Abstract
Acid is an important mediator in the pathogenesis of cough. Inhalation of exogenous acid triggers cough and endogenous acid may contribute to cough in respiratory diseases. Acid directly stimulates vagal bronchopulmonary sensory nerves that regulate the cough reflex. Consistent with their putative role in defence against aspiration and inhaled irritants, Aδ-fibre nociceptors in the large airways are most efficiently stimulated by rapid acidification. In contrast, acid-sensitive properties of the C-fibre nociceptors allow for continuous monitoring of pH which is likely important in inflammation. Acid is also the single most important mediator in the pathogenesis of cough due to gastro-oesophageal reflux (GOR). The cough pathways can be sensitized by the sensory inputs from the oesophagus. This sensitization is likely mediated by a subset of the vagal oesophageal sensory nerves distinguished by discriminative responsiveness to noxious stimuli (nociceptors). The receptors underlying acid-sensitivity of vagal sensory nerves are incompletely understood. The role of TRPV1 has been established but the roles of acid-sensing ion channels (ASIC) and other receptors await more definitive investigation. Here we provide a brief overview of the cough-related acid-sensitive sensory pathways and discuss the mechanisms of acid-sensitivity.
Keywords: cough receptors, acid receptors, aspiration cough, nociceptors
1. Introduction
A decrease in local pH often accompanies inflammation, ischaemia or, in the gastrointestinal system, disorders of acid secretion and containment. Acid has multiple effects in tissues including activation of sensory nerves and/or modulation of sensory excitability. In this review we discuss the effects of acid and the membrane ion channels underlying these effects in the sensory nerves regulating cough. We focus on the vagal sensory nerves in the airways, lungs and oesophagus, thought to be most important pathways mediating acid contribution to the pathogenesis of cough. We also aim to highlight some gaps in the current understanding of the sensory acid-sensitivity and its mechanisms.
2. Peripheral sensory pathways regulating cough
The major pathways for triggering and/or modulation of cough are the vagal sensory nerves innervating the airways and lungs. The vagal innervation of these organs is described in detail elsewhere in this issue [1] and is only briefly outlined below. Another group of vagal afferent pathways important for the modulation of the cough reflex are the sensory nerves innervating the oesophagus. These pathways likely play a major role in the pathogenesis of cough due to gastro-oesophageal reflux (GOR) which ranks among the most important causes of chronic cough [2]. Although cough in GOR can result from (micro) aspiration, GOR-related cough may also be caused by the stimulation of sensory nerves in the oesophagus [3]. Stimulation of sensory nerves in the oesophagus may directly trigger cough or lead to cough by inducing hyperexcitability of the cough reflex via central sensitization [4,5]. Regardless of the mechanisms involved in the pathogenesis of cough in GOR, the key sensory event is the stimulation of sensory nerves. The major reflux component implicated in the cough due to GOR is acid.
3. Classification of vagal sensory nerves
Different classifications have been evolving to categorize sensory nerves in the respiratory and digestive systems [6,7]. The criteria used to categorize the sensory nerves are typically based on their mechanical and chemical responsiveness, presumed location of the nerve terminals in the tissues and/or presumed reflex consequences of the nerve activation. For the purpose of this discussion, we adopt a broad classification of the sensory nerves into two categories: the low threshold mechanosensors and the nociceptors. This classification is based on the discriminative responsiveness to the noxious stimuli, i.e. stimuli associated with impending or actual tissue damage. Thus in this discussion we use the definition of nociceptors as the sensory nerves that have the capacity to discriminate noxious stimuli, while the sensory nerves that lack this capacity are referred to as low threshold mechanosensors. Most sensory nerve types described in the airways, lungs [8] and oesophagus can be categorized as either low threshold mechanosensors or nociceptors. This classification also allows for comparison of the sensory nerves in the respiratory and digestive systems.
Low threshold mechanosensitive nerve terminals are exquisitely sensitive to mechanical forces associated with physiological activity of the innervated tissue. For example, the mechanosensors in the lung (slowly adapting pulmonary stretch receptors, SARs, see below) are activated by lung distention during eupnoeic breathing [9] and the mechanosensors in the oesophagus (tension mechanosensors, see below) are activated by the oesophageal movements during swallowing [10]. The low threshold mechanosensors lack the capacity to discriminate noxious mechanical stimuli and do not directly respond to chemical stimuli including inflammatory autacoids. In contrast, nociceptive nerve terminals are distinguished by the capacity to discriminate noxious mechanical stimuli and detect chemical stimuli associated with tissue injury and inflammation. Thus, the nociceptors (exemplified by the bronchopulmonary C-fibres) are activated by noxious mechanical forces such as noxious levels of tissue distention and are in many instances activated and/or positively modulated by numerous inflammatory autacoids such as bradykinin, prostaglandins, adenosine and others [11]. Typically, although not uniformly, the nociceptive nerve terminals express the capsaicin receptor TRPV1 that confers and/or modulates sensitivity to acid and certain inflammatory autacoids, while low threshold mechanosensors uniformly lack TRPV1. The low threshold mechanosensitive and nociceptive phenotypes also segregate by the expression of other phenotypic markers such as neuropeptide neurotransmitters, certain ion channels and likely specific transcriptional factors. The low threshold mechanosensors are presumed to mediate regulatory reflexes while nociceptors are presumed to mediate defensive reflexes and/or sensations associated with nociception (pain, dyspnoea, discomfort etc.).
4. Vagal sensory innervation of the airways and lungs
Vagal low threshold mechanosensors in the lung comprise the classically described lung “stretch receptors” further subdivided into slowly- and rapidly-adapting receptors (SARs and RARs, respectively) based on the accommodation of the action potential discharge in response to sustained lung inflation. In all species studied thus far the vagal low threshold mechanosensitive nerve fibres in the lung conduct action potentials in the A-fibre range (>10 m/s).
The vagal bronchopulmonary C-fibres are the most numerous group of nociceptors in the lungs and airways (reviewed in [11]). The C-fibre nociceptors are only marginally affected by physiological activity in the airways and lungs. In contrast C-fibres respond vigorously to extreme mechanical forces and numerous autacoids including activators of TRPV1 receptors, which is consistent with their nociceptive phenotype. It is well accepted that at least two subtypes of bronchopulmonary C-fibre nociceptors distinguished by the nerve terminal location and activation profile can be found in higher mammals (i.e. “bronchial” and “pulmonary” C-fibres) [12]. Studies in the guinea pig indicate that the subtype-specific properties of the bronchopulmonary C-fibre nociceptors are determined by the embryonic origin in this species (Fig. 1) [13]. Thus, the placodes-derived neurones in the vagal nodose ganglia and the neural crest-derived neurones in the vagal jugular ganglia project distinct C-fibre nociceptors into the lungs. These observations may also be pertinent to the higher mammals with a well-developed neural crest portion of the vagal afferent system (jugular ganglion neurones). As described below, the embryonic origin also determines the subtype specific properties of the vagal C-fibre nociceptors in the oesophagus. Reflexes triggered by the bronchopulmonary C-fibre nociceptors include cough, apnoea, reflex bronchoconstriction, mucus secretion and bradycardia [14].
Fig. 1.
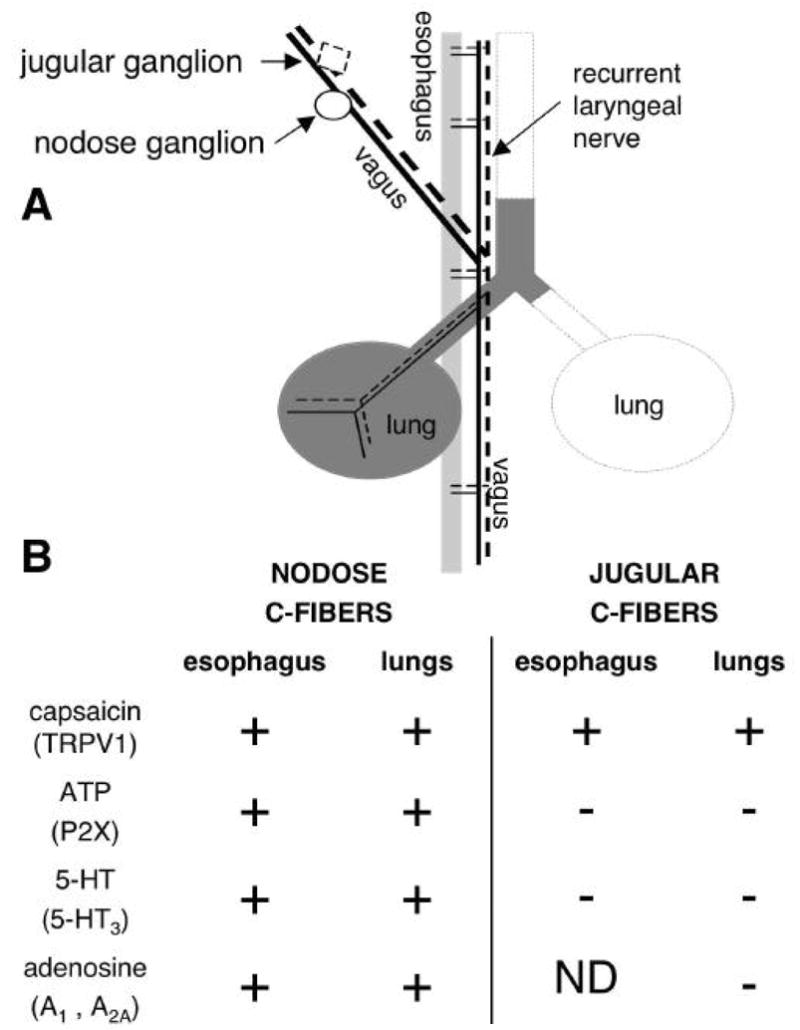
Common pattern of the vagal C-fibre nociceptive innervation of the lungs and oesophagus. (A) Both vagal nodose and jugular sensory ganglia project nociceptive C-fibres into the lungs and oesophagus. RLN, recurrent laryngeal nerve. (B) Responsiveness of the vagal C-fibres to sensory activators. Nodose and jugular C-fibres differ in the activation profile, which reflects their distinct embryonic origin from placodes and neural crest, respectively. Note that the nodose C-fibres in the lungs are similar to the nodose C-fibres in the oesophagus. Likewise, the jugular C-fibres are similar whether they terminate in the lungs or oesophagus. ND, not determined.
The “touch-sensitive” Aδ-fibre described in the large airways of the guinea pig is a unique nociceptive sensory nerve type in that it lacks the most typical nociceptive marker TRPV1 [15]. However, the activation profile of this nerve type is consistent with the nociceptive phenotype. The “touch-sensitive” Aδ-fibers are indifferent to the physiological stimuli in the airways (air flow, changes in tracheal geometry during breathing) but are readily activated by the noxious stimuli associated with aspiration: the localized punctiform mechanical stimuli and acid [16]. Accordingly, activation of the “touch-sensitive” Aδ-fibres triggers cough, an observation which further speaks in support of their nociceptive character [17]. It seems likely that many of the vagal nerve terminals described in the larynx and trachea of larger mammals and termed “irritant receptors” are equivalent to the guinea pig “touch-sensitive” Aδ-fibres [18].
5. Vagal sensory innervation of the oesophagus
Vagal sensory nerves in the oesophagus are often associated only with the regulation of its physiological function and are thought not to directly participate in nociception (i.e. detection of noxious stimuli) [19]. This notion likely relates to the fact that the vagal sensory nerves with nociceptive properties were rarely reported in the oesophagus. Most studies of oesophageal sensory innervation focused on the non-nociceptive low threshold mechanosensors [10,20–22]. However, more recent studies noted that the properties of some oesophageal sensory nerves are consistent with the nociceptive phenotype [23,24]. We have recently contributed to the understanding of oesophageal sensory neurobiology with a detailed description of the vagal nociceptive phenotypes in the oesophagus [25].
Since the early electrophysiological experiments, a descriptive classification has been used to categorize vagal sensory nerves in gastrointestinal system. Based on their apparent most effective mechanical stimuli the “tension receptors” and “mucosal receptors” have been recognized [7]. The “tension receptors” denote the nerve terminals that are most effectively stimulated by oesophageal distention (which increases the tension in the oesophageal wall). In contrast “mucosal receptors” refer to the nerve terminals that are most effectively activated by localized mechanical (“touch”) stimuli delivered to the oesophageal mucosa but are insensitive to oesophageal distention.
In addition to “tension receptors”, “tension mechanoreceptors” or “tension mechanosensors” are used interchangeably to describe the distention-sensitive oesophageal low threshold mechanosensitive phenotype. In this discussion we will refer to this nerve type as the tension mechanosensors. Tension mechanosensors are the best-characterized group of the vagal sensory nerves in the oesophagus and their properties are virtually identical in all species studied. Tension mechanosensors have a low threshold for oesophageal distention and are mechanically activated during the physiological activity of the organ, such as normal peristaltic movements. In the intact oesophagus their activity initially increases with increasing intra-oesophageal pressure, but reaches a plateau (saturates) at a pressure < 60 mm Hg, i.e. within the innocuous pressure range [10,24–27] (Fig. 2). Thus, the tension receptors lack the capacity to discriminate between innocuous and noxious levels of oesophageal distention. The issue of the responsiveness of the oesophageal tension mechanosensors to noxious chemical stimuli is partially clouded because of their exquisite mechanical sensitivity. Pharmacological and chemical stimuli that alter oesophageal muscle activity may also indirectly affect the tension mechanosensors. Indeed, some in vivo studies noted that the typical nociceptive activators such as bradykinin or capsaicin influenced the activity of a proportion of the oesophageal tension mechanosensors [24,28]. However, it has been concluded that these effects are not due to a direct action of the chemical on the nerve terminal [28]. The studies in an isolated ex vivo oesophagus-nerve preparation that allow for better control of the indirect effects found no evidence of direct activation by typical nociceptive activators in guinea pig [25] and mouse (unpublished observation). Nerve terminals of the tension mechanosensors have been morphologically identified and are identical to the classically described intraganglionic laminar endings (IGLE) [22]. The phenotype of the “mucosal receptors” [23] defined by the sensitivity to the low intensity (reported <100 μN) mechanical stimuli applied to the oesophageal mucosa is likely non-nociceptive, but still has to be investigated.
Fig. 2.
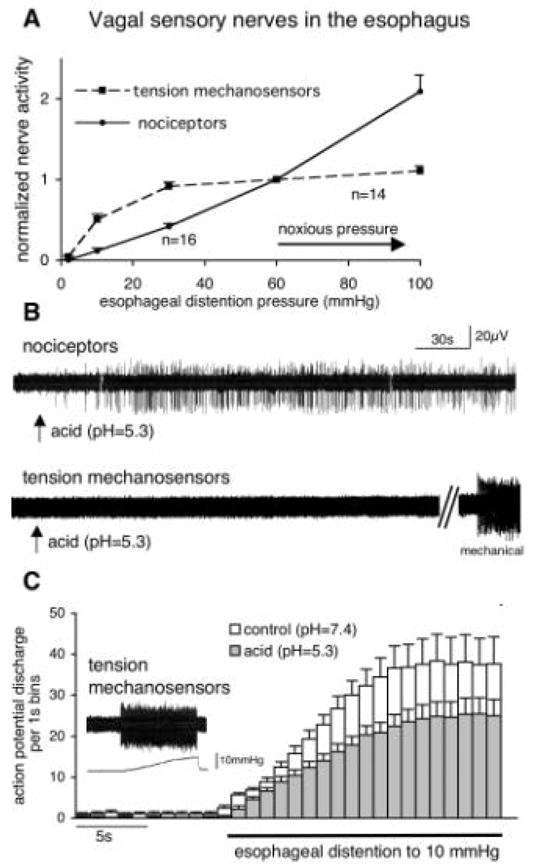
Acid activates nociceptors, but inhibits mechanically-induced activation of tension mechanosensors in the oesophagus. (A) The oesophageal nociceptors have the capacity to discriminate innocuous and noxious distention, while the non-nociceptive tension mechanosensors saturate in the innocuous range. The data are normalized to the nerve activity at the approximate threshold for noxious intra-oesophageal pressure (60 mm Hg). (B) Traces of the vagal single nerve fibre activity recorded extracellularly in the guinea pig innervated oesophagus ex vivo. Acid stimulates C-fibre nociceptors (n=12/13) but not tension mechanosensors* (n=9). (C) Acid inhibits mechanically-induced activation of tension mechanosensors. The time course of the action potential discharge induced by oesophageal distention at control pH and in the presence of acid (n=9). Inset: action potential discharge (top trace) induced by oesophageal distention (bottom trace shows intra-oesophageal pressure).
*Under ex vivo experimental conditions the majority of the oesophageal tension mechanosensors display regular baseline discharge (averaging 4 Hz). When present this discharge was greatly reduced by acid (by ~90%, n=7). A nerve without the baseline activity is presented in (B) for clarity.
We have recently described a large population of the vagal sensory nerves in the guinea pig oesophagus that have a typical nociceptive phenotype [25]. Unlike the oesophageal low threshold mechanosensors these sensory nerves are discriminatively responsive to noxious stimuli. The activity of the vagal oesophageal nociceptors increases linearly with the increasing intra-oesophageal pressure without saturation up to noxious pressures (in some experiments tested up to the integrity limits of the oesophagus) (Fig. 2). Thus, these nerves possess the capacity to discriminate between innocuous and noxious oesophageal distention. Furthermore, this capacity combines with the responsiveness to noxious chemical stimuli. Vagal oesophageal nociceptors express the capsaicin receptor TRPV1 that confers and modulates responsiveness to noxious autacoids such as acid and certain lipid mediators. Other examples of the autacoids associated with the oesophageal tissue damage that stimulate oesophageal nociceptors include bradykinin and adenosine (personal observation). In the guinea pig the electrophysiological properties further segregate oesophageal low threshold mechanosensitive and nociceptive phenotypes. The tension mechanosensors nerve fibres conduct uniformly in the A-fibre range, while the majority of the oesophageal nociceptors are slowly conducting C-fibres.
Because of their responsiveness to noxious stimuli and vagal origin, vagal oesophageal nociceptors are the primary candidates for the pathways that mediate the effects of stimuli in the oesophagus on cough. Consistent with this notion, infusion of the nociceptive activator capsaicin into the oesophagus potentiates cough induced from the airways in the guinea pig (Canning BJ, personal communication).
6. Common pattern of the vagal innervation of the lungs and oesophagus in the guinea pig
The vagal sensory nerves are derived from two embryonic sources (reviewed in [29]). Sensory fibres that have neurones in the vagal nodose ganglia originate from the epibranchial placodes while sensory fibres that have neurones in the vagal jugular ganglia are derived from the neural crest. During their development and maturity the nodose and jugular neurones also differ in their neurotrophin dependency. We have recently evaluated the hypothesis that the nodose and jugular neurones project distinct types of sensory nerves into their target organs [13,25,30,31].
We demonstrated that both the nodose and jugular ganglia project nociceptive nerves into the lungs and oesophagus in the guinea pig (Fig. 1). However, we found that the nodose and jugular nociceptors are distinct, most notably in their activation profile and the neuropeptide content. While both the nodose and jugular nociceptors respond to capsaicin and bradykinin, the activation profile of the nodose nociceptors is wider and includes 5-HT (via 5-HT3 receptors), adenosine (via A1 and A2A receptors) and ATP (and other agonists of the purinergic P2X receptors) (Fig. 1). The majority of jugular C-fibre nociceptors contain substance P, but this neuropeptide is rare in nodose C-fibre nociceptors. The nodose C-fibre nociceptors found in lungs are similar to the nodose C-fibre nociceptors in the oesophagus. Likewise, the jugular C-fibre nociceptors are similar whether they terminate in the respiratory tract or oesophagus. Therefore, the ganglionic (embryonic) origin of a vagal nociceptive C-fibre seems to dictate its properties irrespective of the target organ. This observation further stresses the importance of including embryonic origin into classification of vagal nociceptive subtypes.
Comparative data on the nociceptive vagal innervation of the lungs and oesophagus are not currently available in other species. It was shown that both vagal ganglia contribute to the innervation of the lungs and oesophagus in the rat, however the phenotypes of their nerve terminals have not been characterized in this species [32].
We also noted that the low threshold mechanosensors in both the lungs (SARs and RARs) and oesophagus (“tension mechanosensors”) originate exclusively from the nodose ganglia [17,25]. The nodose origin of the vagal mechanosensors was also demonstrated in other species [20]. The nerve fibres of mechanosensors in both lungs and oesophagus conduct impulses in the A-fibre range. Interestingly, low threshold mechanosensors in the lungs and oesophagus also share responsiveness to P2X receptor agonists [17,25]. Although their role has not yet been clarified, available evidence suggests that the P2X receptors may contribute to mechanotransduction (reviewed in [33]).
7. Acid in the tissue
Detailed discussion of acid production and turnover in different tissues under physiological and pathological circumstances is beyond the scope of this review. In general, the acid-producing pathways that can potentially lead to increased local concentrations of protons have been described. However, the degree of the acidification (pH) at the nerve terminals has not been measured. This is not only due to technical difficulties associated with such studies but also due to our incomplete knowledge as to where the effective acid transducers are located on the nerve terminals. Furthermore, in numerous instances (such as vagal nociceptors in the oesophagus) the exact locations of the nerve terminals and their relationships to potential acid sources are unknown.
Exhaled breath condensate (EBC) studies indicate perturbed turnover of acid in the airways and/or lungs of patients with various respiratory disorders associated with cough. Although methodological issues may still preclude definitive interpretation of these results [34,35], we note that EBC indicating acidification of the airways and lungs was reported in patients with asthma [36], COPD [37] as well as in patients with unexplained chronic cough [38].
An obvious source of acid is acidic gastro-oesophageal reflux (GOR). A certain amount of acid reflux is a physiological phenomenon. However, GOR becomes pathological when linked to pathological consequences including oesophagitis, heartburn, pain and cough. Not all acid in the oesophagus is able to modulate the sensory nerves. The functional integrity of the oesophageal mucosa is an important factor influencing the accessibility of sensory nerves to acid [39]. Thus the acid-triggered sensory input from the oesophagus is likely determined by the amount of acid, the mucosal barrier function and sensitivity of oesophageal sensory nerves to acid. This may contribute to a weak correlation between the pH and cough observed in some patients and emphasizes that under certain circumstances a normal GOR can be causal for chronic cough. In addition, in the oesophageal disorders not associated with pathological acidic GOR (e.g. eosinophilic oesophagitis), other sources of acid, such as inflamed tissue and oesophageal muscle, may be important.
Acid refluxed into the oesophagus may enter large airways by aspiration or microaspiration. Microaspiration is difficult to prove with current pH-monitoring techniques. However, given the high acidity of the refluxed content (pH< 4) and exquisite acid-sensitivity of some cough-triggering sensory nerves in the airways (threshold pH>6.5), acid entering the airways cannot be discarded as a mechanism for cough due to GOR.
Finally, acid has been extensively used to study the cough reflex in both human studies and animal models. Inhalation of acid evokes cough in all coughing species including humans [40], guinea pig [17,41], dog [42] rabbit [43,44]. The interpretation of the information gained in these studies depends on the understanding of the acid-sensitive properties of the sensory nerve subtypes.
8. Acid-sensing mechanisms in sensory nerves
Only a few ion channels expressed in the primary sensory nerves are known to be directly gated by acid and lead to sensory activation. These include the capsaicin receptor TRPV1, certain other members of the Transient Receptor Potential (TRP) superfamily (i.e. TRPV4), and Acid Sensing Ion Channels (ASICs). The examples of the ion channels that are not directly gated, but are modulated by acid in a manner that can increase responsiveness of the sensory nerves include certain purinergic P2X receptors and some two-pore domain potassium channels (TASK) (reviewed in [45]).
TRPV1 is a polymodal non-selective cation channel activated and modulated by diverse stimuli, such as vanilloid compounds (capsaicin, olvanil), heat (>43°C), acid, and numerous endogenous compounds including various endocannabinoids and eicosanoids TRPV1 is an extensively studied receptor described in great detail elsewhere [46,47]. In response to acid TRPV1 mediates sustained non-inactivating currents with a pH threshold about pH=6. Importantly, TRPV1-mediated acid induced-currents are potentiated by other TRPV1 agonists and enhanced by the positive modulation of TRPV1 via intracellular pathways linked to the inflammatory autacoids.
Acid Sensing Ion Channels (ASICs) are members of the DEG/ENaC family of sodium channels that are the cation channels gated by protons. ASICs are widely expressed in the nervous system and are implicated in multiple functions ranging from the peripheral sensory transduction to memory and learning. A comprehensive review of recent progress in ASIC biology can be found elsewhere [48]. Here we focus on the ASIC properties pertinent to the peripheral sensory transduction of acidic stimuli.
Three known ASIC genes produce five subunits, ASIC1a, ASIC1b, ASIC2a, ASIC2b and ASIC3, which assemble into homo- and hetero-multimeric channels gated by acid. The stoichiometry of ASIC channels in not known. Multiple subunits are expressed in the individual peripheral sensory neurones, and some patterns of ASIC subunit expression appear to be associated with certain peripheral sensory phenotypes [49]. Importantly, the subunit composition of an ASIC channel determinates its acid transduction properties, in particular the pH threshold and the time course of acid-induced activation [50]. The pH threshold of ASICs covers a wide range of pH (pH~7 to 4). Most ASIC channels mediate large transient currents in response to rapid acidification, and certain ASICs can support significant sustained currents in the continuous presence of acid [51]. At the level of a nerve terminal the transient and sustained currents would be predicted to translate into rapid transient (seconds) bursts of action potentials in response to rapid acidification and sustained action potential discharge in the continuous presence of acid, respectively. Thus, depending on the subunit composition, the ASICs may confer the capacity to respond at a particular threshold to a particular change in pH. The overall acid responsiveness of a nerve terminal would be, of course, influenced by other channels gated and/or modulated by acid. For example, multiple studies showed co-expression of ASICs and TRPV1 in the same single neurones. The pharmacology of ASIC receptors is undergoing rapid development. Currently available antagonists include a group of ASIC inhibitors based on amiloride and its derivates. Few ASIC subunit-selective antagonists have been described, although certain toxins have been isolated that show selectivity for inhibiting ASIC3 and ASIC1a [52,53]. Finally, a new low molecular weight non-amiloride compound A-317567 has been reported to inhibit ASICs [54].
9. Effects of acid on the vagal sensory nerves
The pH deviation from the normal values indicates impeding or actual tissue damage and acid itself contributes to the tissue damage. Acid is therefore a prototypical noxious stimulus predicted to activate nociceptive nerves. Indeed, acid activates nociceptive nerves in the somatosensory system such as polymodal nociceptors in the skin [55]. Acid also evokes action potential discharge in the vagal nociceptive sensory nerves in the airways, lungs and oesophagus. In contrast to its stimulatory effect on nociceptors, our recent data indicate that acid directly inhibits oesophageal non-nociceptive low threshold mechanosensors. The direct effects of the acid on the low threshold mechanosensors in the lungs (RARs and SARs) have not been to our knowledge reported.
10. Bronchopulmonary C-fibre nociceptors
Bronchopulmonary C-fibre nociceptors are effectively stimulated by acid in all species studied thus far: guinea pig [16,56], rat [57] and mouse [58] (Fig. 3). The C-fibre nociceptors respond to the sustained acidic stimulus with a sustained action potential discharge. In the guinea pig the pH threshold for acid-induced activation is about pH=6.0 [16,56].
Fig. 3.
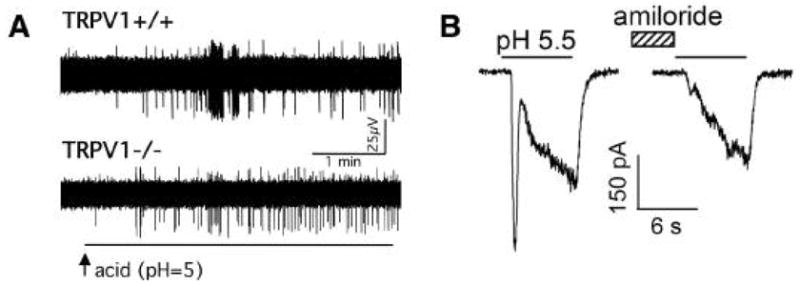
Acid-induced activation of the vagal bronchopulmonary C-fibres is mediated by the TRPV1 and TRPV1-independent receptors, likely the Acid Sensing Ion Channels. (A) Extracellular recordings from the bronchopulmonary C-fibres in the mouse lungs. The response to acid is attenuated (on average by ~50%) but not abolished in the TRPV1 knockout mouse. [From 58] (B) The whole-cell patch clamp recordings from the vagal sensory neurones in the rat. The lung-specific vagal neurones display transient amiloride-sensitive currents that are typical for the ASIC channels. [From 59]
Using the only TRPV1 selective receptor antagonist available at that time, capsazepine, initial studies concluded that the TRPV1 accounts for nearly all acid-induced action potential discharge in nociceptive C-fibres [56]. However, subsequent experiments with the more selective TRPV1 antagonist iodo-resiniferatoxin and studies in the TRPV1 knockout mouse showed that up to 50% of the sustained acid-induced activation is TRPV1-independent [16,58,59] (Fig. 3). In addition, experiments in which the pH was reduced rapidly at the receptive field of the tracheal C-fibres, revealed a TRPV1-independent transient response reminiscent of the ASIC kinetics [16].
The question whether ASICs are expressed in bronchopulmonary sensory neurones was recently more directly addressed in studies by Gu and Lee [60] in rats and Lee et al. [61] in guinea pigs. These studies demonstrated ASIC-like currents in the isolated vagal sensory neurones retrogradely labeled from the lungs (Fig. 3B). This conclusion is based on the kinetics and desensitization studies, selectivity for sodium and pharmacological inhibition by amiloride. Importantly, Gu and Lee with rats [60] reported that ~45% of the bronchopulmonary neurones have acid(pH=5.5)-induced currents consistent with both TRPV1 and ASICs. These capsaicin-sensitive neurones are likely the cell bodies of the C-fibre nociceptors. Thus the data obtained in the isolated lung-specific neurones are in agreement with the studies in the C-fibre nerve terminals in the trachea. In conclusion acid stimulates bronchopulmonary C-fibre nociceptors via TRPV1-dependent and TRPV1-independent mechanisms. Current evidence indicates that the TRPV1-independent acid-sensitivity is mediated by ASICs. However, whether ASICs are the sole mediators of the TRPV1-independent activation and what ASIC subunits are involved remain to be established.
11. Nociceptive “touch-sensitive” Aδ-fibres innervating large airways in the guinea pig
As discussed above, this special nociceptive sensory phenotype, which was extensively studied in the guinea pig, initiates immediate cough even in anaesthetized animals [17]. This nociceptive type is unique among the bronchopulmonary nociceptors in that it does not express the capsaicin receptor TRPV1. The “touch-sensitive” Aδ-fibres uniformly respond to the rapid acidification (a rapid drop in pH) at the tracheal surface with a robust action potential discharge [16]. Their threshold for the acid-induced activation is relatively low (pH50=6.5). However, contrary to the sustained activity observed in the C-fibre nociceptors in the continuous presence of acid, the action potential discharge in the “touch-sensitive” Aδ-fibres rapidly inactivates (within <3 s). Furthermore, in experimental designs in which the rate of the pH reduction is deliberately slow (tested <1 unit/min), the acid fails to activate these sensory nerve terminals. These unique activation kinetics (low threshold, rapid inactivation and absence of activation at a slow pH reduction rate) strongly resembles the whole cell acid-induced currents in the cells expressing certain ASIC subunits, in particular ASIC3. The hypothesis that ASICs mediate the acid-responsiveness of the “touch-sensitive” Aδ-fibres needs to be evaluated in further experiments. The acid-sensitive properties and the exquisite mechanical sensitivity of these fibres are consistent with their putative function as the cough triggers responsive to aspiration that is a mechanical and often acidic stimulus.
12. Oesophageal nociceptors
Oesophageal nociceptors nearly uniformly express the acid-sensitive TRPV1 receptors [23]. We found that nociceptors in the oesophagus are stimulated by acid. Fig. 2B shows the action potential discharge induced by acid (pH=5) in a nodose C-fibre nociceptor with the nerve terminal in the oesophagus. Whether TRPV1 is the sole receptor involved in this response is currently unknown.
13. Vagal low threshold mechanosensors in the oesophagus
The reports on the acid responsiveness of the vagal low threshold mechanosensors in the oesophagus are controversial. While some studies suggest that acid activates this nerve type in vivo in cats [26], others report little excitatory effect in ferrets in vivo [24] and in vitro in ferrets and mouse [23,62]. We have recently addressed this issue in an ex vivo isolated innervated guinea pig oesophagus, a reduced system that allows for better control of indirect effects such as those due to oesophageal muscle activity. Unexpectedly, we found that acid did not activate these nerves, but inhibited mechanical activation of the oesophageal low threshold mechanosensors induced by oesophageal distention (Fig. 2C). The inhibitory effect was observable at the modestly acidic pH=6.5 and fully reversible when the normal pH=7.4 was restored. The inhibitory effects of acid were not limited to the mechanical stimuli. We found that acid (pH=6.5) also effectively inhibited the action potential discharge induced by the P2X receptors agonist α,β-methylene-ATP (by 67±13%, p<0.05, n=7). The inhibition of oesophageal mechanosensors by acid is not unique to the guinea pig since all the above observations could be reproduced in mouse (unpublished observations).
The mechanisms by which acid inhibits the low threshold mechanosensors in the oesophagus are unknown. Although it cannot be excluded that acid inhibits both the putative mechanotransducer channels and purinergic receptors, it is more likely that acid influences low threshold mechanosensors in a more general way by influencing their neuronal excitability. The particularly likely candidate targets for acid are the voltage gated sodium and/or calcium ion channels. These channels are critical for the regulation of sensory excitability and many of them are inhibited by acid. Finally, it appears that acid inhibits low threshold mechanosensors by modulating molecules that are selectively expressed by this phenotype, since the mechanically-induced activation of the oesophageal nociceptors phenotype was not inhibited by acid (n=8).
Whether the inhibition of oesophageal mechanosensors by acid occurs in oesophageal diseases and what are its functional consequences has yet to be investigated. Vagal low threshold mechanosensors mediate the finely tuned reflex regulation of oesophageal motor functions. One might hypothesize that their inhibition could result in the conditions favorable to GOR by, for example, reducing the efficacy of oesophageal clearance or interfering with the proper function of the oesophageal sphincters.
14. Spinal sensory innervation of the lungs and oesophagus
Although not the major focus of this review, the spinal sensory nerves originating in the neurones of spinal dorsal root ganglia (DRG) and projecting fibres to the lungs and oesophagus are worth discussing in the context of sensory responsiveness to acid and cough. Little is known of the roles of spinal sensory pathways as modulators of cough; however, there are well-established links between spinal sensory pathways and sensations. In this capacity, it is possible that the spinal pathways modulate certain conscious aspects of cough. In particular, spinal pathways from the oesophagus may play a role in the modulation of cough as they are important in the generation of the conscious sensation from the oesophagus, such as heartburn and pain. It does not seem unlikely that heartburn and/or oesophageal pain in patients with GOR may influence the cough, an interaction possibly at higher centres within the CNS.
It is appreciated that the lungs are densely innervated by the DRG sensory nerves [63–66]. Unfortunately the role of the spinal pathways in the modulation of cough has not been yet clarified. This is likely, at least in part, attributable to the relatively limited information on the basic neurobiology of the spinal visceral sensory nerves when compared with vagal sensory pathways. Anatomical studies in various species indicate that at least a proportion of spinal sensory nerves projecting to the airways and lungs have nociceptive properties as implicated from expression of typical and putative nociceptive markers (TRPV1, neuropeptides substance P, neurokinin A and others) [64–67]. Directly applicable to the possible role of these pathways in acid-sensing is the recent report by Groth and coworkers [68], which detected expression of receptors potentially conferring acid-sensitivity to sensory terminals (TRPV1 and ASIC3) in nearly 80% of lung-specific DRG neurones. Electrophysiological recordings in the dogs were made from the spinal sensory nerves that are activated by the physiological level of the lung inflation, thus reminiscent of the low threshold mechanosensitive phenotype. However, further studies are needed to characterize the phenotypes of spinal nerves projecting to the lung and airways.
Spinal pathways are believed to be critical in the perception of the sensations from the oesophagus (heartburn, pain). Given the prevalence of acid-related diseases, it is surprising how little information is available on the spinal sensory nerves in the oesophagus and that no study has addressed the mechanisms of their responsiveness to acid. Electrophysiological studies indicate that the majority of the spinal sensory nerves are nociceptive [28]. The expression of the nociceptive markers in the majority of the DRG neurones supplying the oesophagus supports this notion. The analysis of the acid sensitivity was carried out in the neighbouring stomach [69]. A proportion of the DRG neurones traced from the stomach express ASIC currents and the most important ASIC subunits appear to be ASIC1a and ASIC2. It is however not clear to what extent can be these findings extrapolated to the oesophageal sensory nerves.
15. Conclusions
Multiple pathways regulating cough are modulated by acid. Inhaled or aspirated acid, and acid generated in the respiratory system can stimulate bronchopulmonary nociceptors to trigger and/or sensitize cough. The ion channels involved in this stimulation are TRPV1 and most likely the ASICs, but the latter hypothesis still awaits more definitive studies. Acid refluxing from the stomach into the oesophagus can stimulate vagal nociceptive sensory nerves in the oesophagus. This can lead to cough by enhancing the cough reflex via sensitizing mechanisms in the brainstem, or by directly triggering cough. Whether TRPV1 is the sole receptor mediating the response of the oesophageal nociceptors to acid is not known. Development of new drugs targeting TRPV1, ASICs and perhaps other acid-sensitive receptors will likely provide more insight into the role of acid in the pathogenesis of cough and may provide new therapeutic options. Finally, acid can inhibit low threshold mechanosensors regulating the physiological functions of the oesophagus. That this mechanism may result in conditions favorable for reflux and operate in patients with chronic cough is at this point only a speculation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canning BJ. Transduction and encoding of the cough reflex. Pulm Pharmacol Therap. 2007 doi: 10.1016/j.pupt.2006.12.003. in press. This Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:80–94S. doi: 10.1378/chest.129.1_suppl.80S. [DOI] [PubMed] [Google Scholar]
- 3.Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149:160–7. doi: 10.1164/ajrccm.149.1.8111576. [DOI] [PubMed] [Google Scholar]
- 4.Canning BJ, Mazzone SB. Reflex mechanisms in gastroesophageal reflux disease and asthma. Am J Med. 2003;115:45–8S. doi: 10.1016/s0002-9343(03)00192-x. [DOI] [PubMed] [Google Scholar]
- 5.Javorkova N, Pecova R, Varechova S, Demeter M, Hyrdel R, Balaz D, Tatar M, Kollarik M. Acidification of distal esophagus increases cough reflex sensitivity in patients with gastroesophageal reflux disease (GERD) and chronic cough. Gastroenterology. 2006;130(Suppl 2):A105. [Google Scholar]
- 6.Widdicombe J. Airway receptors. Respir Physiol. 2001;125:3–15. doi: 10.1016/s0034-5687(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16 (Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 8.Undem BJ, Kollarik M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:355–60. doi: 10.1513/pats.200504-033SR. discussion 371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol. 2001;125:17–31. doi: 10.1016/s0034-5687(00)00202-4. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–10. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 11.Lee L-Y, Shuei LY, Gu Q, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- 12.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 13.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–17. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Ann Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 15.Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496:521–30. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–58. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223–42. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferents. Gut. 2002;51 (Suppl 1):2–5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falempin M, Mei N, Rousseau JP. Vagal mechanoreceptors of the inferior thoracic oesophagus, the lower oesophageal sphincter and the stomach in the sheep. Pflugers Arch. 1978;373:25–30. doi: 10.1007/BF00581145. [DOI] [PubMed] [Google Scholar]
- 21.Clerc N, Mei N. Vagal mechanoreceptors located in the lower oesophageal sphincter of the cat. J Physiol. 1983;336:487–98. doi: 10.1113/jphysiol.1983.sp014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–55. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512:907–16. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackshaw LA, Page AJ, Partosoedarso ER. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience. 2000;96:407–16. doi: 10.1016/s0306-4522(99)00547-3. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563:831–42. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medda BK, Sengupta JN, Lang IM, Shaker R. Response properties of the brainstem neurons of the cat following intra-esophageal acid-pepsin infusion. Neuroscience. 2005;135:1285–94. doi: 10.1016/j.neuroscience.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Satchell PM. Canine oesophageal mechanoreceptors. J Physiol. 1984;346:287–300. doi: 10.1113/jphysiol.1984.sp015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta JN, Saha JK, Goyal RK. Differential sensitivity to bradykinin of esophageal distension-sensitive mechanoreceptors in vagal and sympathetic afferents of the opossum. J Neurophysiol. 1992;68:1053–67. doi: 10.1152/jn.1992.68.4.1053. [DOI] [PubMed] [Google Scholar]
- 29.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- 30.Chuaychoo B, Lee MG, Kollarik M, Pullmann R, Jr, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol. 2006;575:481–90. doi: 10.1113/jphysiol.2006.109371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuaychoo B, Lee MG, Kollarik M, Undem BJ. Effect of 5-hydroxytryptamine on vagal C-fibre subtypes in guinea pig lungs. Pulm Pharmacol Ther. 2005;18:269–76. doi: 10.1016/j.pupt.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Wank M, Neuhuber WL. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol. 2001;435:41–59. doi: 10.1002/cne.1192. [DOI] [PubMed] [Google Scholar]
- 33.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 34.Effros RM, Casaburi R, Su J, Dunning M, Torday J, Biller J, Shaker R. The effects of volatile salivary acids and bases on exhaled breath condensate pH. Am J Respir Crit Care Med. 2006;173:386–92. doi: 10.1164/rccm.200507-1059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effros RM, Dunning MB, 3rd, Biller J, Shaker R. The promise and perils of exhaled breath condensates. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1073–80. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 36.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 37.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165:1364–70. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 38.Niimi A, Nguyen LT, Usmani O, Mann B, Chung KF. Reduced pH and chloride levels in exhaled breath condensate of patients with chronic cough. Thorax. 2004;59:608–12. doi: 10.1136/thx.2003.012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlow WJ, Orlando RC. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology. 2005;128:771–8. doi: 10.1053/j.gastro.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Morice AH, Kastelik JA, Thompson R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol. 2001;52:365–75. doi: 10.1046/j.0306-5251.2001.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol. 1988;1:33–9. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 42.Tatar M, Sant’Ambrogio G, Sant’Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol. 1994;76:2672–9. doi: 10.1152/jappl.1994.76.6.2672. [DOI] [PubMed] [Google Scholar]
- 43.Tatar M, Karcolova D, Pecova R, Brozmanova M. The role of partial laryngeal denervation on the cough reflex in awake guinea-pigs, rats and rabbits. Pulm Pharmacol. 1996;9:371–2. doi: 10.1006/pulp.1996.0051. [DOI] [PubMed] [Google Scholar]
- 44.Adcock JJ, Douglas GJ, Garabette M, Gascoigne M, Beatch G, Walker M, Page CP. RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol. 2003;138:407–16. doi: 10.1038/sj.bjp.0705056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holzer P. Acid-sensitive ion channels in gastrointestinal function. Curr Opin Pharmacol. 2003;3:618–25. doi: 10.1016/j.coph.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 47.Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–20. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. doi: 10.1016/j.tins.2006.06.014 2006. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA. 2002;99:2326–31. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:1106–15. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 51.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–9. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 52.Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. Embo J. 2004;23:1516–25. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci. 2003;12:1332–43. doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 55.Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995;67:741–52. doi: 10.1016/0306-4522(95)00115-y. [DOI] [PubMed] [Google Scholar]
- 57.Hong JL, Kwong K, Lee L-Y. Stimulation of pulmonary C fibres by lactic acid in rats: contributions of H+ and lactate ions. J Physiol. 1997;500:319–29. doi: 10.1113/jphysiol.1997.sp022023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. J Physiol. 2004;555:115–23. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Undem BJ, Kollarik M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J Pharmacol Exp Ther. 2002;303:716–22. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- 60.Gu Q, Lee L-Y. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2006;291:L58–65. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MG, Undem BJ, Brown C, Carr MJ. Effect of nociceptin in acid-evoked cough and airway sensory nerve activation in guinea pigs. Am J Respir Crit Care Med. 2005 doi: 10.1164/rccm.200507-1043OC. [DOI] [PubMed] [Google Scholar]
- 62.Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- 63.Dalsgaard CJ, Lundberg JM. Evidence for a spinal afferent innervation of the guinea pig lower respiratory tract as studied by the horseradish peroxidase technique. Neurosci Lett. 1984;45:117–22. doi: 10.1016/0304-3940(84)90085-5. [DOI] [PubMed] [Google Scholar]
- 64.Springall DR, Cadieux A, Oliveira H, Su H, Royston D, Polak JM. Retrograde tracing shows that CGRP-immunoreactive nerves of rat trachea and lung originate from vagal and dorsal root ganglia. J Auton Nerv Syst. 1987;20:155–66. doi: 10.1016/0165-1838(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 65.Dinh QT, Groneberg DA, Peiser C, Mingomataj E, Joachim RA, Witt C, Arck PC, Klapp BF, Fischer A. Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiol Neurobiol. 2004;144:15–24. doi: 10.1016/j.resp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–37. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- 67.Oh EJ, Mazzone SB, Canning BJ, Weinreich D. Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol. 2006;573:549–64. doi: 10.1113/jphysiol.2005.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groth M, Helbig T, Grau V, Kummer W, Haberberger RV. Spinal afferent neurons projecting to the rat lung and pleura express acid sensitive channels. Respir Res. 2006;7:96. doi: 10.1186/1465-9921-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–27. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


