SUMMARY
Purpose
To examine antiepileptogenic and antiictogenic potential of retigabine under conditions of rapid kindling epileptogenesis during different stages of development.
Methods
The experiments were performed in postnatal day 14 (P14), P21 and P35 male Wistar rats. After stereotaxic implantation of hippocampal stimulating and recording electrodes, the effects of retigabine on baseline afterdischarge properties were studied. Next, the animals underwent rapid kindling (sixty 10 second trains, bipolar 20 Hz square wave pulses delivered every five minutes). The progression of seizures (kindling acquisition), and responses to test stimulations after kindling (retention) were compared between retigabine and vehicle-treated rats. Additionally, the effects of retigabine on the severity of seizures in previously kindled animals were examined.
Results
When administered intraperitoneally in doses that induced only mild, or no motor deficits, retigabine significantly dampened brain excitability, evident as the increase of afterdischarge threshold and shortening of afterdischarge duration. During kindling, retigabine delayed the development of focal seizures in P14 rats, and prevented the occurrence of full limbic seizures at all three ages. At P14 and P21, but not at P35, pretreatment with retigabine prevented the establishment of kindling-induced enhanced seizure susceptibility. Administration of retigabine to kindled animals decreased the severity of seizures induced by test stimulation. The effect was most prominent at P14.
Discussion
Retigabine exerted both antiepileptogenic and antiictogenic effects under conditions of rapid kindling model. These effects were apparent during post-neonatal, early childhood and adolescent stages of development.
Keywords: Antiepileptic drugs, development, epileptogenesis, kindling, retigabine
INTRODUCTION
Retigabine is a new antiepileptic drug that exhibits broad spectrum anticonvulsant activity under conditions of experimental seizure models. Retigabine inhibited epileptiform activity induced in rat hippocampal slices by 4-aminopiridine, bicuculline, NMDA (Armand et al., 1999) and low Mg2+ (Armand et al., 2000; Dost and Rundfeldt, 2000), as well as in vitro spontaneous bursting in the entorhinal cortex of rats that had been subjected to kainate status epilepticus (Smith et al., 2007). The broad spectrum of activity was evidenced in vivo, by its ability to inhibit acute seizures which were induced in rats by maximal electroshock, pentylenetetrazole, picrotoxin, N-methyl-D-Aspartate (Rostock et al., 1996). The drug was effective against audiogenic seizures in DBA/2 mice (Rostock et al., 1996), and kindled seizures in rats (Nickel et al., 1993; Tober et al., 1996).
Antiepileptic activity of retigabine has been proved in clinical investigations conducted thus far. For example, in Phase II clinical trials adjunctive treatment with retigabine reduced the frequency of partial onset seizures (Porter et al., 2007).
Retigabine exerts its antiepileptic effects mainly through the opening of KCNQ2/3 channels (a.k.a. KV7.2/7.3) and subsequent augmentation of M-type K+ currents (Main et al., 2000; Rundfeldt et al., 2000; Tatulian et al., 2001; Wickenden et al., 2000), which have been implicated in regulating neuronal excitability (Cooper, 2001; Hu et al., 2007; Vervaeke et al., 2002). Particularly high expression of KCNQ2/3 channels is observed in the axon initial segment and the nodes of Ranvier (Devaux et al., 2004; Pan et al., 2006). While the importance of this target is underscored by the contributory role played by mutations in KCNQ2 and KCNQ3 channels in benign neonatal convulsions (Biervert et al., 1998; Singh et al., 1998), these channels are likely involved in other forms of epilepsy, including mesial temporal lobe epilepsy. Indeed, in the hippocampus in vitro, retigabine prevented bursting of hippocampal CA1 pyramidal cells (Yue & Yaari, 2004; 2006). Increase of brain concentration of GABA through the inhibition of the activity of GABA transaminase has also been considered as a mechanism of anticonvulsant effect of retigabine (Kapetanovic et al., 1995; Sills et al., 2000).
While the ability retigabine to inhibit limbic seizures has been shown in some studies (Nickel et al., 1993; Tober et al., 1996), it is not known whether retigabine can interfere with the progression and the establishment of a chronic epileptic state (i.e. epileptogenesis). Meanwhile, the importance of developing of antiepileptogenic therapies, as opposed to merely anticonvulsant agents has been emphasized during recent years (Holtkamp and Meierkord, 2007; Stables et al., 2003).
The main purpose of the current study was to examine the antiepileptogenic potential of retigabine under conditions of animal model of limbic epileptogenesis. As such, we employed a rapid kindling protocol, which might be regarded as a model of “compressed epileptogenesis”. In this process, the animal is brought from the naïve to epileptic state within a matter of several hours (Lothman et al., 1985; Lothman and Williamson, 1994). An important feature of rapid kindling is that it can be induced during early stages of development (Holmes and Thompson, 1987; Michelson and Lothman, 1991), and thus affords examining both epileptogenesis and its modification within an ontogenic window of interest (Mazarati et al., 2007; Zhao et al., 2006). The latter feature is of particular importance when employing rodent models of epileptogenesis, since the maturation of the rodent brain often supersedes the evolution of seizures following a precipitating insult (Sankar et al., 2000; Suchomelova et al., 2006), or during conventional kindling. Our previous studies showed that the same antiepileptic drug may have different effect on rapid kindling in the animals at different stages of brain maturation (Mazarati et al., 2007); such age-discriminating effect of antiepileptic drugs under conditions of rapid kindling opens the possibility to use this model for preclinical evaluation of antiepileptic drugs tailored for certain age populations. Therefore, we studied the effects of retigabine on seizure evolution at three different ages, which corresponded to post-neonatal, early childhood, and adolescent stages of development in humans.
METHODS
Animals
The experiments were performed in male Wistar rats (Charles River, Wilmington, MA). The age of the animals at the beginning of the experiments was postnatal day 14 (P14, post-neonatal), 21 (P21, pre-adolescent), and 35 (P35, adolescent). The experiments were done in accordance with the policies of the National Institutes of Health and the UCLA Office for the Protection of Research Subjects.
Surgery
Animals were anesthetized with Isoflurane and stereotaxically implanted with a twisted bipolar stimulating electrode (Plastics1 Inc., Roanoke, VA) in the left ventral hippocampus according to the coordinates indicated in Table 1. A tripolar recording skull electrode (Plastics1) was placed over the left hemisphere, 2 mm anterior from bregma with the ground connected to a screw in the nasal bone. The experiments began 24 hours after surgery.
Table 1.
Coordinates for stimulating electrode placement.
| Age, postnatal days | mm deep from brain surface | ||
|---|---|---|---|
| P14 | 3.0 | 3.9 | 4.2 |
| P21 | 2.9 | 3.7 | 3.8 |
| P35 | 3.6 | 4.9 | 5.0 |
Kindling procedure
Rapid kindling procedure was performed as previously described (Lothman et al., 1985; Mazarati et al., 2007; Michelson and Lothman, 1991). Animals were placed in Plexiglas observation chambers (Instech Solomon, Plymouth Meeting, PA) with free access to food and water. Stimulating and recording electrodes were connected to the 3 + 2 channel commutator-swivel model SL2X3C (Plastics1). From the swivel, the stimulating electrode was connected to the DS8000 electrical stimulator via DSI100 stimulus isolator (World Precision Instruments, Sarasota, FL). The recording electrode was connected to the MP100/EEG100B system (BIOPAC, Santa Barbara, CA). The latter was connected to a computer equipped with the USB1W external ADD board and AcqKnowledge 3.8 acquisition software (BIOPAC). The acquisition system was configured so that 5 minute-long EEG segments were triggered by stimuli generated via electrical stimulator. Animals’ behavior was digitally captured by a video camera connected to the computer.
At the beginning of the experiment, afterdischarge threshold and duration were detected by applying electrical stimuli of 10 s train duration, 50 ms peak interval, 1 ms pulse duration, square wave biphasic waveform, starting with 0.1 mA, with 0.05 mA increments, delivered every 10 minutes. After the detection of the afterdischarge, evident as a high-frequency response lasting 2 s or more after the end of the train, animals underwent rapid kindling procedure.
Kindling consisted of 60 trains delivered every 5 minutes using the parameters described above and a current of 0.05 mA above the afterdischarge threshold; thus, the whole procedure lasted five hours. Behavioral seizures were scored using a modified Racine (1972) scale: 0, normal behavior (active, or sleep); stage 1, motor arrest with or without facial twitches, chewing; stage 2, head bobbing and/or jerking; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing, and falling.
Twenty four hours after the end of kindling, afterdischarge properties were re-examined. Animals were considered kindled if they exhibited a decrease of afterdischarge threshold as compared to baseline conditions, and developed behavioral seizure in response to the threshold stimulation.
Evaluation of behavioral motor effect of retigabine
Animals received intraperitoneal (i.p., volume 2 ml/kg) injection of retigabine (Valeant Pharmaceuticals International, Aliso Viejo, CA), dissolved in 50% dimethyl sulfoxide (DMSO, Sigma St. Louis MO). Drug doses (2.5 and 5 mg/kg) were chosen based on earlier report (Tober et al., 1996). Control animals were injected with 50% DMSO. Animals were examined every 5–10 minutes fir the presence of motor deficits, for a period of 2 hours after injections. The examination of behavioral effects of retigabine was based on the description provided by Tober et al. (1996). Particularly, we studied two behavioral indices: righting reflex, which was measured as a time required to return to normal position after the animal had been placed on its back; and flat body posture which was expressed using the following ordinal scale: 0: normal behavior; 1: mild – reduced muscle tone, preserved ability to spontaneous movement; 2: moderate – reduced muscle tone, no spontaneous movement, but preserved movement in response to tail pinch; 3: severe – complete loss of muscle tone and movements response to tail pinch is minimal.
Evaluation of antiepileptic effects and the duration of action of retigabine
Antiictogenic effects
Animals were subjected to the rapid kindling. On the next day, afterdischarge properties were examined, and animals received a single bolus of retigabine, dissolved in 50% DMSO. Control animals received DMSO. Based on behavioral effects of retigabine (Results, Fig. 1), P14 animals received 2.5 mg/kg of the drug, while P21 and P35 animals were given 5 mg/kg. Afterdischarge threshold and behavioral seizure response to threshold stimulation were examined 20 min, 1 hour, 2 hours and 4 hours after drug injection.
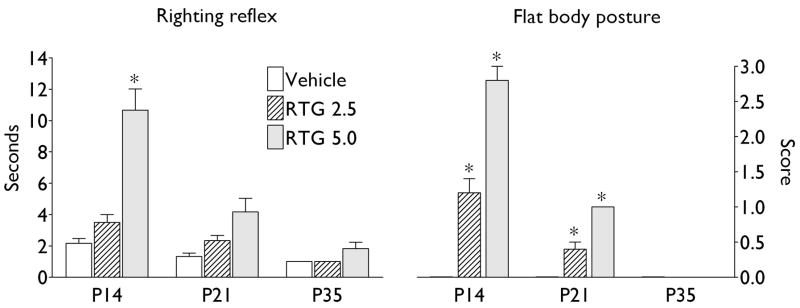
Fig. 1. Motor behavioral effects of retigabine.
Latency of recovery after administration of either vehicle, or retigabine (RTG) under conditions of righting reflex test, and motor impairment score for flat body posture test are presented as Mean ± SEM. Asterisk: p < 0.05 vs. Vehicle (Kruskall-Wallis + Dunn’s post hoc test).
Antiepileptogenic effects: kindling acquisition
After the initial determination of afterdischarge properties, naive animals received i.p. injection of retigabine (P14- 2.5 mg/kg; P21 and P35- 5 mg/kg). Control rats were injected with DMSO. Twenty minutes after the injection, afterdischarge properties were examined again, after which animals were subjected to kindling. For the kindling procedure the stimulation parameters were set at levels that induced an afterdischarge in both control and retigabine-treated rats. Based on the duration of the effects of retigabine obtained during examination of antiictogenic effects of the drug (Results, Fig. 2), all rats received the second injection of the drug in the same dose as initially, after 30 stimulations, that is in the middle of kindling procedure. The following parameters were used to describe kindling progression: number of stimulations required to reach first focal seizure (stage 1); maximal behavioral seizure score; number of full kindled seizures (stage 4–5) recorded in response to 5 consecutive stimulations.
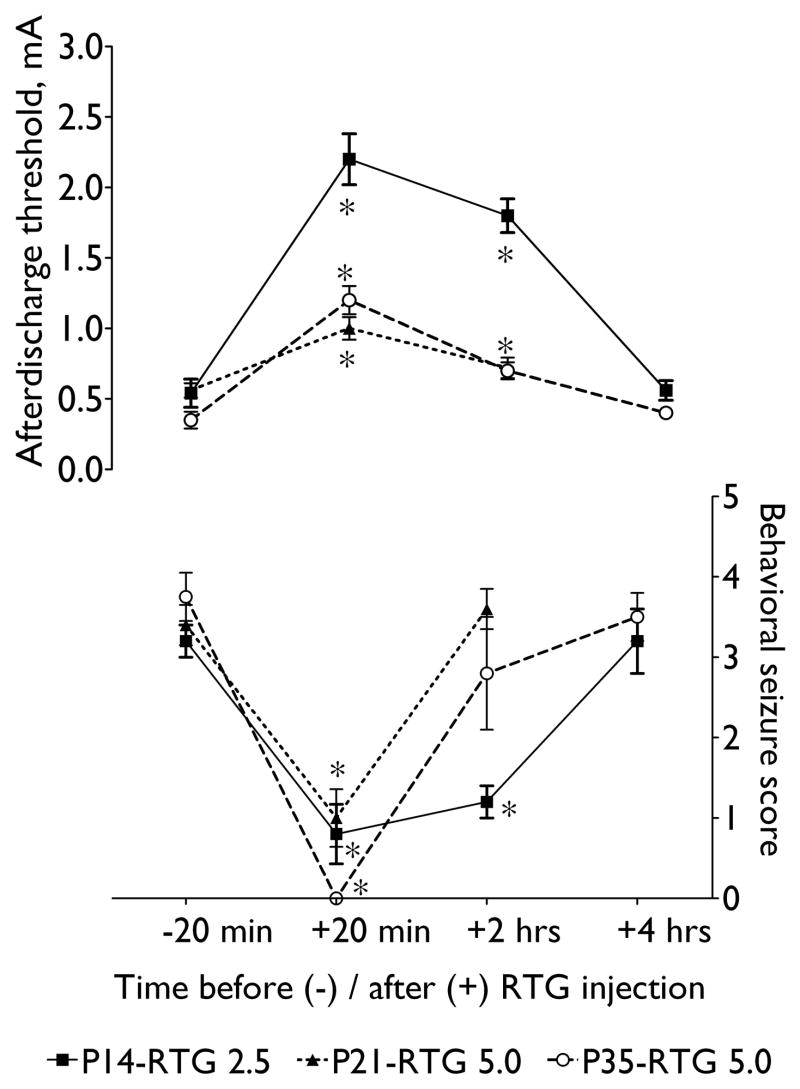
Fig. 2. Antiictigenic effects of retigabine under conditions of the rapid kindling.
Afterdischarge threshold (upper, left Y-axis) and behavioral seizure score in response to threshold stimulation (bottom, right Y-axis) before (-20 min on X-axis) and at different time points after rertigabine administration to kindled animals. Retigabine (RTG) was injected at a dose of 2.5 mg/kg to P14, and 5 mg/kg to P21 and P35 rats. Data are presented as Mean ± SEM. Asterisk- p < 0.05 vs. “−20 min”: repeated measure ANOVA + Bonferroni test for afterdischarge threshold; Friedman + Wilcoxon test for behavioral seizure score.
Antiepileptogenic effects: kindling retention
Twenty four hours after the end of kindling (26 hours after the last retigabine injection), afterdischarge properties and behavioral seizure score in response to threshold stimulation were examined.
Data analysis
Data were analyzed using Prizm 4 software (GraphPad, San Diego, CA). Number of animals in experimental groups and statistical tests used are indicated in respective results sections. For samples that had passed D’Agostino and Pearson omnibus normality test, appropriate parametric analyses were applied; samples that failed normality test were subjected to non-parametric analyses. Statistical tests used for each experiment are indicated in respective figure legends.
RESULTS
Motor behavioral impairments after retigabine injection, and selection of doses for kindling studies
In two-week old animals, strong motor impairments were observed after the administration of retigabine at 5.0 mg/kg; when injected at 2.5 mg/kg, the drug induced only mild motor deficits, (Fig. 1). At the age of three weeks, retigabine induced mild motor impairments when given in both doses. In both two- and three- weeks old subjects, behavioral impairments developed within 5 minutes after drug injection, and were discernable for no more than 30 minutes from the time of the occurrence. No observable alterations in motor behavior were recorded in five-week old rats after the drug was administered at either 2.5, or 5 mg/kg (Fig. 1). Administration of DMSO did not induce motor deficits.
Since at a dose of 5 mg/kg, retigabine induced moderate motor impairments in P14 animals, for further studies the dose of 2.5 mg/kg was selected as the one leading to only mild and short-lasting behavioral alterations. In P21 and P35 rats, we employed a dose of 5 mg/kg, as in this amount of retigabine induced only very mild (P21) or no (P35) motor deficits.
Antiictogenic effects of retigabine
The ability of retigabine to inhibit kindled limbic seizures was examined in the animals which had been subjected to the rapid kindling procedure one day earlier.
As it had been shown earlier (Mazarati et al., 2007; Michelson and Lothman, 1991), rapid kindling lowered afterdischarge threshold, as compared to pre-kindling values. Thus, in P14 pups (n = 8) afterdischarge threshold after kindling was 0.54 ± 0.1 mA as compared with 1.2 ± 0.12 mA (p < 0.05). In P21 animals (n = 7) the value was 0.56 ± 0.5 vs. 0.86 ± 0.1 before kindling (p < 0.01). In P35 rats (n = 7) afterdischarge threshold after kindling was 0.35 ± 0.6 mA as compared to 0.8 ± 0.06 mA prior to kindling (p < 0.05). In addition to changes in afterdischarge properties, threshold stimulation applied to kindled animals induced behavioral seizures with the severity ranging between scores 3 and 4 (Fig. 2).
Administration of retigabine to animals of each of the three ages induced strong anticonvulsant effect. Statistically significant increase of afterdischarge threshold and reduction of the severity of behavioral seizures induced by threshold stimulation peaked during the first test (20 minutes after retigabine treatment, Fig. 2). In P14 animals, the reported changes in afterdischarge and seizure properties were observed during 1-hour (not shown) and 2-hours (Fig. 2) test stimulations. In P21 subjects, anticonvulsant effects of retigabine were present at 1 hour (not shown), but disappeared 2 hours after treatment (Fig. 2). P35 rats exhibited elevated afterdischarge threshold and attenuated seizure severity at 1 hour (not shown). At 2 hours, afterdischarge threshold was significantly lower than at 20 min and 1 hour, but still above pre-treatment value; seizure severity was still lower than before retigabine injection, although the difference was not statistically significant (Fig. 2). Four hours after treatment, both afterdischarge threshold and seizure severity returned to pre-injection values in both P14 and P35 subjects.
In the control experiments, administration of DMSO to separate groups of kindled animals did not affect afterdischarge properties (data not shown).
The effects of retigabine on afterdischarge properties in naïve rats
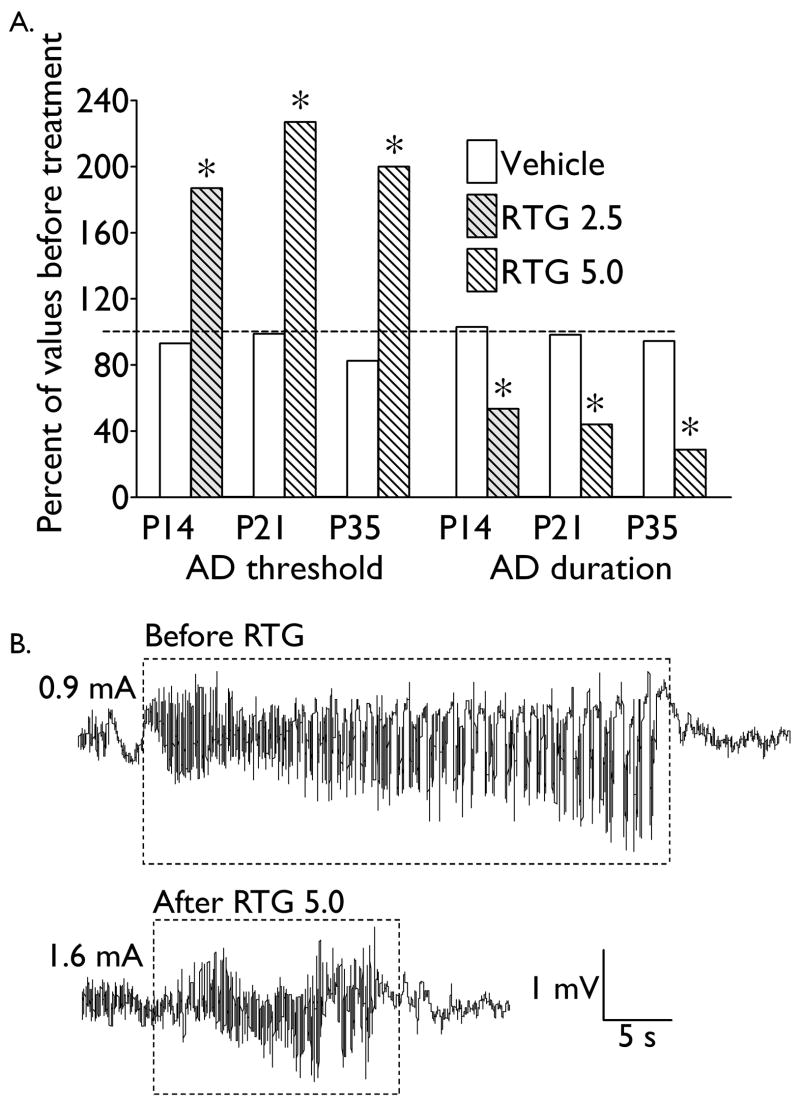
Administration of retigabine to naïve animals strongly suppressed brain excitability at P14 (n = 8, 2.5 mg/kg), P21 (n = 6, 5 mg/kg) and P35 (n = 8, 5 mg/kg). This was manifested by an increase in afterdischarge threshold, and shortening of afterdischarge duration (Fig. 3). The extent of inhibition of afterdischarge was similar across the three ages. No changes in afterdischarge properties were observed in DMSO-treated subjects.
Fig. 3. The effects of retigabine on afterdischarge properties in naïve rats.
A. Afterdischarge (AD) properties are presented as percent of respective values prior to treatment with either DMSO (Vehicle), or retigabine (RTG). Dose of retigabine (mg/kg) for each age is indicated on the figure legend. Asterisk- p < 0.05 vs. values before treatment; paired Student t-test. B. Examples of electrographic responses obtained from a P21 animal before and after retigabine injection (5 mg/kg). Note the increase of afterdischarge threshold (indicated on the left side of each tracing), and the shortening of afterdischarge duration (outlined by the dashed rectangle).
The effects of retigabine on kindling acquisition
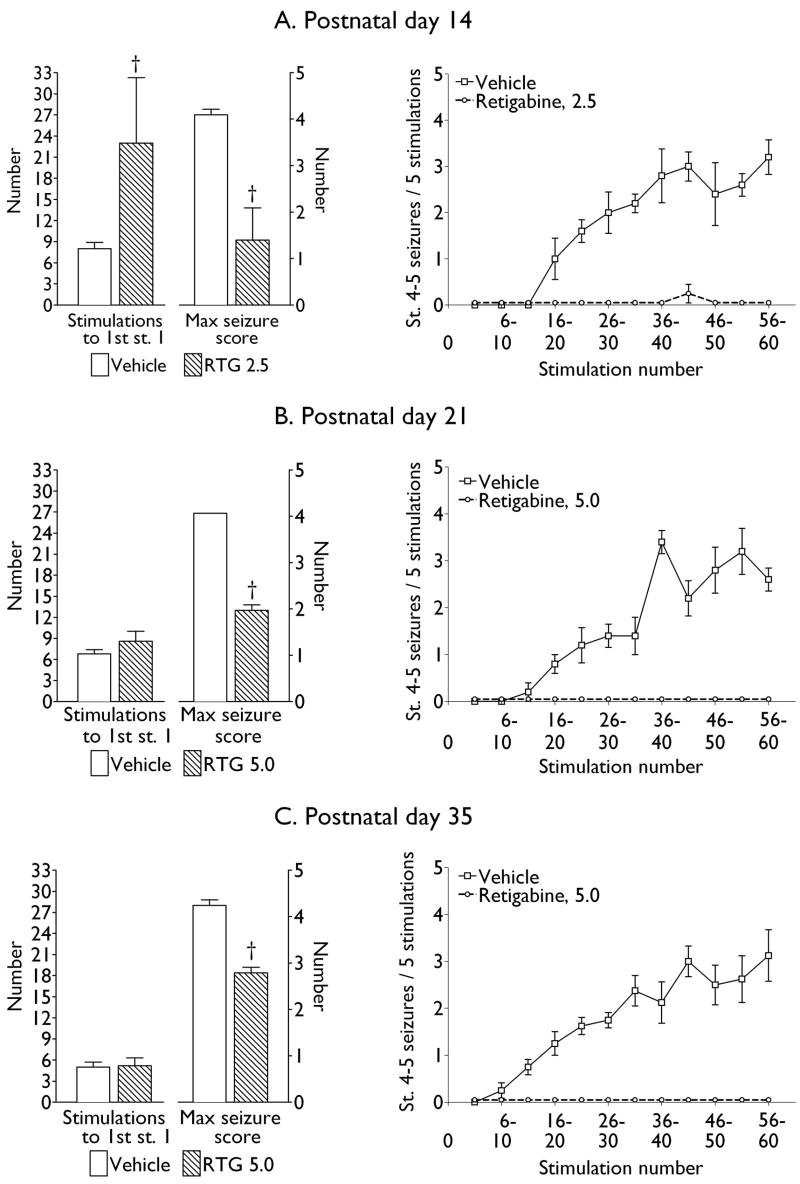
In P14 animals (n = 5), treatment with retigabine (2.5 mg/kg) significantly delayed the development of the first stage 1 seizure, and attenuated the severity of behavioral seizure responses (Fig. 4 A, Left). In P21 (n = 6, Fig. 4 B, Left) and P35 (n = 7, Fig. 4 C, Left) retigabine injection (5 mg/kg) mitigated the severity of behavioral seizures during the course of kindling; however, the occurrence of the first focal seizure was not delayed as compared to controls.
Fig. 4. The effects of retigabine on kindling acquisition.
Left: Bar graphs show number of kindling stimulations required to reach first stage 1 seizure, as well as maximal score of behavioral seizures observed during rapid kindling. Right: Line graphs show the progression of full kindled seizures, expressed as the number of stage 4–5 convulsions recorded in response of 5 consecutive kindling trials. Data are presented as Mean ± SEM. Dagger – p < 0.05 vs. Vehicle (Student t-test).
In control animals, kindling was characterized by the occurrence and progressive increase of the density of stage 4–5 seizures (Fig. 4, A-C, right panels). At the same time, treatment with retigabine prevented the development of full kindled seizures in the animals of all ages (only one of the five P14 rats exhibited a single stage 4 seizure, Fig. 4 A, right panel).
The effects of pre-treatment with retigabine on rapid kindling retention
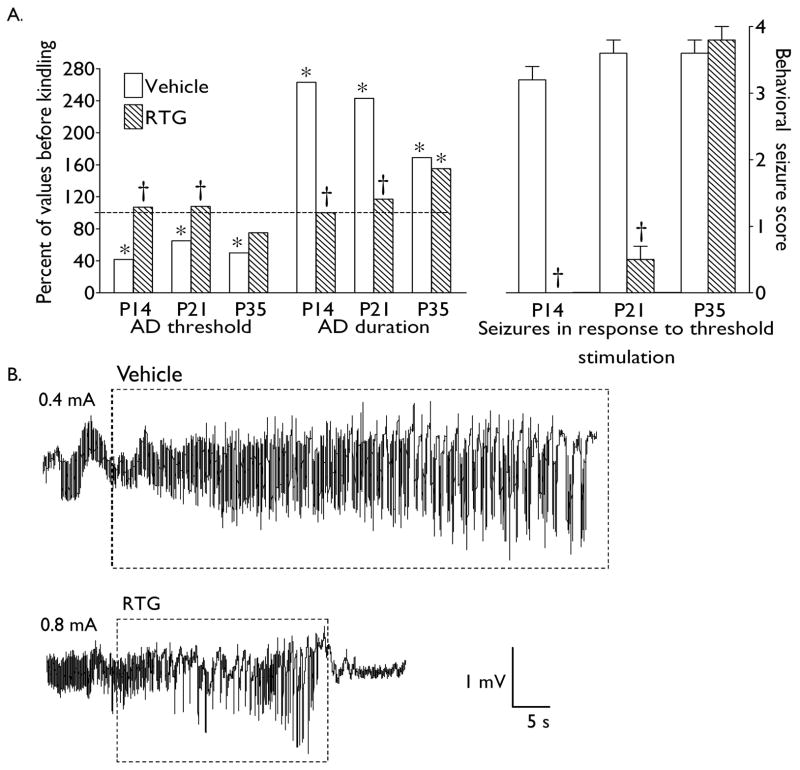
Twenty four hours after the completion of kindling procedure, afterdischarge properties and seizure responses to test stimulations were examined in the same animals which had been used for kindling acquisition studies. Control (Vehicle-treated) animals of each of the three ages, exhibited reduction of afterdischarge threshold and increase of afterdischarge duration; furthermore, animals developed stage 3–4 seizures in response to threshold stimulation (Fig. 5) In contrast to control animals, P14 (n = 5) which had been treated with retigabine prior to, and during the kindling procedure, showed neither a change in afterdischarge properties as compared to pre-kindling value, nor behavioral seizures in response to test stimulations. Likewise, in P21 rats (n = 6) pretreated with retigabine, afterdischarge properties did not differ from pre-kindling indices; seizure responses were limited to focal (stage 1) seizures in 3 out of 6 animals, while 3 rats did not develop seizures at all. Surprisingly, inhibition of kindling acquisition in P35 animals (n = 7), did not prevent post-kindling increase of hippocampal excitability, that was evident as the reduction of afterdischarge threshold, increase of afterdischarge duration, and development of overt limbic seizures in response to threshold stimulation; at this age, changes in afterdischarge properties and seizure response were similar to those in control rats (Fig. 5).
Fig. 5. Afterdischarge properties and seizure responses to test stimulation 24 hours after rapid kindling.
A. Afterdischarge (AD) properties are expressed as percent of respective values prior to kindling for the same animals. Behavioral seizure severity is presented as Mean ± SEM. Asterisk – p < 0.05 vs. respective values before kindling (paired Student t-test); dagger- p < 0.05 vs. Vehicle treated age-matched rats (Student t-test for afterdischarge properties; Mann-Whitney test for behavioral seizure score). B. Examples of electrographic responses obtained 24 hours after rapid kindling from P21 rats which had been treated with either vehicle or retigabine (RTG) prior to kindling procedure. Note that in the vehicle – treated animal, afterdischarge developed in response to a lower-current stimulation (indicated on the left side of each tracing), and that the duration of afterdischarge was longer, as compared to the retigabine – treated rat (afterdischarges are outlined by dashed rectangles). Furthermore, both afterdischarge threshold and duration in the retigabine- treated animal are similar to those observed in retigabine – free animals prior to kindling (compare with the top tracing in Fig. 3 B).
DISCUSSION
Our studies provide evidence that retigabine exerts antiictogenic and antiepileptogenic effects in developing rats under conditions of rapid kindling limbic epileptogenesis.
As was mentioned in the Introduction, retigabine has been reported to possess a broad-spectrum anticonvulsant profile in several animal models. Our studies extends the anticonvulsant efficacy of retigabine to include another seizure model, as the drug reversed rapid kindling-induced decrease of afterdischarge threshold and mitigated the severity of behavioral seizures in kindled animals. This result is in agreement with previously shown effectiveness of the drug in amygdala kindling model (Tober et al., 1996). Furthermore, antiictogenic effects of the drug appeared to be age-dependent. Thus, both the extent (determined by the increase of afterdischarge threshold) and the duration of anticonvulsant effects were more prominent at P14, as compared to both P21 and P35, particularly considering that the dose used in two-week old animals was one-half of that administered to older subjects.
Retigabine significantly decreased brain excitability in naïve rats, which was manifested by an increase in the afterdischarge threshold and shortening of the afterdischarge duration. This effect was consistent with results reported by Tober et al (1996). In contrast to retigabine, topiramate did not modify baseline afterdischarge properties in P14 and P21 animals (Mazarati et al., 2007). At P35 topiramate inhibited ambient afterdischarge, but the extent of this effect was much weaker than that observed for retigabine.
The central finding of the present study was that retigabine strongly antagonized rapid kindling epileptogenesis. The importance of developing of antiepileptogenic therapies has been emphasized in recent years. However, many preclinical studies failed to reveal antiepileptogenic properties for both established and novel antiepileptic drugs (e.g. Glien et al., 2002; Holtkamp and H. Meierkord, 2007; Nissinen et al., 2004; Prasad et al., 2002; Pitkänen et al., 2005; Suchomelova et al., 2006). A significant obstacle in the preclinical development of antiepileptogenic drugs lies in the limitations of existent animal models. Post-status epilepticus chronic epilepsy in rodents has been used for studying antiictogenic therapies (e.g. Grabenstatter et al., 2005, 2007; Nissinen and Pitkänen, 2007). However, high costs, long duration and large sample size due to inter- and intra-animal variability of seizure frequency, make this model quite difficult to use for antiepileptogenic studies (Mazarati, 2007; Nissinen and Pitkänen, 2007). Another important limitation of post-status epilepticus models is that they cannot be used for studying pediatric epileptogenesis: indeed due to rapid maturation, animals that had experienced status epilepticus at young age, become adults by the time they develop spontaneous seizures (Suchomelova et al., 2006).
Rapid kindling which has been described both in adult and immature animals (Holmes and Thompson, 1987; Lothman et al., 1985; Michelson and Lothman, 1981), provides a mechanism in which to study the disease modifying properties of a particular therapy within a very narrow ontogenic window. As such, rapid kindling can be used for screening antiictogenic medications (DeSmedt et al., 2005; Mazarati et al., 2007). In addition, several reports emphasized the possibility of using the model for testing antiepileptogenic therapies. For example, modification of seizure progression under conditions of rapid kindling has been described for uridine (Zhao et al., 2006), the neuropeptide galanin (Mazarati et al., 2006), and topiramate (Mazarati et al., 2007).
While further validation of rapid kindling as an adequate model of epileptogenesis is necessary, the present data provide initial evidence that retigabine may effectively interfere with the evolution of epilepsy. Similarly to its antiictogenic effects, retigabine appeared to be more effective than topiramate at younger ages. Indeed, findings from previous studies (Mazarati et al., 2007) demonstrated that topiramate inhibited rapid kindling epileptogenesis in two- three- and five-week old animals; however, the drug was most effective at the oldest age tested, and was least effective in P14 rats. Unlike topiramate, retigabine was equally effective across all three ages in inhibiting stage 4–5 seizures; in addition, the drug delayed the occurrence of focal (stage 1) seizures selectively in two-week old rats, an effect not observed for topiramate.
The antiepileptogenic effects of retigabine were confirmed in the post-acquisition experiments designed to examine kindling retention. In the rat, retigabine has a half-life of about 2 hours after an i.p. administration (Valeant Pharmaceuticals International, data on file). Thus, hindering of enhanced excitability 24 hours after kindling reflects the drug’s antiepileptogenic effect. While prevention of enhanced seizure susceptibility in P14 and P21 rats further supported the antiepileptogenic efficacy of retigabine, its failure to prevent the establishment of kindling state in P35 rats is somewhat surprising. Indeed, during the kindling experiments under retigabine treatment, stimulation parameters were titrated to elicit afterdischarges and thus to assure adequate electrophysiological response in all ages. While the inability of retigabine to interfere with kindling retention in older animals requires further studies and explanations, it emphasizes higher efficacy of the drug in younger ages.
Intraperitoneal administration of retigabine in therapeutic doses induced none, or mild motor behavioral deficits. The presence of motor impairments after administration of retigabine at a dose of 5 mg/kg contradicts earlier finding that this dose induced no side effects (Tober et al., 1996). A noticeable difference between the present investigation and earlier studies is the age of experimental animals employed. Although Tober et al (1996) did not specify animals’ age, their weight (220–260 g) corresponds to mature rats, aged 10 weeks or more; in contrast we employed subjects that were 5 weeks-old or younger. It is conceivable, that similar to antiictogenic and antiepileptogenic effects, the observed motor deficits were age-dependent. Such suggestion is supported by our observation that the severity of behavioral impairments was most noticeable in P14 animals.
An important question is whether behavioral abnormalities following retigabine administration are mechanistically connected to its anticonvulsant effects. A related technical issue is whether behavioral deficits affect behavioral manifestation of seizures, and thus whether the observed antiepileptic effect represents a false positive result. With this in mind, the behavioral impairments observed in our studies lasted for no longer than 30 minutes after drug administration. At the same time, antiictogenic effects were tested beginning from 20 min after retigabine injection, and persisted between 1 and 2 hours, depending on age. In kindling acquisition studies, the procedure also began 20 min after retigabine treatment, and the second injection was performed 2.5 hours into the kindling procedure. Furthermore, prevention of retention of kindling in P14 and P21 subjects was evident 26 hours after the last retigabine injection. Considering that the half-life of the drug is approximately 2 hours (Valeant Pharmaceuticals International, data on file), this time frame is more than sufficient to completely eliminate retigabine. Finally, in addition to the inhibition of behavioral seizures, retigabine treatment modified electrophysiological correlates of increased brain excitability. All the mentioned facts suggest that antiepileptic effects of retigabine are not related to behavioral abnormalities observed early and for a short time after its administration. Nevertheless, it cannot be excluded that motor deficits and antiepileptic effects of retigabine share common mechanisms; to examine this possibility, it would be interesting to know whether these two phenomena can be dissociated, for example by using KCNQ2/3 channels blockers.
As it has been pointed out throughout the study, many of the effects of retigabine were more pronounced in younger animals, than in their older counterparts. The reasons for stronger and longer-lasting effect of retigabine in younger subjects are not clear. A pharmacokinetic basis for this observation is possible, but seems unlikely. In clinical studies, maximal plasma concentration of retigabine was similar between young (18–40 years) and older (66–81 years) volunteers; half-life of retigabine was around 8 hours at younger age, but was approximately 30% longer in older patients, which was attributed to age-related decline of renal function and hence slower clearance (Hermann et al., 2003). Indeed, clinical experience with other antiepileptic drugs with predominantly renal clearance suggests that a higher dose of the drug is commonly used in young children to achieve clinical efficacy comparable to that in adults. Nevertheless, detailed and age-related pharmacokinetic data in rodents will facilitate future experiments.
At the same time, the reported differences in the effects of retigabine may reflect the importance of KCNQ2/3 channels in regulating brain excitability during development. Thus, under conditions of low Mg2+ model of epileptiform activity in the hippocampus, pharmacological blocking of KCNQ2/3 channels facilitated the transition from interictal to ictal bursting more prominently in immature rats (P8-P15), as compared to adult animals (Qui et al., 2007). Inhibition of KCNQ2/3 channels enhanced depolarization-induced release of GABA and glutamate, and facilitated propagation of neuronal excitability in hippocampal slices from early post-natal, but not older rats (Okada et al., 2003). Finally, prolonged pharmacological blocking of M-currents by linopirdine induced epileptiform activity in hippocampal slices obtained from 1–2 weeks old, but not 8–9 weeks old rats (Peña and Alavez-Pérez, 2006). These data suggest that the excitability of the immature brain is particularly prone to the regulatory effect of M-currents, and hence to the antiepileptic effects of KCNQ2/3 openers. At the same time, reports from both earlier studies and our current data show that retigabine is highly effective in both immature and adult subjects.
In conclusion, our data suggest that retigabine may be effective in inhibiting limbic epileptogenesis, and may be particularly beneficial in pediatric patients. Upon further verification of the observed preferential effectiveness of the drug in the developing brain, retigabine might take its place in the emerging armamentarium of antiepileptic drugs that impact pediatric epileptogenesis.
Acknowledgments
This work was supported by research grants NS059505 (AM) NS046516 from NIH/NINDS, and by DAPA Foundation (RS), and by the scholarship from the Inha University (YK). We are grateful for the critical reading of the manuscript by Dr. H. Steve White, and his suggestions.
Footnotes
The authors confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of conflict of interest. Dr. Jim Wu is an employee of Valeant Pharmaceuticals International, the owner of retigabine. The remaining authors have no conflicts of interest.
References
- Armand V, Rundfeldt C, Heinemann U. Effects of retigabine (D-23129) on different patterns of epileptiform activity induced by 4-aminopyridine in rat entorhinal cortex hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:33–39. doi: 10.1007/pl00005320. [DOI] [PubMed] [Google Scholar]
- Armand V, Rundfeldt C, Heinemann U. Effects of retigabine (D-23129) on different patterns of epileptiform activity induced by low magnesium in rat entorhinal cortex hippocampal slices. Epilepsia. 2000;41:28–33. doi: 10.1111/j.1528-1157.2000.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Cooper EC. Potassium channels: how genetic studies of epileptic syndromes open paths to new therapeutic targets and drugs. Epilepsia. 2001;42(Suppl 5):49–54. doi: 10.1046/j.1528-1157.2001.0420s5049.x. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Vonck K, Raedt R, Dedeurwaerdere S, Claeys P, Legros B, Wyckhuys T, Wadman W, Boon P. Rapid kindling in preclinical anti-epileptic drug development: the effect of levetiracetam. Epilepsy Res. 2005;67:109–116. doi: 10.1016/j.eplepsyres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dost R, Rundfeldt C. The anticonvulsant retigabine potently suppresses epileptiform discharges in the low Ca ++ and low Mg++ model in the hippocampal slice preparation. Epilepsy Res. 2000;38:53–66. doi: 10.1016/s0920-1211(99)00065-0. [DOI] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Loscher W. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43:350–357. doi: 10.1046/j.1528-1157.2002.18101.x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Clark S, Dudek FE. Anticonvulsant Effects of Carbamazepine on Spontaneous Seizures in Rats with Kainate-induced Epilepsy: Comparison of Intraperitoneal Injections with Drug-in-food Protocols. Epilepsia. 2007;48:2287–2295. doi: 10.1111/j.1528-1167.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46:8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- Hermann R, Ferron GM, Erb K, Knebel N, Ruus P, Paul J, Richards L, Cnota HP, Troy S. Effects of age and sex on the disposition of retigabine. Clin Pharmacol Ther. 2003;73:61–70. doi: 10.1067/mcp.2003.12. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Thompson JL. Rapid kindling in the prepubescent rat. Brain Res. 1987;433:281–284. doi: 10.1016/0165-3806(87)90032-0. [DOI] [PubMed] [Google Scholar]
- Holtkamp M, Meierkord H. Anticonvulsant, antiepileptogenic, and antiictogenic pharmacostrategies. Cell Mol Life Sci. 2007;64:2023–2041. doi: 10.1007/s00018-007-7021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27:1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic IM, Yonekawa WD, Kupferberg HJ. The effects of D-23129, a new experimental anticonvulsant drug, on neurotransmitter amino acids in the rat hippocampus in vitro. Epilepsy Res. 1995;22:167–173. doi: 10.1016/0920-1211(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Williamson JM. Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res. 1994;649:71–84. doi: 10.1016/0006-8993(94)91050-2. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF, Conry JA, Moon PF, Perlin JB. Kindling with rapidly recurring hippocampal seizures. Brain Res. 1985;360:83–91. doi: 10.1016/0006-8993(85)91223-5. [DOI] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- Mazarati A. The best model for a cat is the same cat...Or is it? Epilepsy Curr. 2007;7:112–114. doi: 10.1111/j.1535-7511.2007.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Shin D, Auvin S, Sankar R. Age-dependent effects of topiramate on the acquisition and the retention of rapid kindling. Epilepsia. 2007;48:765–773. doi: 10.1111/j.1528-1167.2007.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Sollenberg U, Lundström L, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: the effects of subtype selective agonists and the role of G-protein mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Lothman EW. An ontogenetic study of kindling using rapidly recurring hippocampal seizures. Brain Res Dev Brain Res. 1991;61:79–85. doi: 10.1016/0165-3806(91)90116-z. [DOI] [PubMed] [Google Scholar]
- Nickel B, Shandra A, Godlevsky L, Mazarati A, Kupferberg H. Antiepileptic effects of a new drug D-20443. Epilepsia. 1993;24(Suppl 2):95. [Google Scholar]
- Nissinen J, Pitkanen A. Effect of antiepileptic drugs on spontaneous seizures in epileptic rats. Epilepsy Res. 2007;73:181–191. doi: 10.1016/j.eplepsyres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Large CH, Stratton SC, Pitkanen A. Effect of lamotrigine treatment on epileptogenesis: an experimental study in rat. Epilepsy Res. 2004;58:119–132. doi: 10.1016/j.eplepsyres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Okada M, Zhu G, Hirose S, Ito KI, Murakami T, Wakui M, Kaneko S. Age-dependent modulation of hippocampal excitability by KCNQ-channels. Epilepsy Res. 2003;53:81–94. doi: 10.1016/s0920-1211(02)00249-8. [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Alavez-Perez N. Epileptiform activity induced by pharmacologic reduction of M-current in the developing hippocampus in vitro. Epilepsia. 2006;47:47–54. doi: 10.1111/j.1528-1167.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- Prasad A, Williamson JM, Bertram EH. Phenobarbital and MK-801, but not phenytoin, improve the long-term outcome of status epilepticus. Ann Neurol. 2002;51:175–181. doi: 10.1002/ana.10085. [DOI] [PubMed] [Google Scholar]
- Qiu C, Johnson BN, Tallent MK. K+ M-current regulates the transition to seizures in immature and adult hippocampus. Epilepsia. 2007;48:2047–2058. doi: 10.1111/j.1528-1167.2007.01193.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rostock A, Tober C, Rundfeldt C, Bartsch R, Engel J, Polymeropoulos EE, Kutscher B, Loscher W, Honack D, White HS, Wolf HH. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23:211–223. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C, Netzer R. The novel anticonvulsant retigabine activates M-currents in Chinese hamster ovary-cells transfected with human KCNQ2/3 subunits. Neurosci Lett. 2000;282:73–76. doi: 10.1016/s0304-3940(00)00866-1. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin D, Mazarati AM, Liu H, Katsumori H, Lezama R, Wasterlain CG. Epileptogenesis after status epilepticus reflects age- and model-dependent plasticity. Ann Neurol. 2000;48:580–589. [PubMed] [Google Scholar]
- Sills GJ, Rundfeldt C, Butler E, Forrest G, Thompson GG, Brodie MJ. A neurochemical study of the novel antiepileptic drug retigabine in mouse brain. Pharmacol Res. 2000;42:553–557. doi: 10.1006/phrs.2000.0738. [DOI] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Smith MD, Adams AC, Saunders GW, White HS, Wilcox KS. Phenytoin- and carbamazepine-resistant spontaneous bursting in rat entorhinal cortex is blocked by retigabine in vitro. Epilepsy Res. 2007;74:97–106. doi: 10.1016/j.eplepsyres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkanen A, White HS. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: summary of the NIH/NINDS/AES models II workshop. Epilepsia. 2003;44:1472–1478. doi: 10.1111/j.0013-9580.2003.32803.x. [DOI] [PubMed] [Google Scholar]
- Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–243. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober C, Rostock A, Rundfeldt C, Bartsch R. D-23129: a potent anticonvulsant in the amygdala kindling model of complex partial seizures. Eur J Pharmacol. 1996;303:163–169. doi: 10.1016/0014-2999(96)00073-8. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Gu N, Agdestein C, Hu H, Storm JF. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006;576:235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anticonvulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95:3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Marolewski A, Rusche JR, Holmes GL. Effects of uridine in models of epileptogenesis and seizures. Epilepsy Res. 2006;70:73–82. doi: 10.1016/j.eplepsyres.2006.03.003. [DOI] [PubMed] [Google Scholar]