Abstract
Intergeneric bacterial adherence is responsible for the complexity of the microbiota in human dental plaque and is believed to enable some extraneous bacteria to initially colonize the human oral cavity. Some current evidence indicates that Streptococcus sanguis, an early colonizer of teeth, enhances subsequent colonization by Porphyromonas (Bacteroides) gingivalis, a bacterium associated with advanced adult periodontitis. In this study, selected strains of P. gingivalis and S. sanguis were tested for their adherence activities in vitro. A differential filtration assay was devised in which one member of the test pair was radiolabeled. Heterogeneous aggregates that formed in mixed suspensions were collected on polycarbonate filters (8-microns pore size) and were washed free of individual bacteria and small homologous clumps. P. gingivalis 381, W50, JKG7, and 33277 adhered to S. sanguis G9B, M5, Challis 6, and 38. P. gingivalis A7A1-28 did not adhere well to S. sanguis under these conditions. More precise measurements of intergeneric adherence were obtained with an alternative assay with radiolabeled P. gingivalis and an artificial dental plaque composed of S. sanguis coupled to cyanogen bromide-activated agarose beads. CNBr-agarose was selected as the supporting matrix for the plaque because it was uniformly and permanently coated with S. sanguis and because P. gingivalis had negligible adherence activity for streptococcus-free beads. P. gingivalis W50 grown to the early stationary phase adhered to S. sanguis-coated beads in higher numbers than either midlogarithmic- or late-stationary-phase cells. Intergeneric adherence was not inhibited or reversed by the presence of lactose or other monosaccharides or disaccharides. Pretreatment of either bacterium with trypsin or proteinase K reduced subsequent adherence by 86 to 100%. Neuraminidase treatment of P. gingivalis caused 98% reduction of adherence, whereas similar treatment of S. sanguis caused only a 2% loss. Preincubation of P. gingivalis at 60 degrees C for 30 min decreased subsequent adherence to S. sanguis-coated beads by 94%. Adherence was reduced by 96% when bacteria were assayed while suspended in human whole saliva or when pretreated with saliva and subsequently assayed in buffer. The concentration of whole human saliva required to inhibit 50% adherence in this assay was 23 micrograms per ml (1:200 dilution). Suspension of the bacteria in normal rabbit serum resulted in 94% inhibition of adherence. These data indicate that saliva and serum may be important host defense factors for controlling Porphyromonas-Streptococcus adherence.
Full text
PDF



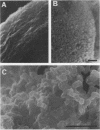
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Schwarz-Faulkner S., Grove D. A. Coaggregation among periodontal pathogens, emphasizing Bacteroides gingivalis--Actinomyces viscosus cohesion on a saliva-coated mineral surface. Can J Microbiol. 1988 Mar;34(3):299–306. doi: 10.1139/m88-055. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. The isolation and partial characterization of a sulphated glycoprotein from human whole saliva which aggregates strains of Streptococcus sanguis but not Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):791–797. doi: 10.1016/0003-9969(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989 Nov;57(11):3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989 Oct;57(10):3204–3209. doi: 10.1128/iai.57.10.3204-3209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Multigeneric aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986 Nov;168(2):851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Phucas C. S. Effect of saliva on coaggregation of oral Actinomyces and Streptococcus species. Infect Immun. 1984 May;44(2):228–233. doi: 10.1128/iai.44.2.228-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ellen R. P. Relative adherence of Bacteroides species and strains to Actinomyces viscosus on saliva-coated hydroxyapatite. J Dent Res. 1989 Sep;68(9):1308–1312. doi: 10.1177/00220345890680090301. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Van der Hoeven J. S. Role of interbacterial adherence in colonization of the oral cavities of gnotobiotic rats infected with Streptococcus mutans and Veillonella alcalescens. Infect Immun. 1981 Aug;33(2):467–472. doi: 10.1128/iai.33.2.467-472.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Ellen R. P., Grove D. A. Bacteroides gingivalis-Actinomyces viscosus cohesive interactions as measured by a quantitative binding assay. Infect Immun. 1987 Oct;55(10):2391–2397. doi: 10.1128/iai.55.10.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Theilade J., Mikkelsen L. Microbiological studies on early dento-gingival plaque on teeth and Mylar strips in humans. J Periodontal Res. 1982 Jan;17(1):12–25. doi: 10.1111/j.1600-0765.1982.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., de Graaff J. The role of black-pigmented Bacteroides in human oral infections. J Clin Periodontol. 1988 Mar;15(3):145–155. doi: 10.1111/j.1600-051x.1988.tb01561.x. [DOI] [PubMed] [Google Scholar]