax polyedra, sowie pinealen und extrapinealen 5-methoxylierten Indolaminen bei Vertebraten. Doctoral thesis; GOttingen: 1992. [Google Scholar]
- 18.Poeggeler B, Cornélissen G, Huether G, Hardeland R, Jozsa R, Zeman M, et al. Chronomics affirm extending scope of lead in phase of duodenal vs. pineal circadian melatonin rhythms. Biomed Pharmacother. 2005;59(Suppl):S220–S224. doi: 10.1016/s0753-3322(05)80035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vakkuri O, Rintamaki H, Leppaluoto J. Presence of immunoreactive melatonin in different tissues of the pigeon (Columba livia) Gen Comp Endocrinol. 1985;58:69–75. doi: 10.1016/0016-6480(85)90136-4. [DOI] [PubMed] [Google Scholar]
- 20.Bubenik GA. Gastrointestinal melatonin: localization, function and clinical relevance. Dig Dis Sci. 2002;47:2336–48. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 21.Zeman M, Cornélissen G, Balazova K, Jozsa R, Olah A, Nagy G, et al. Circadian rhythm of melatonin in rat duodenum. In: Cornélissen G, Kenner R, Fiser B, Siegelova J, editors. Proceedings, Symposium: Chronobiology in Medicine. Dedicated to the 85th Anniversary of Professor Franz Halberg. Brno: Masaryk University; 2004. pp. 95–97. [Google Scholar]