Abstract
Leptin is a centrally-acting hormone controlling metabolic pathways. Recently, it was shown that leptin can reduce amyloid β levels both in vitro and in vivo. Herein, phosphorylation of tau was investigated following treatment of neuronal cells with leptin and insulin. Specifically, phosphorylation of tau at aa residues Ser202, Ser396 and Ser404 were monitored in retinoic-acid induced, human cell-lines: SH-SY5Y and NTera-2. Both hormones induced concentration- and time-dependent reductions of tau phosphoylation, and were synergestic at suboptimum concentrations. Importantly, leptin was 300-fold more potent than insulin (IC50L= 46.9 nM vs IC50I= 13.8 µM). A central role for AMP-dependent kinase as a mediator of leptin’s action is demonstrated by the ability of 5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) to decrease tau phosphorylation and, by blocking leptin in the presence of Compound C. Thus, leptin, which ameliorates both, amyloid β and tau-related pathological pathways, holds promise as a novel therapeutic for Alzheimer’s disease.
Keywords: leptin, tau, Alzheimer’s disease, insulin, AMPK, AICAR
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder of the CNS characterized by distinct pathological hallmarks within the brain. Postmortem examination of AD brains reveals the presence of parenchymal plaques, which consist primarily of the amyloid beta (Aβ) peptide, and neurofibrillary tangles (NFT), which result from hyperphosphorylation of the microtubule-interacting protein, tau.
Leptin is a peptide hormone synthesized by adipocytes that modulates metabolic energy availability [1], thereby leading to fat storage or mobilization, and which also controls insulin sensitivity [2]. Within the central nervous system, leptin crosses the blood brain barrier to bind specific receptors in the brain to mediate food intake, body weight and energy expenditure [3].
Recently, studies have demonstrated that leptin modulates Aβ production and clearance in cell cultures and rodents [4]. Observational studies have shown that a decline of leptin levels is associated with cognitive impairment in the elderly [5]. Also known is that AD patients experience weight loss and a drop in circulating leptin levels [6]. Based on insulin’s known effect on tau phosphorylation [7; 8], we decided to investigate whether leptin had similar activity, alongside or subsequently to its known effects on Aβ homoestasis [4].
We treated primary neurons and neuronal cell lines with a range of concentrations of leptin and insulin, alone or in combination, and for various times. In all cell types, leptin treatment resulted in reduced phosphorylated tau levels at sites relevant to AD pathology. Moreover, leptin was two orders of magnitude more potent than insulin, as determimed by IC50 values.
Materials and Methods
Reagents and Antibodies
Minimum essential medium (MEM) was purchased from ATCC (Manassas, VA). Neurobasal medium, B27 supplement and L-glutamine were purchased from Gibco (Carlsbad, CA). Trypsin-EDTA and penicillin solution were purchased from MP Biomedicals (Solon, Ohio). Fetal bovine serum (FBS), all-trans retinoic acid (RA), human recombinant leptin and insulin were purchased from Sigma-Aldrich (St. Louis, MO). 5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) was purchased from Cell Signaling Technology (Danvers, MA). Compound C was purchased from EMD Chemicals (Gibbstown, NJ).
Rabbit anti-AMPKα (pThr172), -AMPKα (total) and tau (pSer396) mAb were purchased from Cell Signaling Technology. Tau mAb (clone 5E2) was purchased from Upstate Cell Signaling Solutions (Lake Placid, NY). PHF-tau mAb (clone AT8) was purchased from Pierce Biotechnology (Rockford, IL). PHF-1 mAb was a gift from Dr. Peter Davies, Albert Einstein College of Medicine (Bronx, NY). Rabbit anti-leptin receptor and α-tubulin mAb were purchased from Affinity BioReagents (Golden, CO). Insulin receptor (β-subunit) mAb was purchased from Millipore (Billerica, MA).
Culture of Cell Lines
Human neuroblastoma, SH-SY5Y, and embryonal carcinoma, NTera-2 (NT2), cell lines were purchased from ATCC. Cell culture was performed according to manufacturer’s specific guidelines. Cells were propagated in MEM containing 10% FBS until 80–90% confluence then detached from the flask by trypsin-EDTA and sub-cultured at a ratio of 1:5.
Neuronal Induction
1 × 106 SY5Y or NT2 cells were grown in neuronal induction medium (NIM), which consisted of MEM containing 5% FBS supplemented with 10 µM RA. SY5Y were grown in NIM for 6 days, and switched to serum-free NIM prior to treatment and harvesting on day 7. Neuronal differentiation of NT2 cells was based on a previously described protocol [9]. Differentiated NT2 cells (NT2N) were switched to serum-free NIM on the day prior to treatment and harvesting.
Culture of Rat Primary Neurons
Primary rat cortical neurons were purchased from BrainBits LLC (Sprinfield, IL), and cultured as per manufacturer’s instructions. Briefly, tissues were dispersed and supernatant was transferred to a new tube and centrifuged for 1 min at 1100 rpm. Neurons were then seeded in 6-well plates coated with poly-D-lysine (BD Biosciences; San Jose, CA) and grown in Neurobasal medium supplemented with B27 and 0.5mM L-glutamine. Medium was changed after 4 days, and at 7 days in culture the neurons were treated and harvested.
Protein Extraction and Western Blotting
Neuronal cells were harvested by scraping. Cell pellets were resuspended in protease and phosphatase inhibitor-supplemented 1X RIPA lysis/extraction buffer (Pierce), and then subjected to freeze/thaw cycles in a dry ice/ethanol bath. Total protein was determined with the Coomassie (Bradford) Protein Assay Kit (Pierce). Whole cell extracts (25 µg) were analyzed by western blots using 10% SDS-PAGE pre-cast gels (Lonza; Rockland, ME), and the proteins were transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were incubated overnight at 4°C with primary antibodies and then detected the following day with HRP-conjugated IgG. All primary antibodies, except tau-pSer396 (1:500), total tau (1:500) and PHF-tau AT8 (1:200), and secondary antibodies were used at final dilutions of 1:1,000 and 1:10,000, respectively. HRP was developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce), and imaged using a BioRad (Hercules, CA) ChemiDoc XRS System. The membranes were stripped with Restore PLUS Western Blot Stripping Buffer (Pierce) for reprobing with other antibodies.
Statistical Analysis
Statistical data analyses were performed with analysis of variance and Tukey-Kramer multiple comparisons test. Densitometric analyses were performed using the UN-SCAN-IT gel 6.1 software (Silk Scientific; Orem, UT). p<0.05 was considered statistically significant.
Results
Leptin, insulin and tau phosphorylation in RA-induced SY5Y cells
RA induction of the human neuroblastoma cell line, SY5Y, has been reported to increase phosphorylation of tau at AD-related sites [10]. We therefore utilized SY5Y cells induced with retinoic acid (RA-SY5Y) for 7 days as our primary in vitro model to investigate the effects of leptin and other treatments on tau phosphorylation.
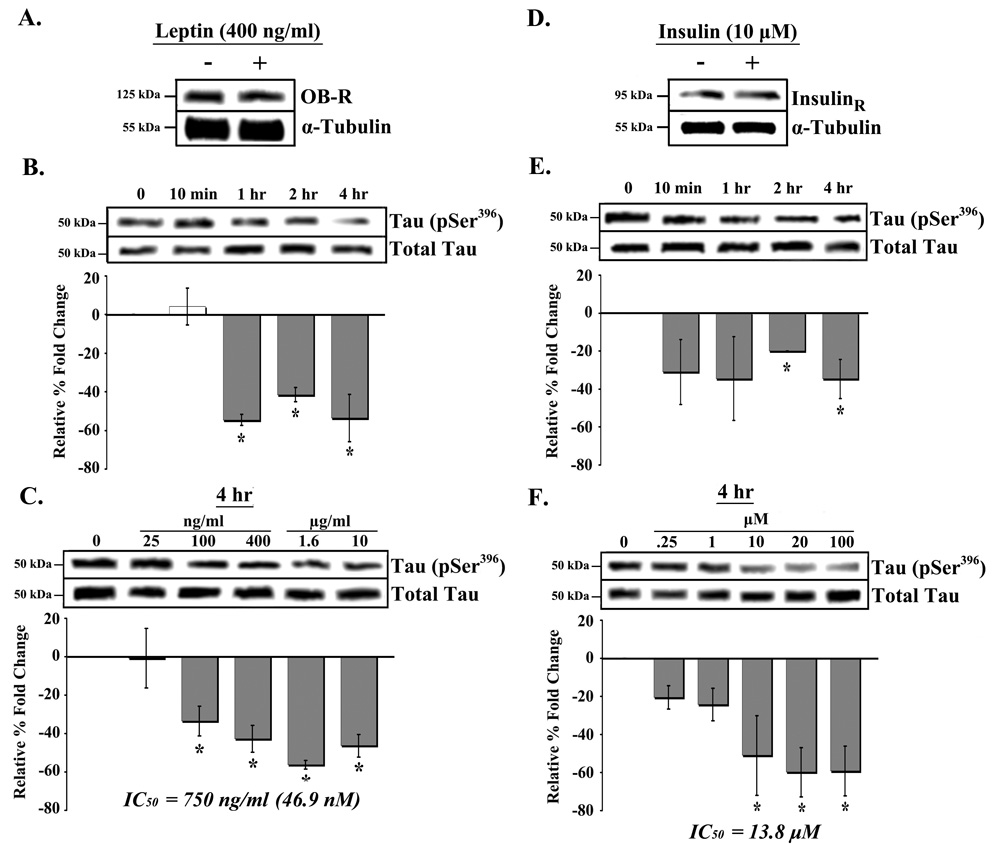
The first set of studies examined expression of the leptin receptor (OB-R) in RA-SY5Y cells treated with 400 ng/ml leptin or placebo. Both treated and placebo cells were found to express relatively high levels of OB-R, however treatment did not significantly alter receptor expression (Figure 1A). We next determined whether leptin had an effect on tau phosphorylation. Cells were treated for a range of time periods with 400 ng/ml leptin or placebo, and phosphorylation of tau at Ser396, a site which affects the ability of tau to bind microtubules when phosphorylated [11], was measured (Figure 1B). Significant (p<0.05) decreases in tau (Ser396) phosphorylation were observed in cells treated with leptin for 1, 2 or 4 hrs compared to placebo (Figure 1B; far right bars). No change in phosphorylation was observed in cells treated with leptin for 24 hrs compared to 4 hrs (data not shown).
Fig. 1.
Time- and dose-dependent dephosphorylation of tau by leptin and insulin in RA-SY5Y. A. RA-SY5Y were treated with leptin (400 ng/ml) for 4 hrs, or non-treated (placebo), and analyzed by western blot using an antibody against OB-R (leptin receptor). Membranes were stripped and re-probed with an antibody against α-tubulin for normalization. Extracts from cells treated for various (B) times with leptin (400 ng/ml), or (C) concentrations (for 4 hr) were prepared and analyzed by western blot using anti-tau (pSer396) antibodies. Membranes were stripped and re-probed with total tau antibody for normalization and analyzed by densitometry. Results are presented as the mean ± SD percent fold change, relative to placebo-treated samples, which were arbitrarily assigned a value of 0. IC50 represents the leptin concentration at which tau (pSer396) phosphorylation is decreased by 50 percent. D. Cells treated with insulin (10 µM) for 4 hrs, or non-treated (placebo), were analyzed by western blot using an antibody against insulin receptor (β-subunit). Membranes were re-probed with anti-α-tubulin antibodies for normalization. Extracts from cells treated for various (E) times with insulin (10 µM), or (F) concentrations (for 4 hr) were prepared and analyzed by western blot using pSer396 antibodies. Experiments were performed as in B and C, respectively. IC50 represents the insulin concentration at which tau phosphorylation is decreased by 50 percent. All blots are representative, n=3.
*p<0.05 vs. non-treated
To determine the dose-response relationship between leptin and tau phosphorylation, RA-SY5Y cells were treated with leptin for 4 hrs at a range of concentrations (Figures 1C). We observed a significant (p<0.05) decrease in tau (Ser396) phosphorylation in cells treated with 100 ng/ml leptin (Figure 1C; second bar from left). Decreasing phosphorylation was observed up to a concentration of 1600 ng/ml leptin (second bar from right), which produced the maximal effect. Estimation of the 50% inhibitory concentration (IC50) of leptin for tau phosphorylation provided a value of 750 ng/ml, or 46.9 nM.
There is increasing evidence to suggest a link between insulin resistance, diabetes mellitus, impaired glucose tolerance and AD [12]. Several reports have demonstrated that insulin treatment reduces the level of phosphorylated tau in both in vitro [7; 13] and in vivo [8] models. We therefore tested the effect of insulin treatment on tau (Ser396) phosphorylation in RA-SY5Y cells and compared it to that of leptin.
Both insulin (10 µM) and placebo-treated cells were found to express high levels of insulin receptor (Figure 1D). Time-course studies with 10 µM insulin treatment produced significant (p<0.05) decreases in tau (Ser396) phosphorylation at 2 and 4 hrs compared to placebo controls (Figure 1E; far right bars). No change in phosphorylation was observed in cells treated with insulin for 24 hrs compared to 4 hrs (data not shown). Dose-response studies with 4 hr insulin treatment produced a significant (p<0.05) decrease in tau phosphorylation at 10 µM insulin concentration (Figure 1F; third bar from right). Further, maximum decrease of phosphorylation was observed at a concentration of 20 µM (second bar from right). Estimation of insulin’s IC50 for tau phosphorylation provided a value of 13.8 µM.
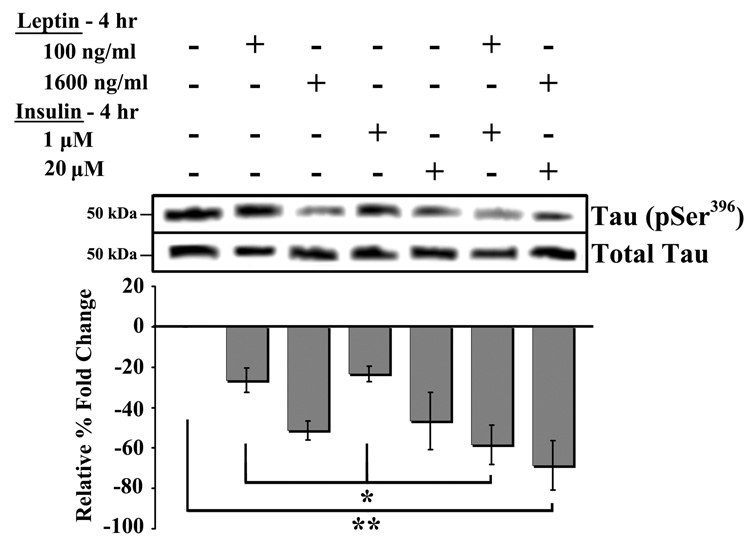
Combined leptin and insulin treatment and tau phosphorylation
RA-SY5Y cells were treated for 4 hrs with sub-optimal or maximum effect doses, either in combination or alone, of leptin and/or insulin, and tau (Ser396) phosphorylation was measured (Figures 2). A significant (p<0.05) decrease in phosphorylation was observed in cells treated with sub-optimal combinations of leptin (100 ng/ml) and insulin (1 µM) compared to either treatment alone (Figure 2; first, third and fifth bars from left). Co-treatment with maximum effect doses of leptin (1600 ng/ml) and insulin (20 µM) produced the most significant (p<0.01) decrease in phosphorylation (first bar from right) compared to placebo-treated. Co-treatment with maximum effect doses of leptin and insulin did not produce a significant (p>0.05) reduction in tau phosphorylation compared to either treatment alone.
Fig. 2.
Combined treatment of leptin and insulin. RA-SY5Y were treated with low or high concentrations of leptin and/or insulin for 4 hrs, or non-treated (placebo), and analyzed by western blot with anti-tau (pSer396) antibodies. Membranes were stripped and re-probed with anti-tau (total) antibodies for normalization and band densities were analyzed by densitometry. Results are presented as the mean ± SD percent fold change, relative to placebo-treated samples, which were arbitrarily assigned a value of 0. Representative blot is shown, n=3.
*p<0.05 vs. group
**p<0.01 vs. group
Tau phosphorylation at other AD-related sites and in other neuronal cultures
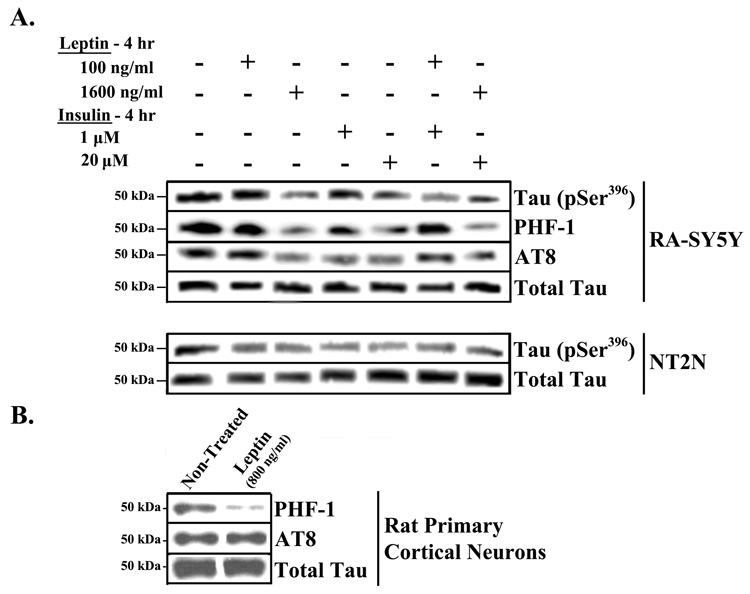
In addition to the phosphorylation of tau at Ser396 (Figure 1 and Figure 2), other sites within tau known to be phosphorylated in paired helical filaments (PHF) were examined. Specifically, the phosphorylation of tau at Ser396/404 and Ser202 was monitored using the PHF-1 and AT8 antibodies, respectively (Figure 3A, top panel; Table 1). RA-SY5Y cells were treated with leptin and/or insulin as in Figure 2. Similar effects were observed using the PHF-1 and AT8 antibodies as with pSer396. Thus it appears that leptin and insulin can modulate phosphorylation of tau at multiple AD-related sites.
Fig. 3.
Effect of leptin and insulin on other phospho-epitopes and other neuronal cell types. A. RA-induced SY5Y and NT2N were treated with low or high concentrations of leptin and/or insulin for 4 hrs, or non-treated (placebo). B. Primary rat cortical neurons were treated with leptin for 24 hrs or placebo. Cell extracts were prepared and analyzed by western blot with antibodies against specific tau phosphoepitopes (pSer396, PHF-1 or AT8). Membranes were stripped and re-probed with antibodies against total tau for normalization. Representative blots are shown, n=3
Table 1.
Relative tau phosphorylation in treated neuronal cultures
| Cell Type | Phospho-Site | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Treated | Leptin 100 ng/ml | Leptin 800 ng/ml | Leptin 1600 ng/ml | Insulin 1µM | Insulin 20 µM | Leptin 100 ng/ml + Insulin 1 µM | Leptin 1600 ng/ml + Insulin 20 µM | ||
| RA-SY5Y | pSer396 | 0 | −26±6* | ND | −51±5* | −23±4* | −47±14* | −58±10* | −69±12* |
| PHF-1 | 0 | −20±19 | ND | −67±4* | −37±11* | −80±7* | −72±3* | −84±6* | |
| AT8 | 0 | −10±5 | ND | −60±19* | −40±13* | −57±14* | −61±17* | −66±21* | |
| NT2N | pSer396 | 0 | −27±6* | ND | −27±5* | −23±6* | −53±10* | −42±7* | −48±1* |
| Rat 1° Neuron | PHF-1 | 0 | ND | −75±18* | ND | ND | ND | ND | ND |
| AT8 | 0 | ND | 5±23 | ND | ND | ND | ND | ND | |
Normalized band densities from Figure 3 were analyzed by densitometry and results are presented as the mean ± SD percent fold change, relative to non-treated samples, which were arbitrarily assigned a value of 0. (ND – Not Determined)
p<0.05 vs. non-treated
In addition to RA-SY5Y cells, two more neuronal cell types were examined: human NT2 cells, which undergo neuronal differentiation with RA treatment (NT2N), and rat primary cortical neurons. NT2N cells were treated with leptin and/or insulin, and the levels of pSer396 were measured (Figure 3A, bottom panel; Table 1). The effects of treatments with insulin alone or in combination with leptin were similar to those seen in RA-SY5Y. For the rat primary neurons, we determined the effect of 24 hr leptin treatment on phosphorylation of tau, as detected by PHF-1 and AT8 antibodies (Figure 3B; Table 1). 800 ng/ml of leptin produced a significant (p<0.05) decrease in tau phosphorylation, as detected by PHF-1 antibody compared to placebo-treated cells. However, in these cells, leptin was unable to reduce the levels of phosphorylated tau as measured by the AT8 antibody (middle row, far right band).
AMPK signaling and tau phosphorylation in RA-SY5Y cells
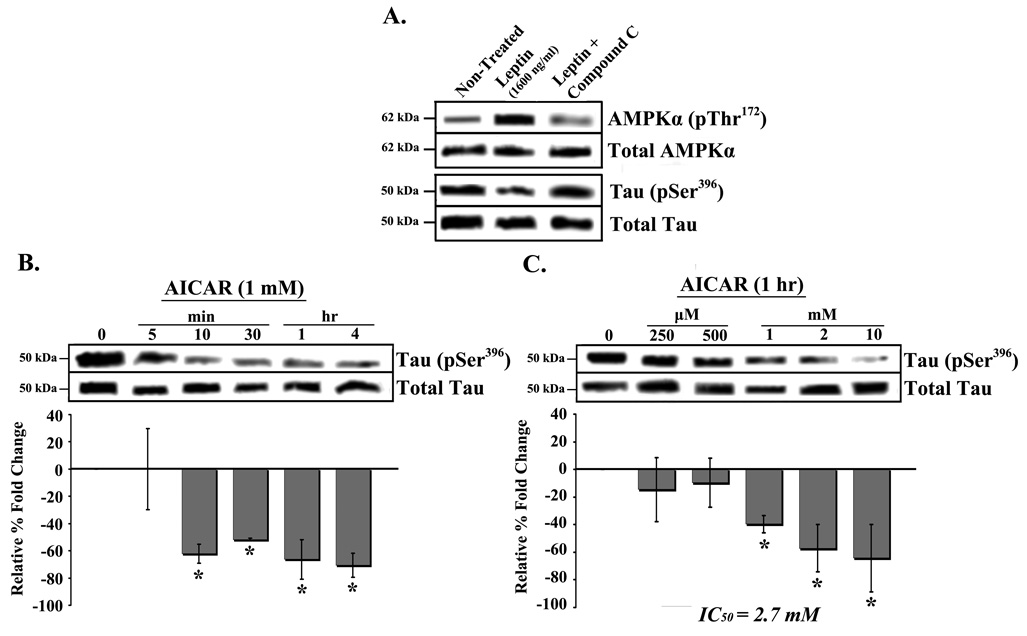
We next explored the post-receptor binding signaling pathway activated by leptin in modulating tau phosphorylation. The energy homeostasis enzyme, AMP-activated protein kinase (AMPK), has been linked to tau phosphorylation via regulation of mammalian target of rapamycin (mTor), PP2A and glycogen synthase kinase-3β (GSK-3β) [14]. As leptin is known to directly activate AMPK [15], we explored the effect of an AMPK activator (AICAR) on tau phosphorylation and compared it to leptin. In addition, we investigated whether Compound C (inhibitor of AMPK) can block leptin’s effect.
Leptin treatment produced a large increase in pThr172 AMPKα band density (Figure 4A, top row, middle band), thus demonstrating activation of AMPKα. The leptin effect was specific, since co-treatment with the AMPK inhibitor, compound C (10 µM), abrogated AMPK activation (top row, far right band) as well as tau Ser396 phosphorylation (second row from bottom, far right band). Treatment with 1 mM AICAR from 10 min to 4 hrs significantly (p<0.05) decreased Ser396 phosphorylation compared to placebo (Figure 4B; gray bars). Dose-response studies with 1 hr AICAR treatment produced a significant (p<0.05) decrease in Ser396 phosphorylation in cells treated with 1 mM AICAR and higher (Figure 4C; right bars). Estimation of AICAR’s IC50 for tauphosphorylation provided a value of 2.7 mM.
Fig. 4.
Dephosphorylation of tau by AMPK activation in RA-SY5Y. A. Induced cells were treated with leptin (1600 ng/ml) with or without the AMPK inhibitor Compound C, for 4 hr, or non-treated (placebo). Whole cell extracts were prepared and analyzed by western blot with antibodies against phospho-AMPKα (pThr172) and –tau (pSer396). Membranes were stripped and re-probed with anti-AMPKα or tau (both total) for normalization. Whole cell extracts from cells treated for various (B) times with AICAR (1 mM), or (C) concentrations (1 hr) were prepared and analyzed by western blot with anti-tau (pSer396) antibodies compared to placebo. Membranes were stripped and re-probed with antibodies against total tau for normalization, which were analyzed by densitometry. Results are presented as the mean ± SD percent fold change, relative t.o placebo-treated samples, which were arbitrarily assigned a value of 0. IC50 represents the AICAR concentration at which tau phosphorylation is decreased by 50 percent. Representative blots are shown, n=3.
*p<0.05 vs. non-treated
In summary, the results suggest that activation of AMPKα by leptin reduces tau phosphorylation at AD-related sites.
Discussion
The utilization of the adipocyte hormone, leptin, in models of AD highlight a unique strategy in addressing the potential underlying causes of the sporadic-onset form of the disease [4][16]. Abnormal lipid levels within the brain have been associated with Aβ production and clearance. Increased production of Aβ has been shown to induce hyperphosphorylation of tau, thereby promoting NFT formation [17]. Leptin, which physiologically functions to modulate lipid homeostasis, has been reported to reduce β-secretase activity and Aβ levels in vitro [4], and brain Aβ load in vivo [4]. Additionally, lower levels of circulating leptin have been observed in AD patients versus healthy controls [6]. In support, a large prospective study involving 2,871 elderly followed over a period of 4 years showed that low leptin levels were associated with a greater cognitive decline [5].
In this study we investigated whether leptin could have a direct effect on the level of tau phosphorylation at sites known to be hyperphosphorylated in AD. Initially using RA-induced, human SY5Y cells, known to express high levels of phosphorylated tau [10] we confirmed previous studies showing that insulin reduces the level of phosphorylated tau [7; 13] and in agreement with in vivo studies [8]. Insulin was able to reduce phospho-tau (Ser396) by 50% at a concentration of 13.8 µM. Strikingly, leptin was 300-fold more potent in this system, being able to achieve the same reduction at a concentration of 46.9 nM (Figures 1). Insulin is considered a potential therapeutic for AD, thus these findings suggest that leptin may represent an attractive alternative with increased potency.
While the signaling pathways through which insulin mediates its specific effects on tau phosphorylation have been studied extensively [8], similar pathways activated by leptin to modulate tau have not been reported. To explore leptin’s post-receptor signaling pathways involved in tau phosphorylation, we focused on the energy homeostasis enzyme AMPK (Figure 4), known to be modulated by leptin [18; 19]. This enzyme is also known to interact with GSK-3β [20], which can potentially phosphorylate all phosphoepitopes studied herein. Activation of AMPK with AICAR produced significant changes in tau phosphorylation within 10 min (Figure 4). In tissues such as skeletal muscle and liver, leptin stimulates fatty acid oxidation via activation of AMPK [19]. However, within the hypothalamus, leptin has been reported to regulate food intake via inactivation of AMPK [18]. In this study leptin was capable of activating AMPK in SY5Y cells differentiated by retinoic acid. It remains to be determined whether AMPK in hippocampal neurons responds similarly. These findings suggest that CNS neuronal AMPK may provide a novel therapeutic target for reducing AD-related tau phosphorylation.
Current research is elucidating the mechanism of action of leptin to reduce tau phosphorylation. We demonstrated here that activation of AMPK mimics the leptin effect (Figure 4). This may be linked to the Akt/GSK-3β pathway that modulates tau phosphorylation; however there are many other kinases and phosphatases that regulate tau at various phosphorylation sites [21].
To clearly show the therapeutic value of leptin in treating or preventing NFT formation, in vivo experiments are necessary. One approach could involve studies with the triple transgenic mouse model of AD, which develop both plaque and tangle pathology [22]. Validation of our current findings, in vivo, would demonstrate leptin’s value in selectively targeting both pathologies of AD.
Leptin has been used in clinical trials extensively and has demonstrated an excellent safety profile, even after prolonged treatments. Taken together, our preclinical data demonstrating that leptin ameliorates both Aβ and tau-related pathologies, along with its pharmacological profile, support its use as a novel therapeutic for Alzheimer’s disease.
Acknowledgements
This work was supported by the National Institute on Aging (SBIR –1R43AG029670) and the New Jersey Commission on Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 3.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 4.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer's Abeta. Faseb J. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 5.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power DA, Noel J, Collins R, O'Neill D. Circulating leptin levels and weight loss in Alzheimer's disease patients. Dement Geriatr Cogn Disord. 2001;12:167–170. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- 7.Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- 8.Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- 10.Jamsa A, Hasslund K, Cowburn RF, Backstrom A, Vasange M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer's disease-like tau phosphorylation. Biochem Biophys Res Commun. 2004;319:993–1000. doi: 10.1016/j.bbrc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 11.Evans DB, Rank KB, Bhattacharya K, Thomsen DR, Gurney ME, Sharma SK. Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau's ability to promote microtubule assembly. J Biol Chem. 2000;275:24977–24983. doi: 10.1074/jbc.M000808200. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer's disease? Trends Pharmacol Sci. 2002;23:288–293. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 13.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 14.Meske V, Albert F, Ohm TG. Coupling of mammalian target of rapamycin with phosphoinositide 3-kinase signaling pathway regulates protein phosphatase 2A- and glycogen synthase kinase-3 -dependent phosphorylation of Tau. J Biol Chem. 2008;283:100–109. doi: 10.1074/jbc.M704292200. [DOI] [PubMed] [Google Scholar]
- 15.Uotani S, Abe T, Yamaguchi Y. Leptin activates AMP-activated protein kinase in hepatic cells via a JAK2-dependent pathway. Biochem Biophys Res Commun. 2006;351:171–175. doi: 10.1016/j.bbrc.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Signore AP, Zhang F, Weng Z, Gao Y, Chen J. Leptin neuroprotection in the CNS: mechanisms and therapeutic potentials. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resende R, Ferreiro E, Pereira C, Oliveira CR. ER stress is involved in Abeta-induced GSK-3beta activation and tau phosphorylation. J Neurosci Res. 2008 doi: 10.1002/jnr.21648. [DOI] [PubMed] [Google Scholar]
- 18.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 19.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 20.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci. 2007;26:3429–3436. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]