Abstract
Colorectal cancer (CRC) is a major cause of death worldwide. Studies suggest that dietary fibre offers protection perhaps by increasing colonic fermentative production of butyrate. This study examined the importance of butyrate by investigating the effects of resistant starch (RS) and butyrylated-RS on azoxymethane (AOM)-induced CRC in rats. Four groups (n = 30 per group) of Sprague–Dawley rats were fed AIN-93G-based diets containing a standard low-RS maize starch (LAMS), LAMS + 3% tributyrin (LAMST), 10% high-amylose maize starch (HAMS) and 10% butyrylated HAMS (HAMSB) for 4 weeks. Rats were injected once weekly for 2 weeks with 15 mg/kg AOM, maintained on diets for 25 weeks and then killed. Butyrate concentrations in large bowel digesta were higher in rats fed HAMSB than other groups (P < 0.001); levels were similar in HAMS, LAMS and LAMST groups. The proportion of rats developing tumours were lower in HAMS and HAMSB than LAMS (P < 0.05), and the number of tumours per rat were lower in HAMSB than LAMS (P < 0.05). Caecal digesta butyrate pools and concentrations were negatively correlated with tumour size (P < 0.05). Hepatic portal plasma butyrate concentrations were higher (P < 0.001) in the HAMSB compared with other groups and negatively correlated with tumour number per rat (P < 0.009) and total tumour size for each rat (P = 0.05). HAMSB results in higher luminal butyrate than RS alone or tributyrin. This is associated with reduced tumour incidence, number and size in this rat model of CRC supporting the important protective role of butyrate. Interventional strategies designed to maximize luminal butyrate may be of protective benefit in humans.
Introduction
Colorectal cancer (CRC) is a common malignancy in Western societies resulting in 8% of all cancer deaths (1) and is emerging as a significant cause of morbidity and mortality in several Asian and Eastern European countries as they adopt western dietary habits and lifestyles. This cancer is considered mostly preventable by appropriate diet and associated lifestyle factors (2).
Resistant starch (RS) is dietary starch that escapes digestion in the small intestine. It is fermented by colonic bacteria with the production of short chain fatty acids (SCFA). Consumption of RS by humans has been shown to improve the colonic environment by softening stools, increasing faecal bulk, decreasing faecal pH, increasing luminal SCFA concentrations (3) and reducing the accumulation of potentially harmful by-products of protein fermentation (4). Recent animal studies show that RS prevents colonic DNA damage in rats fed high protein diets (5).
The main SCFA produced by colonic fermentation of carbohydrate are acetate, propionate and butyrate. These SCFA have important roles in the maintenance of bowel health that include increasing colonic blood flow, improving mineral and water absorption from the colon and the maintenance of low colonic pH (6). Butyrate, which is the preferred energy source for colonocytes (7), may be protective against large bowel cancer by inhibiting histone deacetylase thereby enhancing the apoptotic deletion of genetically damaged cells (8) through a histone hyperacetylation-mediated pathway (9,10). Butyrate has also been shown to inhibit proliferation and induce differentiation of cancer cells in vitro (11,12).
Strategies to increase colonic levels of SCFA, particularly butyrate, may have clinical and public health benefits. However, there may be limits to the usefulness of promoting RS consumption to increase butyrate levels as the microflora of some individuals have limited capacity to ferment certain types of RS, the SCFA profile produced can vary with RS source (13) and dietary fibre is not universally tolerated. Starches acylated to a degree of substitution of 0.2–0.25 (i.e. 0.2–0.25 of the hydroxyl groups on each starch D-glucopyranosyl unit were derivatized or replaced by substituent acids) have been shown to be an effective means of delivering specific SCFA to the colon in both animals (14,15) and humans (16). The SCFA, esterified to the starch carrier molecule, are liberated by bacterial enzymes and are absorbed and utilized by the colonocytes or gut microbes.
This study investigated the importance of butyrate by examining the effects of RS and butyrylated-RS on tumour development in rats treated with azoxymethane (AOM). The effect of systemic butyrate absorbed from the small intestine was also examined by the inclusion of rats fed tributyrin (LAMST).
Materials and methods
Animals and diets
Adult male Sprague–Dawley rats (198 ± 2 g) were purchased from the Animal Resource Centre, Murdoch University, Western Australia. The rats were housed in wire-bottomed cages in a temperature-controlled room (22–24°C) with a 12 h light–dark cycle (lights on 8:00–20:00). They were allocated randomly into four groups (n = 30 per group) with approximately equal body weights and given free access to water and diet throughout the study.
The experimental diets were based on a control AIN-93G (17) diet that contained 530 g standard low-amylose maize starch, 200 g casein, 70 g corn oil and 50 g α-cellulose/kg of diet. The choline, methionine, minerals, vitamins and antioxidant were added as described previously (15). The amount of standard maize starch in the diet was reduced to allow for the addition of experimental starches and tributyrin. The experimental diets were control (LAMS, low RS), low-amylose maize starch with 3% tributyrin (LAMST, low RS), 10% high-amylose maize starch (HAMS, high RS) or 10% butyrylated HAMS (HAMSB, high RS). The latter was manufactured by National Starch Food Innovation and had a degree of substitution of 0.25. HAMS and HAMSB were cooked with water and spray dried (to minimize crystallite formation) as described previously (18) before addition to the diets. This process was used to mimic the condition of starches consumed in foods by humans as cooking changes the production and delivery of butyrate to the colon by starches (15). All diets were prepared regularly at CSIRO and stored at 4°C. The rats were fed fresh diet daily.
After 28 days of feeding the experimental diets rats were injected with 15 mg of AOM/kg (Sigma Chemical Co., St Louis, MO). The rats were maintained on their experimental diets and were injected with the same dose of AOM a week later. They were monitored closely until the termination of the experiment 25–27 weeks post-initial AOM injection. Rats were anaesthetized with halothane and blood collected into heparin-treated tubes from the hepatic portal vein. The small and large intestines and caecum were opened longitudinally, digesta collected and the epithelial surface cleaned with 0.15 mM NaCl/l. The colon was divided into two equal length parts designated proximal and distal colon. The location, number and size of visible tumours were recorded for each animal before the tumours were collected and fixed in 10% buffered formalin, embedded in paraffin blocks and processed by routine histological procedures for subsequent haematoxylin and eosin staining.
All procedures involving animals were approved by the CSIRO Human Nutrition Animal Ethics Committee and complied with the Australian Code of Practice (19).
Analytical procedures
Analysis of SCFA, dry matter and pH was undertaken as described previously (15,20). All tumours were examined histologically by a single independent observer unaware of the dietary treatment and categorized into adenomas and adenocarcinomas (invasive tumours) based on the criteria described previously (21). The ‘tumour incidence’ was defined as the proportion of rats in a group that had at least one tumour in the small or large bowel. The ‘size’ of each tumour was calculated using the formula:
where D1 and D2 are the length and width of each tumour.
Statistical analyses
GraphPad Prism version 4.0 for Windows (http://www.graphpad.com) computer software was used for statistical analyses. Parametric data (body weight, organ weights and digesta measures) were analysed using one-way analysis of variance and Tukey’s post hoc multiple comparison test. Tumour indices were compared between treatment groups using either Kruskal–Wallis test with Dunn’s post hoc tests or Mann–Whitney two-tailed t-tests for non-parametric data. Tumour incidence data were analysed using Pearson’s χ2 test using contingency tables. The relationships between butyrate and tumour measures were determined by non-parametric Spearman-ranked correlations. Data are expressed as mean ± SEM with statistical significance indicated when P < 0.05.
Results
Growth rates were similar in all the experimental groups and there were no significant differences in final body weights between the dietary treatments (mean for all groups 627 ± 7 g, Table I). Two rats were euthanased early due to events unrelated to the experimental procedures (from group LAMS in week 9; HAMSB, week 23). Seven rats were euthanased within 4 weeks of their scheduled dates because they showed possible symptoms of CRC including weight loss and rectal bleeding; tumour end points were obtained from these animals.
Table I.
Final measures of rats fed diets containing low-amylose maize starch (LAMS), LAMS with 3% tributyrin (LAMST), HAMS and butyrylated HAMS (HAMSB)
| LAMS | LAMST | HAMS | HAMSB | |
| Body weight (g) | 635 ± 15 | 621 ± 18 | 638 ± 14 | 616 ± 25 |
| Caecal tissue weight (g) | 0.84 ± 0.02a | 0.78 ± 0.02ab | 0.93 ± 0.03a | 1.19 ± 0.03 |
| Caecal digesta (g) | 1.46 ± 0.07a | 1.41 ± 0.09a | 1.70 ± 0.09a | 2.27 ± 0.08 |
| Caecal digesta pH | 7.12 ± 0.04ac | 7.07 ± 0.04ad | 6.92 ± 0.03a | 6.69 ± 0.04 |
| Distal colonic digesta pH | 6.97 ± 0.05b | 6.85 ± 0.07 | 6.66 ± 0.05 | 6.77 ± 0.06 |
Values are mean ± SEM, n = 28–30.
Significantly different from HAMSB, P < 0.001.
Significantly different from HAMS, P < 0.01.
Significantly different from HAMS, P < 0.001.
Significantly different from HAMS, P < 0.005 within each row of data.
Caecal tissue and digesta weights were higher in rats fed HAMSB than all other groups (P < 0.001), and caecal tissue weight was higher in HAMS rats compared with LAMST (P < 0.01) (Table I). Caecal digesta pH was different between groups with pH in HAMSB-fed rats lower than the other groups (P < 0.001), and the pH in the HAMS group lower than the LAMS (P < 0.001) and LAMST (P < 0.05) groups (Table I). The digesta pH fell from caecum to distal colon in all groups except HAMSB; in the distal colon, the digesta pH of rats fed LAMS was higher than HAMS (P < 0.01).
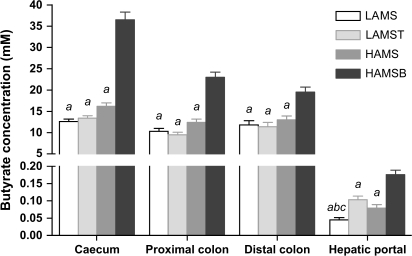
Caecal and proximal and distal colonic digesta butyrate concentrations were higher in rats fed HAMSB than other groups (P < 0.001) and were similar in HAMS, LAMS and LAMST groups (Figure 1). Digesta SCFA pool sizes, calculated by multiplying the digesta wet weights by the concentrations of each of the fatty acids measured (acetate, propionate and butyrate), are shown in Table II. Digesta butyrate pool sizes were consistently larger throughout the large bowel in rats fed HAMSB (P < 0.05–0.001). Caecal pools of acetate and propionate and proximate and distal colonic pools of propionate were also larger in rats fed HAMSB (P < 0.001–0.05). Caecal and distal pools of propionate were higher in HAMS than LAMST or LAMS groups (P < 0.05–0.001), and proximal pools of propionate were higher in HAMS than LAMST (P < 0.001).
Fig. 1.
Concentration of butyrate in the digesta of the caecum, proximal and distal colon (mM; n = 28–30) and hepatic portal plasma (mM; n = 26–30) of rats fed diets containing low-amylose maize starch (LAMS; open bars), LAMS with 3% tributyrin (LAMST; light grey bars), (HAMS; dark grey bars) and butyrylated HAMS (HAMSB; black bars) (mean ± SEM). aSignificantly different from HAMSB, P < 0.001; bsignificantly different from HAMS, P < 0.05 and csignificantly different from LAMST, P < 0.001 within each gut region or hepatic portal plasma.
Table II.
Digesta SCFA pools (micromoles) of rats fed diets containing low-amylose maize starch (LAMS), LAMS with 3% tributyrin (LAMST), HAMS and butyrylated HAMS (HAMSB)
| Diet | Caecal SCFA pools |
Proximal colon SCFA pools |
Distal colon SCFA pools |
||||||
| Acetate | Propionate | Butyrate | Acetate | Propionate | Butyrate | Acetate | Propionate | Butyrate | |
| LAMS | 107.7 ± 7.2a | 30.0 ± 1.8ab | 21.3 ± 1.6a | 44.1 ± 4.2 | 11.4 ± 1.1a | 7.1 ± 0.7a | 40.9 ± 3.8 | 9.5 ± 0.8ac | 7.8 ± 0.8a |
| LAMST | 99.8 ± 7.6a | 27.8 ± 2.3ab | 22.0 ± 1.7a | 31.6 ± 3.1b | 8.1 ± 0.7ac | 5.3 ± 0.6a | 36.7 ± 4.1c | 8.7 ± 0.9ac | 7.6 ± 1.0a |
| HAMS | 132.2 ± 11.8a | 53.5 ± 4.6a | 36.2 ± 4.0a | 47.2 ± 4.8 | 17.2 ± 1.6a | 9.2 ± 0.9a | 55.4 ± 5.7 | 20.8 ± 2.1d | 12.8 ± 1.9d |
| HAMSB | 237.2 ± 26.8 | 125.7 ± 11.1 | 125.5 ± 12.5 | 45.5 ± 4.3 | 23.5 ± 2.3 | 18.7 ± 2.0 | 51.1 ± 5.0 | 29.5 ± 2.9 | 19.4 ± 2.0 |
Values are mean ± SEM, n = 28-30 for caecal; 24–30 for proximal colon; 24–27 for distal colon.
Significantly different from HAMSB, P < 0.001.
Significantly different from HAMS, P < 0.05.
Significantly different from HAMS, P < 0.001.
Significantly different from HAMSB, P < 0.05 within each column of data.
Hepatic portal plasma butyrate concentration was higher (P < 0.001) in the HAMSB compared with the other groups (Figure 1). Dietary tributyrin (LAMST) increased hepatic portal butyrate concentration compared with LAMS to levels similar to HAMS (P < 0.001).
More large bowel tumours were found in the distal colon rather than the proximal colon (P < 0.02); however, a greater proportion of proximal tumours was adenocarcinomas (13 of 32) compared with the distal colon, where most tumours were adenomas (55 of 67) (P < 0.02). The size of caeco-colonic adenomas was positively and significantly correlated to the distance of the adenomas from the rectum (Spearman r = 0.310, P < 0.008, n = 74) with larger adenomas tending to be closer to the caecum. The reverse was the case for adenocarcinomas, with larger adenocarcinomas tending to be closer to the rectum (Spearman r = −0.403, P < 0.05, n = 25). There was no effect of diet on the distribution of tumours based on size or tumour type i.e. the ratio of adenomas to adenocarcinomas in the proximal and distal colons was not significantly different between treatment groups.
Large bowel tumour incidence (proportion of rats per group with tumours) was lower in HAMSB and HAMS compared with LAMS group (P < 0.05). Tumour number (number of tumours per group) was lower in HAMSB compared with LAMS (P < 0.05). There was an apparent trend towards reduced large bowel number for the HAMS group compared with the LAMS (P < 0.055). Caecal butyrate pools and concentrations were significantly and negatively correlated with the number of large bowel tumours per rat (P < 0.03, r = −0.206 and P < 0.04, r =−0.198, respectively), whereas there was no relationship between distal butyrate levels and tumour number. The total tumour area for each rat was lower in the HAMBS than LAMS group (P < 0.05). The caecal butyrate pools and total large bowel tumour area for each rat were significantly and negatively correlated (P < 0.05, r = −0.185). Hepatic portal plasma butyrate concentration was negatively correlated with tumour number per rat (Spearman r = −0.243, P < 0.009) and total tumour area index for each rat (Spearman r = −0.184, P = 0.05).
Most small intestinal adenomas were found in the proximal small intestine (data not shown). There were no significant differences in the incidence, numbers and ratio of adenomas to adenocarcinomas in the small intestine between groups (Table III). Tumours in the small intestine of rats fed LAMST were significantly larger than those in the rats fed LAMS (P < 0.05) but the total small intestinal tumour area per rat was not significantly affected by diet.
Table III.
Indices of AOM-induced small and large bowel tumours in rats fed diets containing low-amylose maize starch (LAMS), LAMS with 3% tributyrin (LAMST), HAMS and butyrylated HAMS (HAMSB) (mean ± SEM)
| LAMS | LAMST | HAMS | HAMSB | |
| Number of rats | 29 | 30 | 30 | 29 |
| Incidence of tumours (%)a | ||||
| Small intestine | 27.6 | 16.7 | 30.0 | 20.7 |
| Large bowelb | 65.5cd | 56.7 | 40 | 37.9 |
| Number tumours per rat | ||||
| Small intestine | 0.4 | 0.2 | 0.4 | 0.2 |
| Large bowele | 1.3c | 0.9 | 0.6 | 0.5 |
| Average tumour area (tumour area index) | ||||
| Small bowel | 35.9 ± 11.5f | 146.9 ± 70.5 | 49.0 ± 10.2 | 51.8 ± 11.0 |
| Large bowel | 26.2 ± 8.8 | 21.9 ± 4.6 | 49.5 ± 14.7 | 18.0 ± 5.1 |
| Total tumour area per rat | ||||
| Small bowel | 13.6 ± 5.8 | 29.4 ± 17.3 | 19.6 ± 6.9 | 10.7 ± 4.5 |
| Large bowele | 34.3 ± 12.7c | 19.8 ± 4.7 | 31.4 ± 12.7 | 9.3 ± 3.5 |
| Tumour type (large intestine) | ||||
| Adenoma (number)a | 27 | 24 | 12 | 11 |
| Adenocarcinoma (number)a | 11 | 3 | 7 | 4 |
Analysed using Pearson’s χ2 test.
Contingency comparison between two groups.
Significantly different from HAMSB, P < 0.05.
Significantly different from HAMS, P < 0.05.
Analysed using Mann–Whitney non-parametric t-tests between two groups.
Significantly different from LAMST, P < 0.05.
Discussion
Acylated starches were designed to promote large bowel health by providing a vehicle for the rapid and sustained delivery of SCFA, particularly butyrate, to the colon (22). Low levels of colonic SCFA in individual humans may result either from low consumption of dietary fibre or from an inadequate fermentative capacity of the colonic microflora. Earlier animal studies have shown that acylated starches are twice as effective as HAMS in raising large bowel SCFA levels of the acid that has been esterified (14,15). The present data confirm those previous experiments and show that while both HAMS and HAMSB raise total SCFA, the greatest increase in butyrate was with the butyrylated SCFA. HAMSB was particularly effective in raising butyrate concentrations in the distal colon, which is the site of most CRC in humans.
This study examined the importance of butyrate by investigating the effects of RS (a dietary fibre) and butyrylated-RS on levels of butyrate in the large bowel and their impact on intestinal tumorigenesis in an established animal model of CRC. The results from this study are consistent with previous experiments showing that dietary RS reduces CRC incidence in rats treated with AOM (23–26). The importance of butyrate to this protection is demonstrated by the ability of an RS-equivalent HAMSB to achieve greater protection and higher butyrate levels. Statistical examination of the data showed that this effect correlated with caecal butyrate pools and concentrations and hepatic portal butyrate concentrations.
The total tumour size per rat was also significantly lower in the HAMSB than LAMS group, which reflects a lower incidence and smaller size of tumours in rats fed HAMSB. This further supports the argument that butyrate absorbed from the lumen of the large bowel may have a role regulating the initiation and growth of large bowel tumours.
The relationship between tumour size and butyrate pool was stronger for the caecum than the colon, which may reflect the large bowel physiology of the rat in which bacterial activity occurs principally in the caecum.
There was no effect of tributyrin on tumour development in the large bowel. Tributyrin (glycerol tributyrate) is absorbed in the small intestine, raising free butyrate concentrations in peripheral plasma for up to 4 h (27). Tributyrin has been recommended for clinical evaluation as a possible treatment for leukaemia (28) but has not been found to reduce the incidence of AOM-induced CRC in mice (29). At the level of tributyrin included in the LAMST diet in this study (3%), hepatic portal plasma butyrate concentrations were similar to those that resulted from ingesting the HAMS diet. In a previous study (unpublished data), hepatic portal butyrate concentrations up to 1.0 mM were recorded in individual rats fed 3% tributyrin; however, these levels were not associated with increased butyrate concentrations in peripheral plasma. These data suggest that 3% tributyrin may not increase levels of systemically delivered butyrate to the large bowel and that butyrate absorbed from the small intestine in the rat is ineffective in altering large bowel CRC induced by AOM.
Genomic instability is a prerequisite for oncogenesis and the genotoxic effects of AOM are thought to be responsible for tumour initiation leading to the development of aberrant crypts, adenomas and eventually invasive adenocarcinoma. Butyrate could inhibit this process either by promoting apoptosis (8) or repair in damaged colonocytes during the initiation phase or by suppressing the growth of cells with damaged DNA (30). Alternatively, butyrate may be reducing tumour growth by inhibiting hypoxia-induced angiogenesis (31,32). It is not clear which mechanism(s) is operating. Tumour numbers and incidence were lower in HAMSB than in LAMS. The size of neoplastic lesions is an important factor clinically as large adenomas are more likely to progress to adenocarcinomas (33). Progression to adenocarcinoma may be the consequence of adenoma cells acquiring resistance to butyrate due to the deregulation of genes involved with the processes of apoptosis, proliferation and differentiation (34), resulting in cells with increased transformation potential (35). However, the number of adenomas relative to adenocarcinomas in this experiment was not affected by dietary treatment, suggesting that increased luminal butyrate suppressed oncogenesis at all stages. This conclusion is similar to that drawn from studies on dietary protein-induced genetic damage in rats (36) where feeding HAMS opposed double-stranded DNA break formation in colonocytes.
RS has been reported to reduce the number of colonic adenocarcinomas in AOM-treated rats (24) fed diets containing no fibre and high fat levels. This may be attributed to the high ratio of colonic adenocarcinomas in the control animals in this trial compared with the current trial, whereas the levels of adenomas and adenocarcinomas in the RS-fed animals were almost identical in both studies.
The present data also confirm the findings of other studies that intake of RS increases caecal digesta weight and decreases digesta pH, which can be interpreted as improving bowel health (3). Butyrylation leads to increased resistance to small intestinal amylolysis and increased passage of starch to the caecum. Furthermore, the HAMS and HAMSB used in this study were cooked, and as HAMSB appears to be more resistant to small intestinal digestion following cooking than HAMS (15), more fermentative substrate would be delivered to the large bowel of rats fed this diet.
The size of individual tumours, but not incidence, was increased in the small intestine of the LAMST group. This effect was unexpected and was not reported in the one other study we are aware of involving dietary tributyrin in AOM-treated rodents (29). In humans, sporadic cancers are normally rare in the small compared with the large intestine, which may be due to a range of factors including the high level of spontaneous apoptosis in this organ protecting against low-level DNA damage (37). Glutamine and glucose are preferred ahead of SCFA as energy substrates by jejunal enterocytes (38) that would normally be exposed to low-digesta butyrate levels, suggesting nutritional factors controlling cellular apoptosis may differ in the small and large intestine. Other workers have reported an increase in small intestinal tumour incidence, but not size, in response to RS (39) in mice with a targeted mutant gene resulting in spontaneous small intestinal tumours. In the AOM-treated rats in this study, HAMS did not increase small intestinal tumour size or incidence, supporting the epidemiological evidence that high-RS diets do not enhance the risk of tumourigenesis in the small intestine (40).
The current study demonstrates that large bowel and hepatic portal venous butyrate levels correlated negatively with tumour indices in an accepted animal model of CRC. Butyrate was raised by HAMS relative to the LAMS control, but the highest butyrate levels were achieved with HAMSB, indicating the greater potential effectiveness for this modified starch to significantly improve bowel health and reduce the risk of developing CRC. Further work is necessary to determine the effectiveness of HAMSB in opposing initiation or progression of experimental carcinogenesis.
Acknowledgments
The authors wish to thank National Starch Food Innovation for providing the HAMS and HAMSB and gratefully acknowledge Julie Dallimore, Ben Scherer, Jessica Winkler and Peter Royle for their contributions to the study. In addition, the statistical advice of Dr Ian Saunders of CSIRO Mathematical and Information Sciences (Urrbrae, South Australia) was greatly appreciated.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- CRC
colorectal cancer
- CSIRO
Commonwealth Scientific and Industrial Research Organisation
- HAMS
high-amylose maize starch
- HAMSB
butyrylated high-amylose maize starch
- LAMS
low-amylose maize starch
- RS
resistant starch
- SCFA
short chain fatty acids
References
- 1.Garcia M, et al. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; [Google Scholar]
- 2.World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 3.Young GP, et al. Resistant starch and colorectal neoplasia. J. AOAC Int. 2004;87:775–786. [PubMed] [Google Scholar]
- 4.Birkett A, et al. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 1996;63:766–772. doi: 10.1093/ajcn/63.5.766. [DOI] [PubMed] [Google Scholar]
- 5.Toden S, et al. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol Ther. 2006;5:267–272. doi: 10.4161/cbt.5.3.2382. [DOI] [PubMed] [Google Scholar]
- 6.Topping DL, et al. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 7.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- 8.Hague A, et al. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int. J. Cancer. 1993;55:498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- 9.Medina V, et al. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res. 1997;57:3697–3707. [PubMed] [Google Scholar]
- 10.Hinnebusch BF, et al. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002;132:1012–1017. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead RH, et al. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215) Gut. 1986;27:1457–1463. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young GP, et al. Dietary fibre and colorectal cancer: a model for environment—gene interactions. Mol. Nutr. Food Res. 2005;49:571–584. doi: 10.1002/mnfr.200500026. [DOI] [PubMed] [Google Scholar]
- 13.Mortensen PB, et al. Fermentation to short-chain fatty acids and lactate in human faecal batch cultures. Intra- and inter-individual variations versus variations caused by changes in fermented saccharides. Scand. J. Gastroenterol. 1991;26:1285–1294. doi: 10.3109/00365529108998626. [DOI] [PubMed] [Google Scholar]
- 14.Annison G, et al. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J. Nutr. 2003;133:3523–3528. doi: 10.1093/jn/133.11.3523. [DOI] [PubMed] [Google Scholar]
- 15.Bajka BH, et al. Butyrylated starch is less susceptible to enzymic hydrolysis and increases large-bowel butyrate more than high-amylose maize starch in the rat. Br. J. Nutr. 2006;96:276–282. doi: 10.1079/bjn20061807. [DOI] [PubMed] [Google Scholar]
- 16.Clarke JM, et al. Excretion of starch and esterified short chain fatty acids by ileostomists after the ingestion of acylated starches. Am. J. Clin. Nutr. 2007;86:1146. doi: 10.1093/ajcn/86.4.1146. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, et al. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 18.Brown MA, et al. Cooking attenuates the ability of high-amylose meals to reduce plasma insulin concentrations in rats. Br. J. Nutr. 2003;90:823–827. doi: 10.1079/bjn2003958. [DOI] [PubMed] [Google Scholar]
- 19.National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. 2004. [Google Scholar]
- 20.McIntosh GH, et al. Wheat aleurone flour increases cecal beta-glucuronidase activity and butyrate concentration and reduces colon adenoma burden in azoxymethane-treated rats. J. Nutr. 2001;131:127–131. doi: 10.1093/jn/131.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, et al. Sulindac corrects defective apoptosis and suppresses azoxymethane-induced colonic oncogenesis in p53 knockout mice. Int. J. Cancer. 2005;116:870–875. doi: 10.1002/ijc.21107. [DOI] [PubMed] [Google Scholar]
- 22.Annison G, et al. Fatty Acid Delivery System. CSIRO Human Nutrition; 1995. [Google Scholar]
- 23.Bauer-Marinovic M, et al. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis. 2006;27:1849–1859. doi: 10.1093/carcin/bgl025. [DOI] [PubMed] [Google Scholar]
- 24.Le Leu RK, et al. Effect of dietary resistant starch and protein on colonic fermentation and intestinal tumourigenesis in rats. Carcinogenesis. 2007;28:240–245. doi: 10.1093/carcin/bgl245. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen H, et al. Carbohydrate digestibility predicts colon carcinogenesis in azoxymethane-treated rats. Nutr. Cancer. 2006;55:163–170. doi: 10.1207/s15327914nc5502_7. [DOI] [PubMed] [Google Scholar]
- 26.Le Leu RK, et al. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol. Ther. 2007;6:1621–1626. doi: 10.4161/cbt.6.10.4764. [DOI] [PubMed] [Google Scholar]
- 27.Egorin MJ, et al. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother. Pharmacol. 1999;43:445–453. doi: 10.1007/s002800050922. [DOI] [PubMed] [Google Scholar]
- 28.Newmark HL, et al. Butyrate and phenylacetate as differentiating agents: practical problems and opportunities. J. Cell. Biochem. Suppl. 1995;22:247–253. doi: 10.1002/jcb.240590831. [DOI] [PubMed] [Google Scholar]
- 29.Deschner EE, et al. Dietary butyrate (tributyrin) does not enhance AOM-induced colon tumorigenesis. Cancer Lett. 1990;52:79–82. doi: 10.1016/0304-3835(90)90080-h. [DOI] [PubMed] [Google Scholar]
- 30.Wachtershauser A, et al. Expression of 5-lipoxygenase by human colorectal carcinoma Caco-2 cells during butyrate-induced cell differentiation. Biochem. Biophys. Res. Commun. 2000;268:778–783. doi: 10.1006/bbrc.2000.2213. [DOI] [PubMed] [Google Scholar]
- 31.Zgouras D, et al. Butyrate impairs intestinal tumor cell-induced angiogenesis by inhibiting HIF-1 alpha nuclear translocation. Biochem. Biophys. Res. Commun. 2003;300:832–838. doi: 10.1016/s0006-291x(02)02916-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, et al. Inhibition of hypoxia-induced angiogenesis by sodium butyrate, a histone deacetylase inhibitor, through hypoxia-inducible factor-1 alpha suppression. Oncol. Rep. 2007;17:793–797. [PubMed] [Google Scholar]
- 33.Morson BC. The polyp-cancer sequence in the large bowel. Proc. R. Soc. Med. 1974;67:451–457. [PMC free article] [PubMed] [Google Scholar]
- 34.Daly K, et al. The importance of colonic butyrate transport to the regulation of genes associated with colonic tissue homoeostasis. Biochem. Soc. Trans. 2005;33:733–735. doi: 10.1042/BST0330733. [DOI] [PubMed] [Google Scholar]
- 35.Olmo N, et al. Acquisition of resistance to butyrate induces resistance to luminal components and other types of stress in human colon adenocarcinoma cells. Toxicol. In Vitro. 2007;21:254–261. doi: 10.1016/j.tiv.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Toden S, et al. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: attenuation by high amylose maize starch. Carcinogenesis. 2007;28:2355–2362. doi: 10.1093/carcin/bgm216. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS. Epithelial cell growth and differentiation. 2. Intestinal apoptosis. Am. J. Physiol. 1997;273:G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- 38.Fleming SE, et al. Nutrient utilization by cells isolated from rat jejunum, cecum and colon. J. Nutr. 1991;121:869–878. doi: 10.1093/jn/121.6.869. [DOI] [PubMed] [Google Scholar]
- 39.Williamson SLH, et al. Intestinal tumorigenesis in the Apc1638N mouse treated with aspirin and resistant starch for up to 5 months. Carcinogenesis. 1999;20:805–810. doi: 10.1093/carcin/20.5.805. [DOI] [PubMed] [Google Scholar]
- 40.Cassidy A, et al. Starch intake and colorectal cancer risk: an international comparison. Br. J. Cancer. 1994;69:937–942. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]