Abstract
There are profound gender-related differences in the incidence, presentations and outcomes of Coronary Artery Disease (CAD). These differences are not entirely explained by traditional cardiovascular risk factors. Non-traditional risk factors such as psychological traits have increasingly been recognized as important contributors to the genesis and outcomes of CAD. Mental stress induces significant peripheral arterial vasoconstriction with consequent increases in heart rate, and blood pressure. These changes are thought to underlie the development of myocardial ischemia and other mental stress-induced adverse cardiac events in patients with CAD. In this study we examined for gender-related differences in the peripheral arterial response to mental stress in a cohort of CAD patients using a novel peripheral arterial tonometry (PAT) technique. Participants were 211 patients [77 (37%) females] with documented history of CAD and a mean age of 64±9 years. Patients were enrolled between August 18th 2004 and February 21st 2007. Mental stress was induced using a public speaking task. Hemodynamic and PAT measurements were recorded during rest and mental stress. The PAT response was calculated as a ratio of stress to resting pulse wave amplitude. PAT responses were compared between males and females. We found that the PAT ratio (stress to rest) was significantly higher in females compared to males. The mean PAT ratio was 0.80±0.72 in females compared to 0.59±0.48 in males (p=0.032). This finding remained significant after controlling for possible confounding factors (p=0.037). In conclusion, peripheral vasoconstrictive response to mental stress was more pronounced in males compared to females. This finding may suggest that males have higher susceptibility to mental stress-related adverse effects. Further studies are needed to determine the significance of this finding.
Little has been reported regarding gender related differences in mental stress-induced vascular reactivity. However, there is consistent evidence that females have reduced sensitivity to the vasoconstrictor effects of norepinephrine.1–6 Females have also been shown to have higher basal nitric oxide levels compared to males.7, 8 Collectively, these observations suggest that males may have more intense vascular reactivity to mental stress compared to females. In patients with coronary artery disease (CAD) these changes may increase vulnerability to mental stress-induced myocardial ischemia and other mental stress related adverse events.9–12 In this study, we sought to examine for gender-related differences in the peripheral arterial response to mental stress in a cohort of CAD patients using a novel non-invasive peripheral arterial tonometry (PAT) technique.
Methods
Participants in this study were recruited from outpatient clinics affiliated with university based medical centers. Eligibility criteria included age above 18 years and a documented clinical diagnosis of CAD supported by: 1) angiographic evidence of >50% stenosis in one or more coronary arteries or previous percutaneous intervention (PCI) or coronary artery bypass graft surgery (CABG), 2) previous myocardial infarction (MI) documented with elevated troponin level in the range typical of MI, Q-wave abnormalities on Electrocardiogram (ECG), or fixed perfusion abnormalities on nuclear scan, or 3) a positive radionuclide pharmacologic or exercise stress test. Patients were excluded if they had unstable angina or acute MI within the two months preceding enrollment, a severe co-morbid medical problem restricting life-expectancy to less than 5 years, pregnancy or body weight over 400 lbs.
Study procedures were performed in the morning after and an over night fast. Beta-blockers, calcium-channel blockers and long acting nitrates were withheld the night before testing. Demographic and psychosocial characteristics were obtained prior to study procedures. Patients were initially rested for 30 minutes in a temperature controlled (21–23 °C), dark and quiet room. Their heart rate (HR) and blood pressure were obtained every 5 minutes using an ECG monitor and automatic oscillometric device (Dinamap; Critikon Inc, Tampa, Florida), respectively. Mental stress was then induced via a public speaking task performed in front of a small white coated audience, as in prior research.13 Participants were given a scenario describing a real life stressful event and were asked to make up a realistic story around it. Participants were given two minutes to prepare their speech and three minutes to speak. They were told that their speech would be video-taped and the laboratory staff would replay the tape to rate it for content, quality and duration of the speech. Hemodynamic measurements were obtained every minute during the preparation and the speech periods and at 1, 3, 5 and 10 minutes into the recovery period. Systolic blood pressure (SBP) and HR were used to calculate the double product (DP) value (DP=SBP×HR).
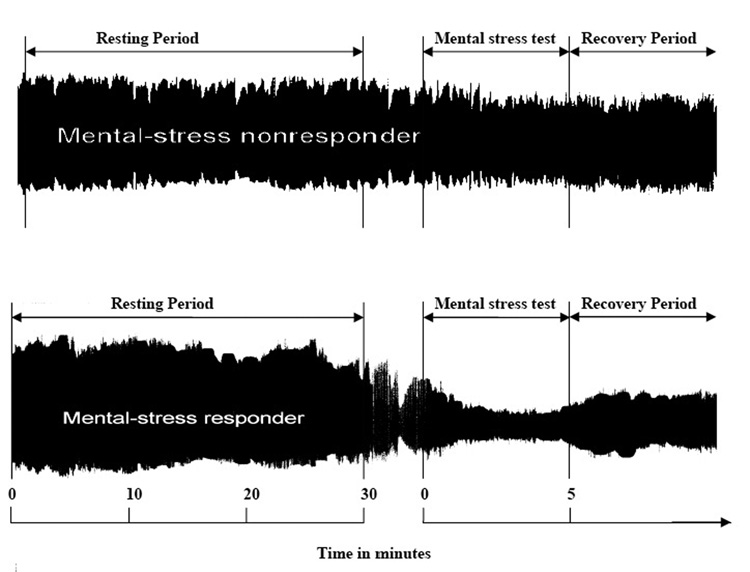
The PAT device (Itamar-Medical, Caesarea, Israel), was used to continuously measure pulse wave amplitude in the finger during rest and mental stress, as in prior research.14 It applies a constant pressure of 40–70 mm/Hg to eliminate venous stasis and unload arterial wall tension within the probed part of the finger. The device is connected via thin flexible tubing to an isolated volume reservoir to buffer pressure changes within the probe. Another volume reservoir not connected to the probe serves as a pressure reference. The distal compartment of the device is connected to a pressure transducer which senses pulsatile fluctuations exerted by blood volume changes in the digital arteries. In this study, the device was applied to the index finger. Attention was paid not to use the same extremity for blood pressure measurements. PAT measurements were recorded during rest and mental stress (2 minutes speech preparation and 3 minutes speaking task). The pressure changes were fed to a personal computer where the signal is filtered (0.3–30 Hz), amplified, stored and analyzed in an operator-independent manner. Noise was removed from the tracing by electronic filtering. The baseline pulse wave amplitude was determined by averaging over the entire rest period. The stress amplitude was determined by averaging over the 5 minutes stress period. Using these measurements the PAT response was calculated as a ratio of the stress to the resting pulse wave amplitude. Representative PAT signals and the time course of the study are shown in figure 1.
Figure 1.
Representative examples of PAT tracing. The top tracing shows a patient with no significant response to mental stress. The bottom tracing shows significant peripheral arterial vasoconstrictive response to mental stress. The PAT response was calculated as a ratio of stress to the resting pulse wave amplitude.
Results were expressed as means ± standard deviations for continuous variables and frequencies and percentages for categorical variables. Stress hemodynamic responses were calculated as the difference between the stress and rest measurements. Statistical significance was considered as p<0.05. Univariable and multivariable analyses were used to examine the influence of gender on the PAT response. In additions to gender, the latter analysis included, as covariates, co-morbid conditions (diabetes, hypertension and smoking status), medication status (beta blockers, calcium channel blockers and angiotensin converting enzyme inhibitors) and body mass index (BMI). Log transformation of the PAT ratio was conducted to satisfy the Gaussian distribution assumption of the model. All analyses were done using SAS statistical software (SAS Inc., Cary, NC).
Results
A total of 211 patients were studied, of which 134 (63%) were males and 77 (37%) were females. The baseline demographics by gender groups are shown in table 1.
Table 1.
Baseline demographic and clinical characteristics in males and females
| Variable | Males (N=134) | Females (N=77) | p value |
|---|---|---|---|
| Mean age (years) | 64±9 | 64±9 | NS |
| Ethnicity (Caucasian) | 118 (89%) | 68 (91%) | NS |
| (African American) | 6 (5%) | 7 (9%) | NS |
| Number of diseased coronary vessels* | 2.2±0.82 | 1.9±0.93 | 0.034 |
| Previous myocardial infarction | 26 (19%) | 13 (17%) | NS |
| Previous percutaneous coronary intervention | 59 (44%) | 32 (42%) | NS |
| Hx of exercise or adenosine-induced myocardial ischemia | 43 (32%) | 31 (40%) | NS |
| Smoking status: | |||
| Never | 23 (17%) | 40 (52%) | |
| Current | 22 (17%) | 8 (10%) | <0.001 |
| Former | 89 (66%) | 29 (38%) | |
| Hypertension | 104 (78%) | 63 (82%) | NS |
| Diabetes mellitus | 48 (46%) | 20 (26%) | NS |
| Angina pectoris | 83 (62%) | 54 (70%) | NS |
| Hyperlipidemia | 122 (91%) | 68 (88%) | NS |
| On Beta Blockers | 111 (82%) | 49 (64%) | 0.002 |
| On ACE Inhibitors | 79 (59%) | 31 (40%) | 0.009 |
| On Calcium Channel Blockers | 21 (16%) | 24 (31%) | 0.008 |
| On Hormone replacement therapy | 5 (6.5%) | ||
| Body mass index (Kg/m2) | 30±4 | 30±7 | NS |
Values expressed as mean±standard deviation
Frequencies expressed as N (Percentage)
NS=statistically non significant
This information was only available in 143 patients (97 males and 46 females)
Mental stress induced significant changes in SBP, diastolic blood pressure (DBP), HR and DP compared to the resting condition in the study population combining both males and females (p<0.001). Comparing hemodynamic responses to mental stress across gender groups did not show any differences in SBP, DBP, HR or DP between males and females (p values 0.89, 0.53, 0.11 and 0.13, respectively). Hemodynamic responses to mental stress by gender groups are shown in table 2.
Table 2.
Mental stress-induced hemodynamic and peripheral arterial tonometry (PAT) responses in males and females.
| Males | Females | p value | |
|---|---|---|---|
| (N=134) | (N=77) | ||
| Hemodynamic responses: | |||
| Increase in Systolic Blood Pressure (mm/Hg) | 44±20 | 43±20 | 0.89 |
| Increase in Diastolic Blood Pressure (mm/Hg) | 28±11 | 29±12 | 0.53 |
| Increase in Heart Rate (beats/min) | 19±12 | 22±14 | 0.11 |
| Increase in Double Product | 5682±2965 | 6353±3308 | 0.13 |
| PAT response: | |||
| Unadjusted | 0.59±0.48 | 0.80±0.72 | 0.032 |
| Adjusted* | 0.48 (0.38–0.48) | 0.61 (0.38–0.58) | 0.037 |
Values expressed as mean±standard deviation or mean (95% confidence interval)
Frequencies expressed as N (Percentage)
Controlling for co-morbid conditions (diabetes, hypertension and smoking status), Body Mass Index (BMI) and medication status (beta blockers, calcium channel blockers and angiotensin converting enzyme inhibitors). Values expressed as mean (95% CI).
Most of the patients [180 (85%)] developed a vasoconstrictive PAT response to mental stress (PAT<1), 31 patients (15%) developed a vasodilative response (PAT ratio >1). The rate of vasoconstrictive response was significantly higher among males compared to females (91% versus 75%; p=0.002)
We observed that the PAT ratio was significantly higher in females compared to males. The mean PAT ratio was 0.80±0.72 in females compared to 0.59±0.48 in males (p=0.032). Using multivariable analysis to control for possible confounding factors (see the statistical section), this gender effect remained significant with females having higher PAT ratios than males (p=0.037). The adjusted mean PAT ratio in females was 0.61 (95% CI 0.43–0.79) compared to 0.48 (95% CI 0.38–0.58) in males (see Table 2). These findings suggest that males exhibited a more pronounced vasoconstrictive response during mental stress compared to females.
Discussion
In this study we examined for gender-related differences in the peripheral arterial response to mental stress. Our findings indicate that males exhibit a more pronounced peripheral vasoconstrictive response to mental stress compared to females. The average PAT ratio (stress to rest) was 0.59 in males compared to 0.80 in females. These findings are consistent with previous literature suggesting that females have reduced sensitivity to the vasoconstrictor effects of norepinephrine as well as higher basal nitric oxide levels.1–8
We explored the relationship between gender and vascular reactivity to mental stress in an effort to unravel mechanisms that might explain the observed gender related differences in prevalence, clinical presentation and outcomes of CAD. Mental stress has been shown to induce transient myocardial ischemia and other adverse cardiac events in patients with CAD. Peripheral arterial vasoconstriction has consistently been reported as an underlying mechanism for ischemia development in this setting. Laboratory-induced mental stress is a direct simulation of everyday life stress.15–19 Thus it is possible that the vascular responses to mental stress observed in this study could represent frequent daily life occurrences. There is also evidence that situational stressors similar to the one used in this study, could lead to transient endothelial dysfunction lasting up to 4 hours.20 Potentially, these observations could provide a mechanistic link between mental stress and myocardial ischemia and other cardiac events. The fact that males had more vasoconstrictive response to mental stress may imply higher susceptibility to mental stress-related adverse events. This needs to be validated in a follow up study.
Arguably many factors might be operative in explaining the gender differences observed in this study. We believe that the findings we observed are due to true physiologic differences between males and females. Estrogen is a known vasodilator and mediates this effect, in part, by stimulating the release of nitric oxide and prostacyclin from the vessel wall.21–23 It is intriguing that we found remarkable differences in this study despite the fact that the majority of our female participants were postmenopausal. Another unique and relevant correlate is the existence of some reports suggesting that females have better coping skills with situational stressors than males.24–26 This could potentially attenuate their physiologic adrenergic response to psychological stress.
We did not find any significant differences in BP or HR response to mental stress between males and females. Similar findings have been reported in other studies.27 Furthermore; we also found that the differences in the mental stress PAT response between males and females did not translate into differences in HR or BP responses. This is not surprising as the PAT dose not measure pressure; instead it measures peripheral blood volume. Hence changes in peripheral blood volume may not necessarily reflect changes in BP. Another potential reason for this discrepancy is the BP measurement techniques used in this study. Using the traditional sphygmomanometeric brachial BP technique may miss rapid changes that would only be captured by beat-by-beat measurements. Another potential explanation pertains to the fact that in our cohort, males were more likely to be tested under the effects of beta blocker or calcium channel blockers (see Table 1) which might have blunted their BP or HR response to mental stress.
The gender groups in our study were not prospectively matched in terms of their CAD severity and other co-morbid conditions. The males in this cohort tended to have more severe CAD disease compared to females. This is probably related to the fact that the mean age for participants in this study was similar for males and females. Typically, age matched females are expected to have less severe atherosclerotic CAD disease compared to their male counterparts.28, 29 However, in our analysis, the differences in PAT measurements between males and females remained significant after statistically controlling for CAD severity factors and other co-morbid medical conditions.
Acknowledgments
Funding Sources
This study was supported by grants HL 070265 and HL 072059 of the National Heart Lung and Blood Institute. This material is also the result of work supported with resources and the use of facilities at the Department of Veterans Affairs Medical Center, Gainesville FL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest to be reported.
References
- 1.Kneale BJ, Chowienczyk PJ, Cockcroft JR, Coltart DJ, Ritter JM. Vasoconstrictor sensitivity to noradrenaline and NG-monomethyl-L-arginine in men and women. Clin Sci. 1997;93:513–518. doi: 10.1042/cs0930513. [DOI] [PubMed] [Google Scholar]
- 2.Colucci WS, Gimbrone MA, McLaughlin MK, Halpern W, Alexander RW. Increased vascular catecholamine sensitivity and α-adrenergic receptor affinity in female and estrogen-treated male rats. Circ Res. 1982;50:805–811. doi: 10.1161/01.res.50.6.805. [DOI] [PubMed] [Google Scholar]
- 3.Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585. doi: 10.1161/01.res.61.4.581. [DOI] [PubMed] [Google Scholar]
- 4.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Duckles SP. Influence of gender on vascular reactivity in the rat. J Pharmacol Exp Ther. 1994;286:1426–1431. [PubMed] [Google Scholar]
- 6.Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- 7.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- 8.Forte P, Copland M, Smith LM, Milne E, Sutherland J, Benjamin N. Basal nitric oxide synthesis in essential hypertension. Lancet. 1997;349:837–842. doi: 10.1016/S0140-6736(96)07631-3. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, O'Connor CM, Blumenthal JA. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 10.Jain D, Burg M, Soufer A, Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 11.Krantz DS, Santiago HT, Kop JW, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 12.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann PG, McMahon RP, Becker LC, Bertolet B, Bonsall R, Chaitman B, Cohen JD, Forman S, Goldberg AD, Freedland K, Ketterer MW, Krantz DS, Pepine CJ, Raczynski J, Stone PH, Taylor H, Knatterud GL, Sheps DS. The Psychophysiological Investigations of Myocardial Ischemia (PIMI) study: objective, methods, and variability of measures. Psychosom Med. 1998;60:56–63. doi: 10.1097/00006842-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Goor DA, Sheffy J, Schnall RP, Arditti A, Caspi A, Bragdon EE, Sheps DS. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27:137–141. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottdiener JS, Krantz DS, Howell RH, Hecht GM, Klein J, Falconer JJ, Rozanski A. Induction of silent myocardial ischemia with mental stress testing: relation to the triggers of ischemia during daily life activities and to ischemic functional severity. J Am Coll Cardiol. 1994;24:1645–1651. doi: 10.1016/0735-1097(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 16.Gullette EC, Blumenthal JA, Babyak M, Jiang W, Waugh RA, Frid DJ, O'Connor CM, Morris JJ, Krantz DS. Effects of mental stress on myocardial ischemia during daily life. JAMA. 1997;277:1521–1526. [PubMed] [Google Scholar]
- 17.Gabbay FH, Krantz DS, Kop WJ, Hedges SM, Klein J, Gottdiener JS, Rozanski A. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: physical and mental activities, anger and smoking. J Am Coll Cardiol. 1996;27:585–592. doi: 10.1016/0735-1097(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 18.Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, Taylor HA, Cohen JD, Freedland KE, Bertolet BD, Coughlan C, Pepine CJ, Kaufmann PG, Sheps DS. Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: the Psychophysiologic Investigations of Myocardial Ischemia (PIMI) study. J Am Coll Cardiol. 1999;33:1476–1484. doi: 10.1016/s0735-1097(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, Hanson M, Babyak M, Thyrum ET, Krantz DS, O'Connor C. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Circulation. 1995;92:2102–2108. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 20.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Lüscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 21.Mendelsohn ME. Nongenomic, ER-mediated activation of endothelial nitric oxide synthase: how does it work? What does it mean? Circ Res. 2000;87:956–960. doi: 10.1161/01.res.87.11.956. [DOI] [PubMed] [Google Scholar]
- 22.Arnal JF, Clamens S, Pechet C, Negre-Salvayre A, Allera C, Girolami JP, Salvayre R, Bayard F. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine aortic endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Natl Acad Sci USA. 1996;93:4108–4113. doi: 10.1073/pnas.93.9.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- 24.Martin P, Lee H-S. Indicators of active and passive coping in myocardial infarction victims. Journal of Gerontology: Psychological Sciences. 1992;47:238–241. doi: 10.1093/geronj/47.4.p238. [DOI] [PubMed] [Google Scholar]
- 25.Bogg J, Thornton E, Bundred P. Gender variability in mood, quality of life and coping following primary myocardial infarction. Coronary Health Care. 2000;4:163–168. [Google Scholar]
- 26.LaCharity LA. The experiences of younger women with coronary artery disease. Journal of Women's Health and Gender-Based Medicine. 1999;8:773–785. doi: 10.1089/152460999319101. [DOI] [PubMed] [Google Scholar]
- 27.York KM, Hassan M, Li Q, Li H, Fillingim RB, Lucey D, Bestland M, Sheps DS. Do men and women differ on measures of mental stress-induced ischemia? Psychosom Med. 2007;69:918–922. doi: 10.1097/PSY.0b013e31815a9245. [DOI] [PubMed] [Google Scholar]
- 28.Kannel WB, Abbott RD. Incidence and prognosis of myocardial infarction in women. The Framingham Study. In: Eaker ED, Packard B, Wenger NK, Clarkson TB, Tyroler HA, editors. Coronary Heart Disease in Women. New York: Haymaker Doymer; 1987. pp. 208–214. [Google Scholar]
- 29.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;3:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]