Abstract
Chronic low dose exposure to organophosphorus poisons (OP) results in cognitive impairment. Studies in rats have shown that OP interfere with microtubule polymerization. Since microtubules are required for transport of nutrients from the nerve cell body to the nerve synapse, it has been suggested that disruption of microtubule function could explain the learning and memory deficits associated with OP exposure. Tubulin is a major constituent of microtubules. We tested the hypothesis that OP bind to tubulin by treating purified bovine tubulin with sarin, soman, chlorpyrifos oxon, diisopropylfluorophosphate, and 10-fluoroethoxyphosphinyl-N-biotinamidopentyldecanamide (FP-biotin). Tryptic peptides were isolated and analyzed by mass spectrometry. It was found that OP bound to tyrosine 83 of alpha tubulin in peptide TGTYR, tyrosine 59 in beta tubulin peptide YVPR, tyrosine 281 in beta tubulin peptide GSQQYR, and tyrosine 159 in beta tubulin peptide EEYPDR. The OP reactive tyrosines are located either near the GTP binding site or within loops that interact laterally with protofilaments. It is concluded that OP bind covalently to tubulin, and that this binding could explain cognitive impairment associated with OP exposure.
Keywords: Tubulin, Organophosphate, Tyrosine, Nerve agent, Mass spectrometer
1. Introduction
Acute toxicity from organophosphorus poisons (OP) is mainly due to inhibition of acetylcholinesterase [1]. However, low dose exposure that causes minimal inhibition of AChE and no obvious cholinergic symptoms has been linked to memory loss, sleep disorder, depression, learning and language impairment, and decreased motor skills in humans [2–4]. Rats treated with low doses of chlorpyrifos have behavioral deficits in a water-maze hidden platform task and in prepulse inhibition [5]. The mechanism to explain cognitive deficits from low dose exposure is thought to be inhibition of fast axonal transport [5]. Axonal transport was impaired in sciatic nerves isolated from chlorpyrifos treated rats [5, 6]. Transport of nutrients to nerve endings is accomplished via microtubules that serve as the highway on which kinesin molecules carry their cargo [7]. When microtubule function is disrupted, neurons lose viability. Microtubules are polymers of alpha and beta tubulin. Prendergast et al have shown that polymerization of tubulin is inhibited by low doses of chlorpyrifos and diisopropyl fluorophosphate DFP [8]. The goal of the present work was to identify the amino acid residues modified by reaction of tubulin with OP. Mass spectrometry identified 4 covalent binding sites, all of them tyrosines.
2. Materials and methods
2.1. Materials
Bovine tubulin (TL238) >99% pure, isolated from bovine brain, was from Cytoskeleton, Inc (Denver, CO). This tubulin preparation contains both alpha and beta-tubulin. Chlorpyrifos oxon (MET-674B) was from Chem Service Inc. (West Chester, PA). 10-Fluoroethoxyphosphinyl-N-biotinamidopentyldecanamide (FP-biotin) was custom synthesized in the laboratory of Dr. Charles M. Thompson at the University of Montana, Missoula, MT [9]. Diisopropylfluorophosphate (D0879) was from Sigma/Aldrich (St. Louis, MO). The nerve agents sarin and soman were from CEB (Vert-le-Petit, France). Sequencing grade modified porcine trypsin (V5113) was from Promega (Madison, WI). Slide-A-Lyzer 7K dialysis cassettes (No. 66370) and ImmunoPure immobilized monomeric avidin (#20228) were from Pierce Biotechnology Inc. (Rockford, IL).
2.2. OP-labeled tubulin tryptic peptides
Bovine tubulin (2 mg/ml) was dissolved in either 50 mM ammonium bicarbonate pH 8.3 or in 80 mM PIPES, 0.5 mM EGTA, 0.25 mM MgCl2 buffer pH 6.9, or in 10 mM TrisCl pH 8.0. The 0.5 ml of 2 mg/ml tubulin (40 µM) was treated with a 20-fold molar excess of FP-biotin dissolved in dimethyl sulfoxide, or a 200-fold molar excess of diisopropyl fluorophosphate (DFP), or a 20-fold molar excess of chlorpyrifos oxon (CPO) dissolved in dimethyl sulfoxide, or a 5-fold molar excess of soman and sarin dissolved in isopropanol. The reaction mixtures were incubated at 37°C for 16 to 24 hours. The proteins were denatured by boiling in a water bath for 10 min. Excess OP was removed by dialysis against 10 mM ammonium bicarbonate. The 1 mg of dialyzed tubulin was digested with 0.02 mg of Promega trypsin at 37°C for 16 hours.
2.3. Purification of FP-biotinylated peptides on monomeric avidin beads
The trypsin-digested, FP-biotinylated tubulin was boiled for 10 min to denature trypsin. This prevented digestion of avidin protein by trypsin. The digest was loaded on a 1 ml column of monomeric avidin beads. The column was washed with 20 ml of 1 M TrisCl pH 8.5 to wash off unbound peptides, followed by 20 ml of 0.1 M TrisCl pH 8.5, and 20 ml of 10 mM ammonium bicarbonate. Salts were washed off with 20 ml water, before the peptides were eluted with 10 ml of 10% acetic acid. One ml fractions were collected.
2.4. HPLC purification
Peptides intended for infusion on the Q-Trap mass spectrometer were purified by reverse phase HPLC. The advantage of offline HPLC purification was the large amount of peptide sample that could be loaded on the C18 column (Phenomenex Prodigy 5 micron ODS size 100 × 4.60 mm). Peptides from a 1 mg tubulin digest were eluted with a gradient that started with 100% of 0.1% trifluoroacetic acid and increased to 60% acetonitrile/40% 0.1% trifluoroacetic acid in 60 min on a Waters 625 LC system. Fractions of 1 ml were collected, analyzed by MALDI-TOF mass spectrometry, and dried in a SpeedVac.
2.5. MALDI-TOF mass spectrometry
The MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems) was used for analysis of tryptic peptides prior to more rigorous analysis with the Q-Trap. This mass spectrometer was also used for analysis of tryptic peptides. A 0.5 µl sample was spotted on a 384 well Opti-TOF plate (P/N 1016491, Applied Biosystems) and the air dried spot was overlaid with 0.5 µl of 10 mg/ml -cyano-4-hydroxycinnamic acid dissolved in 50% acetonitrile, 0.1% trifluoroacetic acid. Mass spectra were collected in positive ion reflector mode on a MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems, Foster City, CA). The final spectrum was the average of 500 laser shots. Masses were calibrated using CalMix 5 (Applied Biosystems).
2.6. Q-Trap 4000 mass spectrometry
Peptides from selected HPLC fractions were dissolved in 100 µl of 50% acetonitrile, 0.1% formic acid and infused into the Q-Trap 4000 linear ion trap mass spectrometer (Applied Biosystems) via a nanospray source, using a continuous flow head, a flow rate of 0.30 µl/min, and an ion spray potential of 1900 volts. Spray was through a distal coated silica tip emitter FS360-75-15-D (New Objective, Woburn, MA). Mass spectra were obtained using the trap function at 4000 amu/sec with dynamic fill to determine the filling time for the trap. One hundred to 350 spectra were averaged. Peptide fragmentation also employed the trap. MS/MS spectra were obtained by collision induced dissociation (CID) at a nitrogen gas pressure of 40 µTorr and a collision energy of 30–60 volts. The spectrometer was calibrated on selected fragments from the MS/MS spectrum of [Glu]-fibrinopeptide B.
3. Results
3.1. Strategy for identifying labeled residues in tubulin
The strategy is to first use FP-biotin to label the tubulin. FP-biotinylated peptides are easy to find because the biotin tag gives a signature fragmentation pattern. Masses of 227, 312, and 329 amu are always present in the MS/MS scan of an FP-biotin labeled peptide [9]. We use the MS/MS function of the MALDI-TOF-TOF mass spectrometer to screen for FP-biotin labeled peptides. Then we use the Q-Trap 4000 mass spectrometer to fragment the peptides for de novo sequencing to identify the site of covalent attachment of FP-biotin.
In a second phase, the protein is labeled with other OP. In the first round of screening for peptides labeled with these other OP, the assumption is made that the sites labeled by FP-biotin are also labeled by other OP. This assumption allows one to calculate theoretical OP-peptide masses and to look for the presence of these masses in the HPLC-fractionated, tryptic digest using the MALDI-TOF-TOF mass spectrometer. However, this assumption may not hold for all OP. Therefore a second strategy is used. Peptide masses observed in the MS scan for OP-labeled peptides are compared with theoretical masses for unlabeled peptides. The list of theoretical masses is generated with Protein Prospector software (UCSF). This free software is available at http://prospector.ucsf.edu. Candidates for OP-labeled peptides are chosen when their masses are equal to the sum of the known peptide mass and the added mass from the OP. These putative, OP-labeled peptides are further tested by CID fragmentation in the Q-Trap 4000 mass spectrometer, followed by manual de novo sequencing to identify the site of covalent, OP attachment.
3.2. Four tubulin peptides are labeled by OP
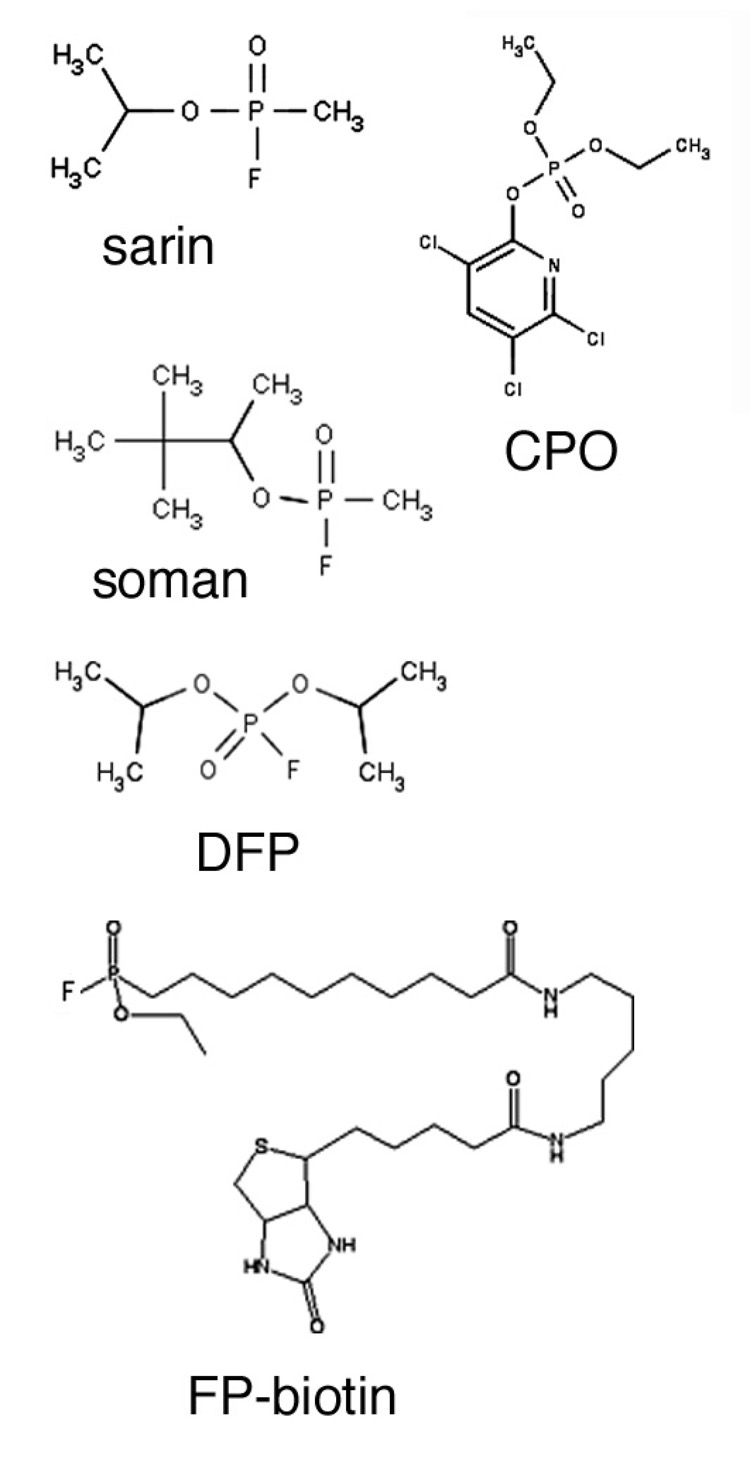
The structures of the OP studied in the present report are shown in Figure 1. A portion of each OP and the phenolic proton from the labeled tyrosine are displaced when the OP makes a covalent bond with tubulin, so that the mass added to tubulin is less than the mass of the OP. The added masses are 120 amu for sarin, 136 amu for CPO, 162 amu for soman, 164 amu for DFP, and 572 amu for FP-biotin. The leaving group is fluoride ion for sarin, soman, DFP and FP-biotin, and is 3,5,6-trichloro-2-(O)-pyridine for CPO.
Figure 1.
OP structures.
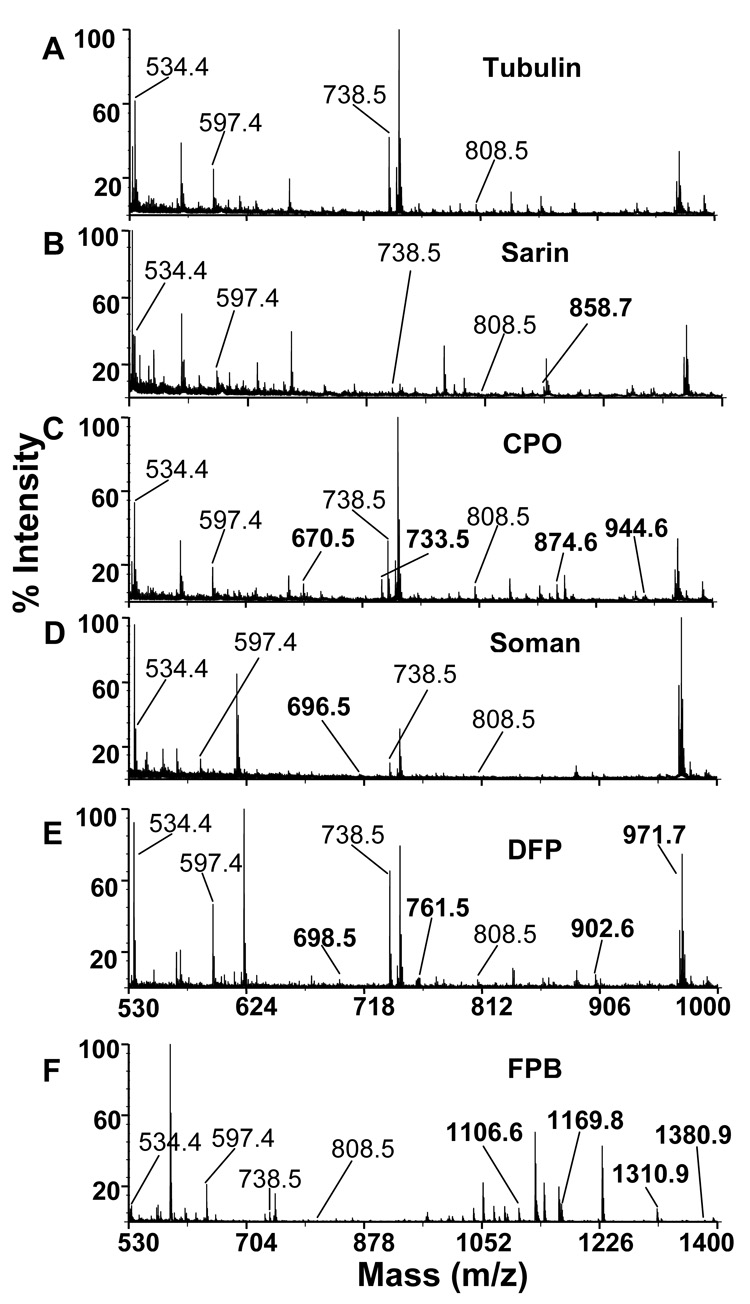
Figure 2 shows the tubulin tryptic peptides that are targets for OP-labeling, before (panel A) and after treatment with OP (panels B–F). The peaks at 534.4, 597.4, 738.5 and 808.5 m/z in panel A are unlabeled peptides with the sequences YVPR, TGTYR, GSQQYR, and EEYPDR. After treatment of tubulin with OP, new peaks appear whose masses correspond to some of the expected, theoretical masses for OP-labeled peptides (Table 1).
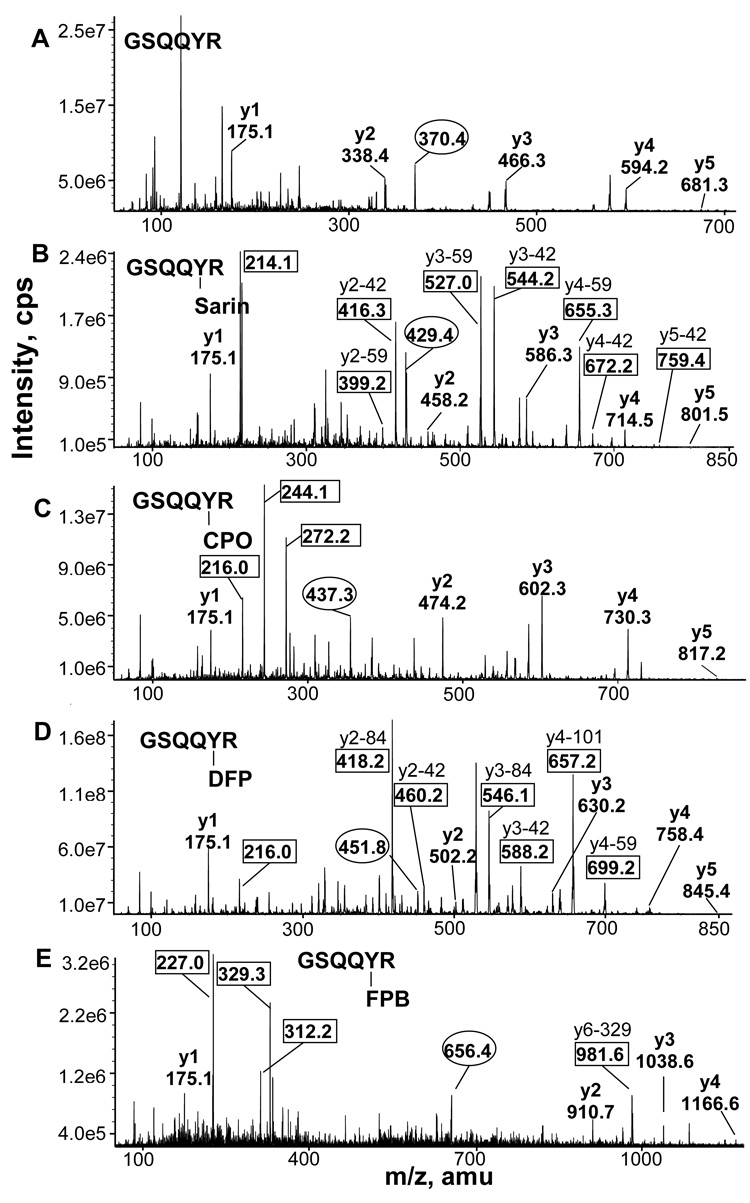
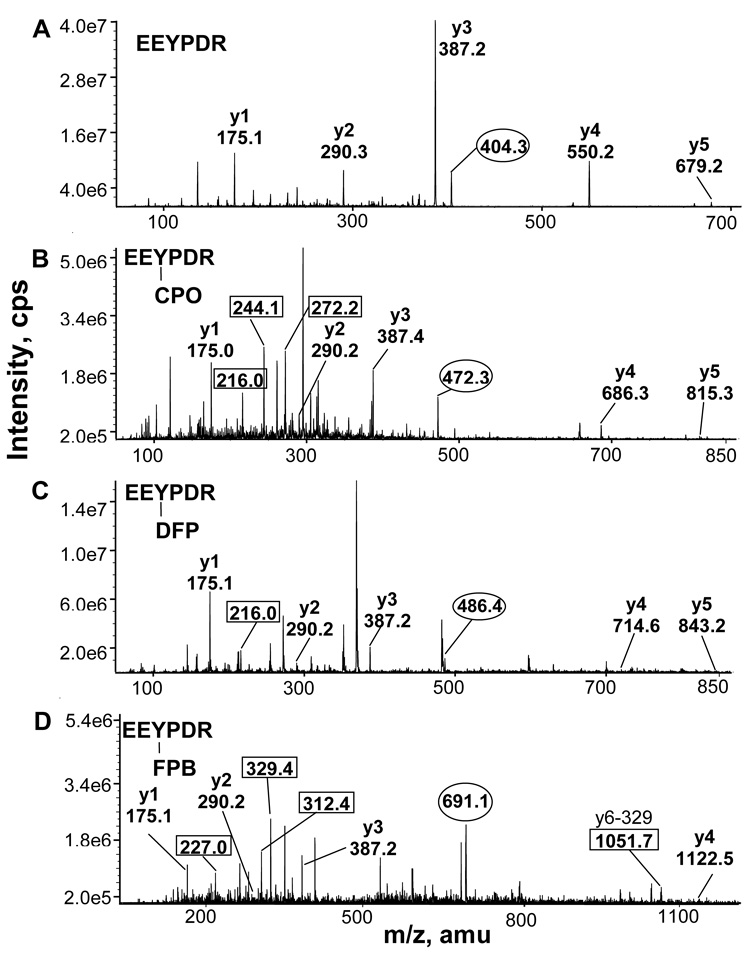
Figure 2.
Mass spectra of tryptic peptides of bovine tubulin, before and after labeling with OP. Peptides from A) control bovine tubulin, B) sarin treated tubulin, C) CPO treated tubulin, E) DFP treated tubulin, D) soman treated tubulin, F) FP-biotin treated tubulin. OP-labeled peptide masses are in bold. One peptide was labeled with sarin and soman; 4 peptides were labeled with CPO, DFP, and FP-biotin.
Table 1.
Theoretical masses of bovine tubulin tryptic peptides covalently labeled by OP
| tubulin chain | sequence | mass, m/z | +120 sarin | +136 CPO | +162 soman | +164 DFP | +572 FP-biotin |
|---|---|---|---|---|---|---|---|
| alpha | TGTYR | 597.3 | 717.3 | 733.3 | 759.3 | 761.3 | 1169.3 |
| beta | YVPR | 534.3 | 654.3 | 670.3 | 696.3 | 698.3 | 1106.3 |
| beta | GSQQYR | 738.4 | 858.4 | 874.4 | 900.4 | 902.4 | 1310.4 |
| beta | EEYPDR | 808.3 | 928.3 | 944.3 | 970.3 | 972.3 | 1380.3 |
Accession # gi: 73586894 for alpha-tubulin and gi: 75773583 for beta-tubulin in the NCBInr database.
Unlabeled active site peptides were present in each digest, indicating that modification by OP was incomplete. The relative amount of labeled and unlabeled peptide was calculated from isotope cluster areas. The results are summarized in Table 2. FP-biotin, DFP, and CPO reacted with all 4 peptides, whereas sarin and soman reacted with only one peptide. Soman and sarin concentrations were significantly lower than the concentrations of the other OP during the labeling reaction, which might explain why fewer peptides were labeled.
Table 2.
Percent of each peptide labeled by OP
| % labeled | |||||
|---|---|---|---|---|---|
| sequence | sarin | CPO | soman | DFP | FP-biotin |
| TGTYR | 43 | 7 | 70 | ||
| YVPR | 19 | 2 | 6 | 54 | |
| GSQQYR | 54 | 23 | 13 | 66 | |
| EEYPDR | 21 | 12 | 62 | ||
3.3. Tyrosine covalently modified by OP
Collision induced fragmentation in the Q-Trap mass spectrometer conclusively identified the amino acid sequence of each labeled peptide and the residue covalently modified by OP. The MS/MS spectra in Figure 3, Figure 4, Figure 5, and Figure 6 show the y ions of each unlabeled peptide (panel A), and the same peptide after covalent modification by sarin, CPO, soman, DFP, and FP-biotin (panels B–F). The masses exactly fit the indicated sequence and fit the interpretation that the OP is attached to tyrosine (Table 3).
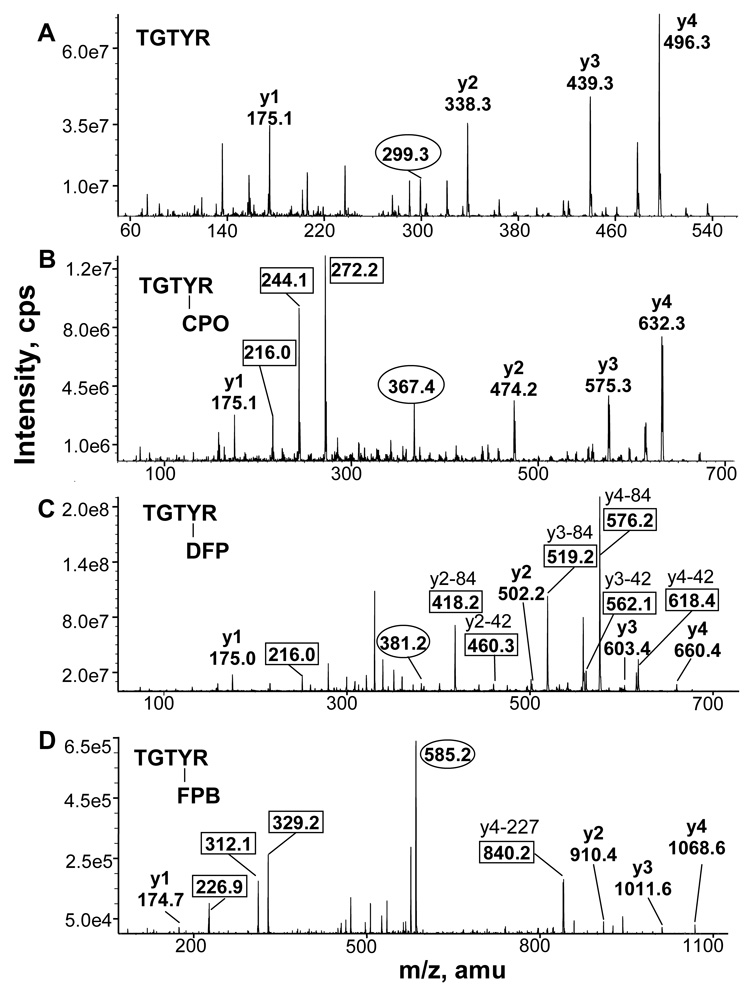
Figure 3.
MS/MS spectra of the TGTYR peptide of alpha tubulin. A) Singly charged y ions derived from the doubly-charged, unlabeled parent ion at 299.3 m/z are shown. B) CPO-labeled TGTYR has a doubly charged parent ion of 367.4 m/z. The y ion masses are consistent with diethylphosphate attached to tyrosine. The mass at 244 m/z is phosphotyrosine, the mass at 216 m/z is the immonium ion of phosphotyrosine, and the mass at 272 m/z is monoethylphosphotyrosine. C) DFP-labeled TGTYR has a doubly charged parent ion of 381.2 m/z. The y ion masses are consistent with diisopropylphosphate attached to tyrosine. Masses enclosed in boxes are y ions that have lost one (42 amu) or both (84 amu) isopropyl groups. The ion at 216 m/z is the immonium ion of phosphotyrosine. D) FP-biotin labeled TGTYR has a doubly charged parent ion of 585.2 m/z. The y ion masses are consistent with FP-biotin attached to tyrosine. The ions at 227, 312, and 329 m/z are fragments of FP-biotin. The ion at 840.2 m/z is the y4 ion that has lost 227 amu from FP-biotin.
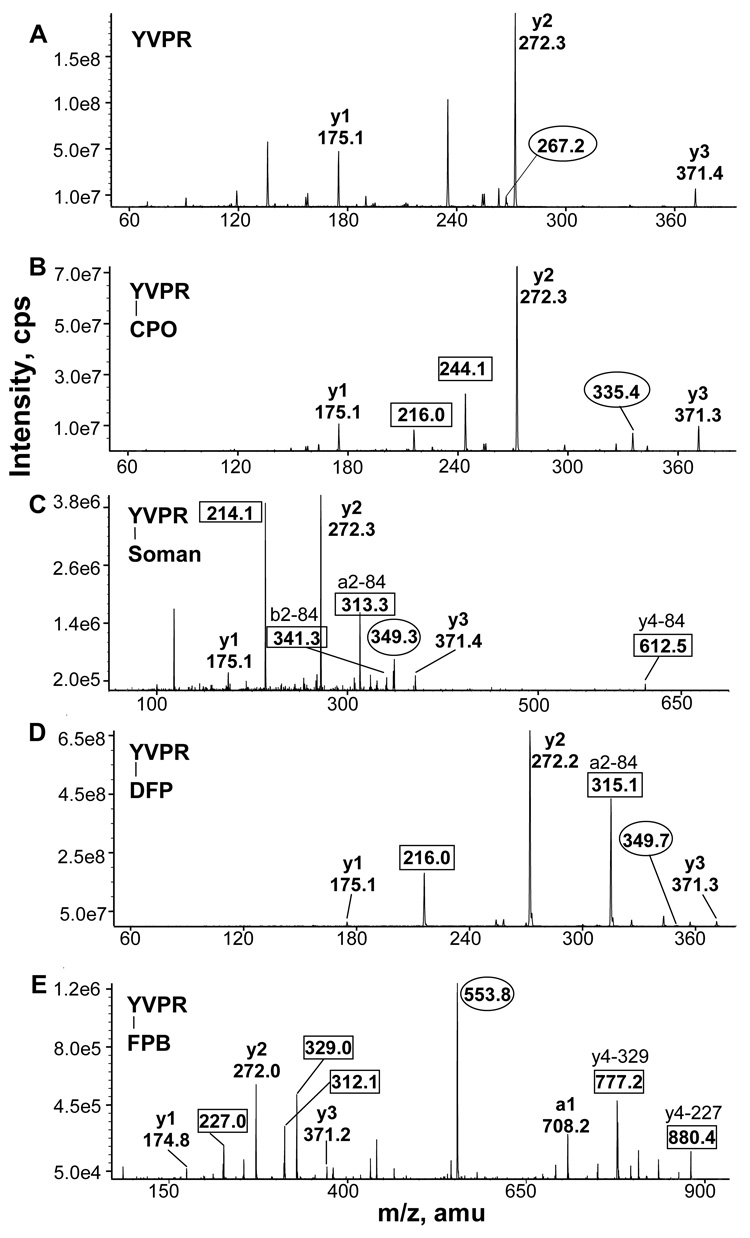
Figure 4.
MS/MS spectra of the YVPR peptide of beta tubulin. A) Singly charged y ions derived from the doubly charged, unlabeled parent ion at 267.2 m/z are shown. B) CPO-labeled YVPR has a doubly charged parent ion of 335.4 m/z. The y ion masses are consistent with diethylphosphate attached to tyrosine. The 216 m/z ion is the immonium ion of phosphotyrosine. The 244.1 m/z ion is phosphotyrosine. C) Soman labeled YVPR had a doubly charged parent ion at 349 m/z. The 214 m/z ion is the immonium ion of methylphosphotyrosine. Loss of the pinacolyl group of soman reduces the mass of the singly charged parent ion by 84 amu to yield the 612 m/z, ion. Loss of the pinacolyl group also yields the b2 ion at 341 m/z, and the a2 ion at 313.3 m/z. D) DFP-labeled YVPR has a doubly charged parent ion at 349.7 m/z. The a2 ion has lost both isopropyl groups (84 amu) to yield 315.1 m/z. E) FP-biotin labeled YVPR has a doubly charged parent ion at 553.8 m/z. Ions at 227, 312, and 329 m/z are fragments of FP-biotin. The ions at 777.2 and 880.4 m/z are the parent ion that has lost 329 or 227 amu from FP-biotin, respectively.
Figure 5.
MS/MS spectra of the GSQQYR peptide of beta tubulin. A) Singly charged y ions derived from the doubly charged, unlabeled parent ion at 370.4 m/z are shown. B) Sarin-GSQQYR had a doubly charged parent ion at 429.4 m/z and the immonium methylphosphotyrosine ion at 214 m/z. Loss of the isopropyl group reduces the y ion masses by 42 amu. Loss of the isopropyl group plus loss of an NH3 group reduces the y ion masses by 59 amu. C) CPO-GSQQYR had a doubly charged parent ion at 437.3 m/z. The immonium ion of phosphotyrosine is at 216 m/z. The phosphotyrosine ion is at 244.1 m/z. The monoethylphosphotyrosine ion is at 272.2 m/z. D) DFP-GSQQYR has a doubly charged parent ion at 451.8 m/z and the immonium phosphotyrosine ion at 216 m/z. Loss of one or both isopropyl groups from diisopropylphosphate yields y ions whose masses are reduced by 42 or 84 amu. An additional loss of 17 amu yields y ions that have lost 101 or 59 amu. E) FP-biotin-GSQQYR had a doubly charged parent ion at 656.4 m/z. Fragments derived from FP-biotin are at 227, 312, and 329 m/z. The singly charged ion at 981.6 m/z is consistent with the parent ion after loss of 329 amu from FP-biotin.
Figure 6.
MS/MS spectra of the EEYPDR peptide of beta tubulin. A) Singly charged y ions derived from the doubly charged, unlabeled parent at 404.3 m/z are shown. B) CPO-EEYPDR has a doubly charged parent ion at 472.3 m/z. The immonium phosphotyrosine ion is at 216 m/z. Phosphotyrosine is at 244 m/z and monoethylphosphotyrosine is at 272 m/z. C) DFP-EEYPDR has a doubly charged parent ion at 486.4 m/z and an immonium phosphotyrosine ion at 216 m/z. D) FP-biotin EEYPDR has a doubly charged parent ion at 691.1 m/z. Fragment ions of FP-biotin are present at 227, 312, and 329 m/z. The singly charged parent ion that has lost 329 amu from FP-biotin is at 1051.7 m/z.
Table 3.
OP-labeled tyrosines in bovine tubulin
| tubulin chain | sequence | OP-labeled tyrosine | location in crystal structure |
|---|---|---|---|
| alpha | TGTYR | Tyr 83 | loop between H2 and S3 |
| beta | YVPR | Tyr 59 | part of the loop between H1-S2; makes lateral contact between protofilaments [10] |
| beta | GSQQYR | Tyr 281 | part of the M loop between S7 and H9; makes lateral contact between protofilaments [10] |
| beta | EEYPDR | Tyr 159 | at the C-terminus of helix H4: residues 157–176 bind ribose of GTP [11] |
Ions at 214, 216, 244, and 272 m/z provide additional evidence that the OP bind to tyrosine (see the figure legends for details).
3.4. Fragmentation patterns characteristic of a particular OP
The data from Figure 3, Figure 4, Figure 5 and Figure 6 reveal CID fragmentation patterns that are characteristic of particular OP. For example, DFP-peptides readily release one or both isopropyl to yield y ions missing either 42 or 84 amu. CPO-labeled peptides yield intense peaks at 216, 244 and 272 m/z that are consistent with phosphotyrosine immonium ion, phosphotyrosine and monoethylphosphotyrosine, respectively. The FP-biotinylated peptides release the characteristic fragments of FP-biotin at 227, 312, and 329 m/z. Singly charged ions missing either 227 or 329 amu were common. The pinacolyl group of soman was released from soman labeled YVPR peptide (Fig 4C) to yield ions missing 84 amu. The isopropyl group of sarin was released from sarin labeled GSQQYR peptide (Fig 5B) to yield ions missing 42 amu. Peptides that had lost 42 amu fragmented further to release NH3 (17 amu), yielding an ion pair characteristic of sarin labeling.
In no case was the entire OP released from tyrosine. The phosphate group remained bound to tyrosine during CID fragmentation. This contrasts with OP bound to serine where the fragmentation process releases the entire bound OP, leaving no trace of the OP behind, and yielding dehydroAlanine in place of the OP-labeled serine [12].
3.5. No aging
When soman, sarin, or DFP are bound to acetylcholinesterase or butyrylcholinesterase they rapidly lose an alkyl group in a process called aging [13–15]. An aged soman labeled peptide would have an added mass of 78 amu rather than 162; an aged sarin labeled peptide would have an added mass of 78 amu rather than 120; an aged DFP labeled peptide would have an added mass of 122 amu rather than 164 in the MS spectrum. No evidence of aging was found in OP labeled tubulin peptides as no masses representing aged OP-peptides were found in MS scans. We conclude that tubulin OP adducts on tyrosine do not age.
4. Discussion
4.1. Tyrosine as a motif for OP labeling
The literature overwhelmingly supports the fact that OP bind covalently to an active site serine within the consensus sequence GXSXG. Enzymes are defined as serine hydrolases when their activity is inhibited by OP. On the other hand, covalent binding of OP to tyrosine has previously been reported only for albumin [16–19], papain [20] and bromelain [21]. Our previous mass spectrometry work identified the OP binding site in human albumin as Tyr 411, and in bovine albumin as Tyr 410 [9, 16]. The present report of OP binding to tyrosine in alpha and beta tubulin is novel. No covalent attachment site for OP binding to tubulin has previously been reported.
No obvious consensus binding site can be deduced from the 4 peptides reported here, though each target peptide contains a positively charged arginine within 3 residues of the labeled tyrosine which may serve to reduce the pKa of the tyrosine hydroxyl group, and thereby activate it.
4.2. Significance of OP-labeling of tubulin
The crystal structure of tubulin shows that each of the OP-labeled tyrosines is located in a region where protofilaments interact laterally, or bind GTP [10, 11]. OP-binding to tubulin could therefore alter tubulin conformation or GTP binding. A change in tubulin conformation or GTP binding could explain the observation of Prendergast et al that CPO inhibits tubulin polymerization [8]. When tubulin does not polymerize, microtubules do not form, and nutrient transportation from the nerve cell body to the nerve synapse is disrupted. It is concluded that OP bind covalently to tubulin, and that this binding could explain the axonal transport deficits and cognitive impairment previously associated with OP exposure [5, 6].
Acknowledgements
Supported by U.S. Army Medical Research and Materiel Command W81XWH-07-2-0034 (to OL), W81XWH-06-1-0102, Eppley Cancer Center grant P30CA36727, DGA grant 03co010-05/PEA 01 08 7 to (PM), NIH/NIEHS) 1 R01 ES012241-01A1 (to AT), and NIH ES016102 (to CMT).
Abbreviations
- amu
atomic mass units
- CPO
chlorpyrifos oxon
- DFP
diisopropyl fluorophosphate
- FP-biotin
10-fluoroethoxyphosphinyl-Nbiotinamidopentyldecanamide
- FPB
FP-biotin
- OP
organophosphorus poison
- CID
collision induced dissociation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maxwell DM, Brecht KM, Koplovitz I, Sweeney RI. Acetylcholinesterase inhibition: does it explain the toxicity of organophosphorus compounds? Arch. Toxicol. 2006;80:756–760. doi: 10.1007/s00204-006-0120-2. [DOI] [PubMed] [Google Scholar]
- 2.Roldan-Tapia L, Parron T, Sanchez-Santed F. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol. Teratol. 2005;27:259–266. doi: 10.1016/j.ntt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345:1135–1139. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- 4.London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am. J. Ind. Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- 5.Terry AV, Jr, Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J. Pharmacol. Exp. Ther. 2007;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- 6.Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J. Pharmacol. Exp. Ther. 2003;305:375–384. doi: 10.1124/jpet.102.041897. [DOI] [PubMed] [Google Scholar]
- 7.Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol. Appl. Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146:330–339. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schopfer LM, Champion MM, Tamblyn N, Thompson CM, Lockridge O. Characteristic mass spectral fragments of the organophosphorus agent FP-biotin and FP-biotinylated peptides from trypsin and bovine albumin (Tyr410) Anal. Biochem. 2005;345:122–132. doi: 10.1016/j.ab.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Lowe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 11.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 12.Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem. Res. Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- 13.Michel HO, Hackley BE, Jr, Berkowitz L, List G, Hackley EB, Gillilan W, Pankau M. Ageing and dealkylation of Soman (pinacolylmethylphosphonofluoridate)-inactivated eel cholinesterase. Arch. Biochem. Biophys. 1967;121:29–34. doi: 10.1016/0003-9861(67)90006-9. [DOI] [PubMed] [Google Scholar]
- 14.Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- 15.Nachon F, Asojo OA, Borgstahl GE, Masson P, Lockridge O. Role of water in aging of human butyrylcholinesterase inhibited by echothiophate: the crystal structure suggests two alternative mechanisms of aging. Biochemistry. 2005;44:1154–1162. doi: 10.1021/bi048238d. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Schopfer LM, Hinrichs SH, Masson P, Lockridge O. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay for organophosphorus toxicants bound to human albumin at Tyr411. Anal. Biochem. 2007;361:263–272. doi: 10.1016/j.ab.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Means GE, Wu HL. The reactive tyrosine residue of human serum albumin: characterization of its reaction with diisopropylfluorophosphate. Arch. Biochem. Biophys. 1979;194:526–530. doi: 10.1016/0003-9861(79)90647-7. [DOI] [PubMed] [Google Scholar]
- 18.Williams NH, Harrison JM, Read RW, Black RM. Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch. Toxicol. 2007;81:627–639. doi: 10.1007/s00204-007-0191-8. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Nachon F, Froment M-T, Verdier L, Debouzy J-C, Gillon E, Brasme B, Schopfer L, Lockridge O, Masson P. Binding and hydrolysis of soman by human serum albumin. Chem. Res. Toxicol. 2007 doi: 10.1021/tx700339m. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 20.Chaiken IM, Smith EL. Reaction of a specific tyrosine residue of papain with diisopropylfluorophosphate. J. Biol. Chem. 1969;244:4247–4250. [PubMed] [Google Scholar]
- 21.Murachi T, Inagami T, Yasui M. Evidence for alkylphosphorylation of tyrosyl residues of stem bromelain by diisopropylphosphorofluoridate. Biochemistry. 1965;4:2815–2825. doi: 10.1021/bi00888a036. [DOI] [PubMed] [Google Scholar]