Abstract
Mechanisms underlying axon degeneration in peripheral neuropathies and during normal remodeling are poorly understood. Because estrogen induces widespread sympathetic axon degeneration within female reproductive tract smooth muscle, we surveyed estrogen-regulated genes in rat myometrium. Microarray analysis revealed that the neural cell adhesion protein neurotrimin (Ntm) was markedly upregulated at 6h and down-regulated by 24h after injection of 17β-estradiol and real time RT-PCR confirmed this pattern of expression. Protein analysis by western blotting showed that uterine Ntm protein is also upregulated in vivo at 6−24h following estrogen injection, and that Ntm protein is increased selectively in the myometrium during the high-estrogen phase of the estrous cycle. Cultured myometrial smooth muscle cells display peri-nuclear accumulations of Ntm protein, and 17β-Estradiol also increases intracellular levels of Ntm and its secretion into the culture medium. To determine if neurotrimin is required for estrogen-induced sympathetic pruning, sympathetic neurons were co-cultured with uterine smooth muscle cells transfected with siRNA directed against Ntm. While estrogen inhibited neurite outgrowth in non-transfected co-cultures, estrogen's ability to reduce sympathetic outgrowth was impaired substantially following Ntm downregulation. This supports a role for neurotrimin in mediating estrogen-induced sympathetic pruning in some peripheral targets. Together with earlier studies, these findings support the idea that physiological sympathetic axon degeneration is a multifactorial process requiring dynamic regulation of multiple repellant proteins.
Keywords: neurodegeneration, estrogen, sympathetic, IgLON
Introduction
Peripheral neuropathies are common disorders characterized by abnormal degenerative loss of motor and sensory axons. They arise from multiple causes including diabetes, environmental toxin exposure, infections, alcohol abuse, and as a complication of medications such as chemotherapeutic agents. Sensory and somatic motor axon neuropathies are associated with motor weakness, sensory loss, and pain (Wokke and van Dijk 1997), whereas autonomic neuropathies lead to disturbances such as orthostatic hypotension, anhydrosis, incontinence and impotence (Freeman 2005).
While neuropathic degeneration is a significant medical problem, discrete and limited axon degeneration occurs normally. ‘Physiological’ axon degeneration is essential during development for eliminating inappropriate projections (O'Leary and Koester 1993) and in the adult as a component of autonomic axon remodeling in some targets such as the reproductive tract (Marshall 1981; Zoubina et al. 1998; Ting et al. 2004). Both neuropathic and physiologic degeneration appear to have features in common and may arise through similar mechanisms (Raff et al. 2002).
The cellular and molecular mechanisms underlying axon degeneration are poorly understood, in part because there are relatively few tractable model systems. In studies following nerve transection, Wallerian degeneration is characterized by distal axon compartmentalization and degradation, which is a caspase-independent programmed process (Finn et al. 2000). The discovery of the Wlds spontaneously mutant mouse with greatly retarded Wallerian degeneration was important for illustrating mechanistic similarities between dying- back and Wallerian degeneration (Lunn et al. 1989; Gillingwater and Ribchester 2001; Coleman 2005). Degeneration also can be elicited in compartmented culture system where withdrawal of neurotrophic support from distal neurites induces ‘dying back’ of the sympathetic neurite while sparing the soma (Campenot 1994), similar to that seen in distal neuropathies.
Studies of innervation remodeling in the rodent female reproductive tract provide new insight into mechanisms underlying axon pruning. During pregnancy, uterine sympathetic innervation undergoes widespread degeneration, followed by reinnervation after parturition (Sjoberg 1967; Sporrong et al. 1978). Similar remodeling occurs during the rodent estrous cycle, where a surge in serum estrogen at proestrus elicits widespread degeneration of myometrial sympathetic fibers, which regenerate rapidly during the low estrogen stages (Zoubina et al. 1998; Zoubina and Smith 2000). Similar degenerative changes can be induced in ovariectomized (OVX) rats by a single injection of 17β-estadiol (Zoubina et al. 2001). Explant culture studies show that estrogen acts directly on the myometrium to render it inhospitable to sympathetic axons, thus preventing the normally robust outgrowth induced by this target (Krizsan-Agbas and Smith 2002). Because estrogen-induced uterine sympathetic axon degeneration is selective, synchronous, rapid, and reproducible, this system offers significant advantages over other existing models for studying mechanisms underlying axon degeneration.
Our previous culture studies revealed that brain derived neurotrophic factor (BDNF) is important in eliciting uterine sympathetic denervation (Krizsan-Agbas et al. 2003). However, BNDF neutralization fails to fully reverse estrogen's effects, suggesting that additional target-derived factors also participate.
In the present study we sought to identify novel target-derived, estrogen-regulated factors implicated in peripheral sympathetic pruning. Here we introduce evidence that neurotrimin (Ntm), a neural cell adhesion molecule, is present in uterus and is regulated by estrogen. Further, reductions in Ntm synthesis abrogate estrogen's ability to convert myometrium from hospitable to repulsive for sympathetic neurites. These data support the idea that Ntm is a participant in a multifactorial process that dynamically governs the integrity of peripheral sympathetic target innervation.
Materials and methods
Sample preparation
Fifty six adult virgin female Spague-Dawley rats (Harlan) were used in the studies. Cycling female rats were staged according to established criteria (Long and Evans 1922) or ovariectomized under anesthesia (27.5 mg/kg ketamine, 25 mg/kg xylazine, and 0.24 mg/kg atropine sulfate, ip injection). Ovaries were removed via bilateral dorsal incisions (Zoubina et al. 2001). After a week of recovery animals were injected subcutaneously with 10 μg/kg β-estradiol benzoate (estrogen) or sesame oil vehicle. Animals were sacrificed 6 or 24 hrs later and the distal halves of uterine horns harvested. All experimental protocols conformed to NIH guidelines and were approved by the University of Kansas Medical Center Animal Care and Use Committee.
The myometrium was stripped from the endometrium and stroma and placed into RNAlater (Ambion) for total RNA isolation or in ice cold RIPA buffer (Upstate) supplemented with protease and phosphatase inhibitors for protein extraction. Samples for protein were homogenized (Polytron) and centrifuged at 13,000×g for 30 min at 4°C. The protein content of the supernatant was estimated by the DC method (BioRad).
Microarray analysis
Total RNA was extracted from myometria of ovariectomized and estrogen-treated rats using Trizol (Invitrogen). After total RNA quantitation, ds cDNA was reverse transcribed, and biotinylated cRNA was prepared by in vitro transcription according to Affymetrix protocols. cRNA was hybridized to 4 Affymetrix GeneChip arrays (RG_U34A) containing 8,799 probes for the rat genome. The data were processed by MAS5 and probesets that were up-or down-regulated by 2-fold or greater were selected for analysis.
Quantitative and semi-quantitative RT-PCR
An RNA sense probe was generated using PCR and in vitro transcription as follows. Primers to Ntm (NM_017354) were designed with T7 and T3 promoters (sense: AAT TAA CCC TCA CTA AAG GGA GAG AGA TTG TCG ATT GAG GAA CCG TAG ACC, antisense: TAA TAC GAC TCA CTA TAG GGA GAG AGA TTG TGA CCC AGC GAT GAA CTA GC) to synthesize a 400bp PCR product. The product was verified with gel electrophoresis, and sense RNA was prepared by in vitro transcription using T3 phage RNA polymerase (Maxiscipt, Ambion) and gel purified. Sense probe and total RNA samples were quantified using an Agilent 2100 Bioanalyzer (Quantum Analytics). Twenty ng of sense probe and 200 ng of tRNA from samples were reverse transcribed using QuantiTect RT kit (Qiagen). A standard curve was prepared from the sense probe and total RNA equivalent to 50ng cDNA was tested from each sample with a QuantiTect SYBR Green PCR kit (Qiagen) using an iCycler iQ real time cycler (Biorad). Melt curve analysis confirmed the specificity of primers, and PCR efficiencies were between 91.6−96.7%.
For the evaluation of siRNA treatment RT-PCR was employed. Primer pairs were: Ntm, (sense: CTCGTGGTCGTGTCTCTC, antisense: ATGTCTCCAGGTTACTGTAGG); MAPK1, (sense: GAGAACATCATCGGCATCAATG, antisense: TCAGCAGGAGGTTGGAAGG); and actin, (sense: TGGGTATGGAATCCTGTGG, antisense: CATCCTGTCAGCAATGCC), which yielded 474bp, 232bp and 140bp products, respectively. PCR was run at 30 cycles for Ntm and MAPK1 and 20 cycles for actin (linear range), and products were evaluated on 2% agarose gel stained with Sybersafe (Invitrogen).
Western blots
SDS-PAGE electrophoresis was carried out on 12% Tris/HCl ready gels (Biorad) with a loading of 20 μg of total protein, and the proteins were electroblotted onto PVDF membrane. The membrane was probed with 1/1000 dilution of goat anti-Ntm (R&D Systems) primary antibody and alkaline phosphatase conjugated rabbit anti-goat IgG secondary antibody or using AttoglowAdvance western blot kit (BioChain Institute). Bands were detected by chemoluminescence using a DCC camera (Biorad,). Band areas and intensities were analyzed by QuantityOne software (Biorad).
Cell cultures
Primary smooth muscle cell cultures were prepared from rat uterine myometrium as follows. Uterine horns from OVX adult rats terminally anesthetized with Nembutal (100mg/kg, ip) were collected aseptically into Leibovitz (L15) culture medium, and the myometrium was separated from endometrium and stroma and cut into small pieces. Tissue was incubated first at 4°C for 30 min, then twice for 30 min at 37°C with collagenase (1mg/ml, Sigma) in L15 medium. The digested tissue was triturated with a fire-polished Pasteur pipette and filtered through a 40μm pore size cell strainer. The cells were washed and spun down at 300g 7 min and resuspended in growth medium consisting of DMEM/F12 supplemented with 10% FBS and Primocin (InvivoGene) and plated onto collagen coated dishes. The cultures were maintained at 5% CO2, 37° C and medium was changed every 3−5 days.
Sympathetic neuronal cultures were prepared from superior cervical ganglia harvested from newborn rat pups by mechanical disaggregation (Bray 1970). Briefly, ganglia were placed in L15 media, teased apart with fine forceps, and triturated with fire-bored Pasteur pipettes. The cell suspension was filtered through 40 μm mesh and centrifuged at 300g for 7 min. The collected cells were resuspended in plating medium (DMEM/F12, 2% FBS, ± 2×10−8 M estrogen) and seeded on top of cultured smooth muscle cells in 48 well culture plates (approx 0.5 ganglion/well).
Immunohistochemistry
Freshly harvested uterine horns were snap frozen and cryosectioned at 15μm. For Ntm staining, thaw-mounted sections or cultures were fixed with 4% PFA for 10 min, rinsed with PBST 3 times, blocked with 1% BSA and 5% donkey serum in PBST for 1 hr, and incubated with goat anti-Ntm IgG (1/20, R&D Systems) overnight. Sections were incubated with biotin-conjugated donkey anti-goat IgG (1/500, Jackson) for 1 h, followed by labeling for 30 min with CY3-conjugated streptavidin (1/3000, Jackson).
For double staining with peripherin and α-smooth muscle actin, cultures were fixed with 4% PFA for 10 min. After blocking with 1%BSA and 5% goat serum, cultures were incubated overnight with a mixture of rabbit anti-peripherin (1/400, Chemicon) and mouse anti-α smooth muscle actin (1/300, Sigma), A secondary antibody mixture consisting of Alexa 488-conjugated goat anti-mouse IgG (1/500, Invitrogen) and CY3-conjugated goat anti-rabbit IgG (1/200, Jackson) was used with 1h incubation. Omission of primary antibodies eliminated staining in all cases.
Ntm knockdown with siRNA
Predesigned siRNAs for Ntm (Ntm si1; sense: GCA GAA UUU CAG UGG UUC AdTdT; antisense: UGA ACC ACU GAA AUU CUG CdTdG, and Ntm Si2; sense: AGU GCU UAC UCC UGU CCA AdTdT; antisense: UUG GAC AGG AGU AAG CAC UdTdT), MAPK1 control siRNA and Allstars-si negative control siRNA were purchased from Qiagen. In preliminary experiments, transfection parameters, transfection efficiency and knockdown efficiency were established. Different concentrations (5, 10, 20nM) of Alexa-488 conjugated negative control siRNA were used with varying amounts of HiPerfect (0.75, 1.5 and 3μl/well in a 24-well plate) to transfect cultured smooth muscle cells. Cells were monitored for fluorescence inclusion and for signs of toxicity (trypan blue staining). Three μl /well HiPerfect with 5nM siRNA concentration showed optimal fluorescence inclusion with minimal toxicity. Knockdown efficiencies were further evaluated using positive control siRNA directed at MAPK1, and for Ntm siRNAs. After preliminary testing, Ntm si1 was used for the transfection experiments. Smooth muscle cells were replated onto collagen-coated 48 well plates in growth medium (as above) a day before transfection. Cells were transfected for 6 hr with 5nM siRNA (control or Ntm siRNA) with HiPerfect transfection agent in serum free media according to the manufacturer's instructions. After 6 hr transfection, the medium was removed and freshly prepared neurons in plating medium (with or without estrogen) were plated onto the smooth muscle cells.
Measurement of neurite outgrowth
Neurite outgrowth by neurons co-cultured for 3 d was evaluated following fixation with 4%PFA and staining for peripherin. Ten representative images of neurons were captured from each well; an area containing the full neurite arbor for individual neurons was selected randomly and bracketed with a 10x or 20x objective. Total neurite length for each neuron was measured using AnalySis software and was expressed as neurite length per neuron.
Imaging
Epifluorescence microscopic images lof neurite outgrowth were obtained using a Nikon TE3000 inverted microscope with 4x/0.10 Plan, 10x/0.30 Plan Fluor and 20x/0.45 Plan Fluor objectives and a MagnaFire camera (Optronics).
Confocal images were obtained with a Nikon C1+ confocal system equipped with 543 nm HeNe, 488 nm Ar and 405 nm diode lasers integrated into an Eclipse TE2000-U microscope with 20x/0.75 Plan apochromat and 60x/1.40/0.21 oil PlanApochromat objectives. Image acquisition and volume renderings of z stacks were performed using Nikon EZC1 software.
Data analysis
Values are expressed as mean ± SEM, and statistical comparisons between groups were made by using the Student t-test or one way ANOVA with Student-Newman-Keuls post-hoc comparisons; significance was set at p<0.05.
Results
Estrogen regulates myometrial neurotrimin expression in vivo
To investigate genes that are potentially involved in estrogen-initiated sympathetic axon pruning, we conducted exploratory microarray analyses on OVX myometria at 6 and 24h after estrogen or vehicle injection. From the set of 8,799 probes, 306 and 505 genes were upregulated and 274 and 415 genes were downregulated at 6 and 24 h, respectively (data accessible at NCBI GEO, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9117). Many of these regulated genes appeared to be involved with smooth muscle structural remodeling that is known to occur when estrogen is elevated (Ross and Klebanoff 1967), and we focused specifically on estrogen-upregulated genes with recognized effects on the nervous system. Ntm, a glycophosphatidylinositol (GPI)-anchored cell adhesion molecule, fulfilled these criteria. It was upregulated by estrogen in a temporally appropriate manner; intensity of the U16845 probe set corresponding to Ntm mRNA was increased 5 fold at 6 h and decreased 13 fold at 24h relative to vehicle-injected controls. While Ntm is present within the central nervous system and has been shown to adversely affect neurite formation by cultured sympathetic neurons (Gil et al. 1998), its expression in peripheral sympathetic target tissues and regulation by estrogen have not been previously recognized.
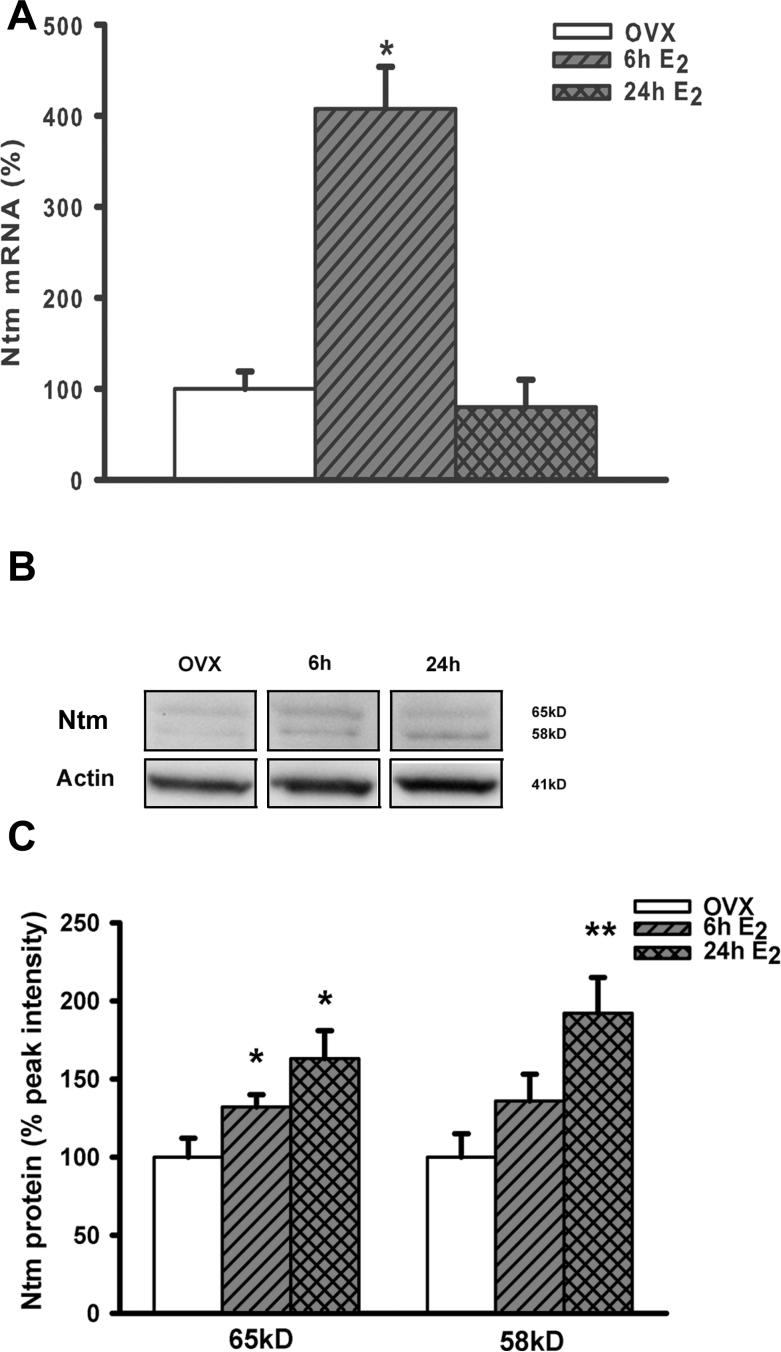
To validate microarray findings we performed real time RT-PCR for Ntm mRNA using myometria harvested from OVX or estrogen treated rats. Real time RT-PCR confirmed the presence of Ntm mRNA in the myometrium, and showed that estrogen upregulated expression in a manner consistent with the microarray data; tissue harvested after 6h of estrogen treatment showed a 4 fold increase in Ntm mRNA content, which declined to baseline 24h following treatment (Fig. 1A).
Figure 1. Estrogen upregulates uterine Ntm mRNA and protein.
(A) The effect of estrogen on myometrial Ntm mRNA content was analyzed by real time PCR. In vivo treatment with estrogen benzoate (E2, 10 μg/kg) resulted in a significant elevation of Ntm mRNA at 6 but not at 24 h relative to ovariectomized (OVX) controls (p<0.001, 1-way ANOVA, n=3). (B) Western blots showed low levels of Ntm protein in OVX rats, and increases at 6 and 24 h after estrogen administration. (C) Ntm band intensities were normalized to actin band intensities in the same lane and expressed as percent change. The 65 kD isoform was significantly increased at both 6 and 24h after estrogen administration (p<0.05, 1- way ANOVA), while the 58kD protein was more abundant 24h after treatment (p<0.05, 1- way ANOVA, n=6).
To determine whether Ntm mRNA is translated to protein, we conducted immunoblotting for Ntm. Blots revealed 2 weak but distinct Ntm bands at ∼58 kD and at ∼65kD in OVX myometria (Fig. 1B); these presumably represent 2 glycosylated isoforms that predominate within this tissue. Ntm protein levels increased progressively at 6h and 24h after estrogen treatment, with the 65 kD band upregulated at 6h and both isoforms significantly elevated at 24h (Fig. 1C).
To define the cellular distribution of Ntm protein in vivo, we conducted immunohistochemical staining on uterine tissue sections. We assessed whether Ntm levels vary as a function of the normal physiological changes in estrogen that occur during the estrous cycle. Vaginal smears were obtained to determine estrous cycle stage, and uterine tissue harvested from animals at estrus (following the estrogen surge of proestrus) and diestrus (serum estrogen nadir). In rats at diestrus when sympathetic axon density is high, Ntm staining was weak in the myometrial circular and longitudinal smooth muscle, prominent in perivascular tissues, luminal epithelium and epithelium of the endometrial glands, and essentially absent in endometrium and stroma (Fig. 2A). In uteri of rats at estrus when sympathetic innervation density is low, Ntm staining was markedly increased in circular and longitudinal smooth muscle (Fig. 2B) but apparently unchanged in other cellular compartments. These data indicate that Ntm protein is present in uterine smooth muscle and undergoes cyclic variations in association with fluctuations in estrogen that occur normally during the estrous cycle.
Figure 2. Estrogen selectively increases myometrial Ntm.
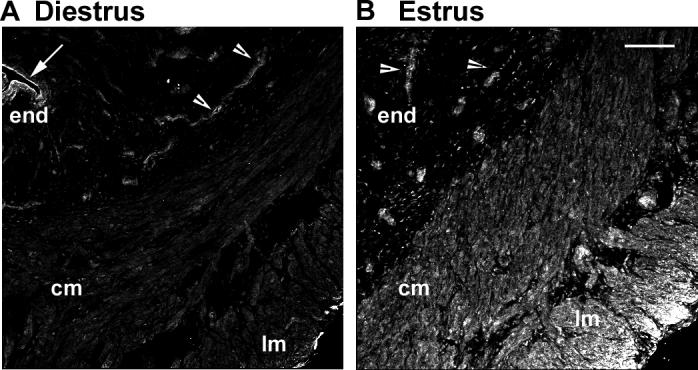
Uterine tissue frozen sections from rats at diestrus or estrus were immunostained for Ntm. (A) At diestrus, Ntm-ir was evident in the endometrium (end, arrow) and endometrial gland epithelium (arrowheads), but largely absent from the stroma and myometrial circular (cm) and longitudinal smooth muscles (lm). (B) At estrus, Ntm-ir was maintained in epithelial cells (arrowheads) but markedly increased in circular and longitudinal smooth muscle. Bar = 100 μm.
Cultured myometrial smooth muscle cells express and secrete neurotrimin
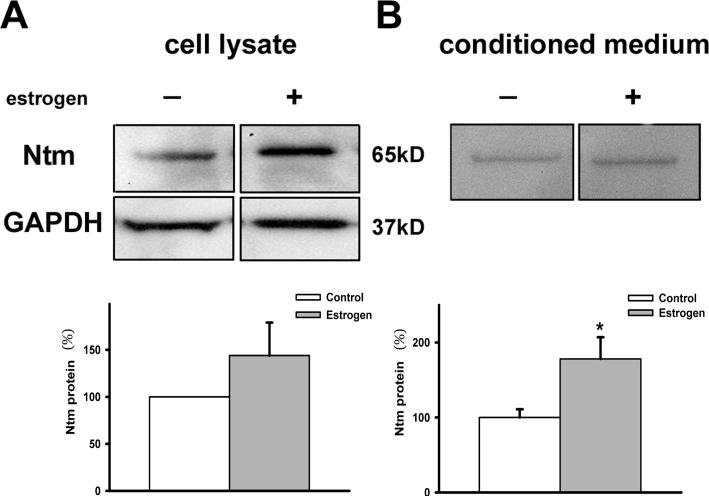
To assess the dynamics of Ntm expression within smooth muscle cells, we developed an in vitro culture system consisting of dissociated smooth muscle cells from the myometrium of adult female virgin rats. We first sought to confirm that cultured uterine smooth muscle cells express Ntm and that it is upregulated by estrogen in vitro (Fig. 3A). Cell lysates from cultures lacking estrogen revealed a 65 kD band corresponding to Ntm. Treating smooth muscle cultures with estrogen for 24h increased Ntm protein in the lysate, suggesting a direct effect of estrogen on smooth muscle Ntm content in vitro.
Figure 3. Ntm is secreted by cultured uterine smooth muscle cells.
(A) Cell lysates were obtained from cultures of untreated myometrial cells (control) and from cells 24h after addition of 2×10−8 M 17 β-estradiol to the culture medium (estrogen). In the presence of estrogen Ntm expression tended toward an increase, (p=0.1, n=3 in each group), as assessed by immunoblotting . (B) Conditioned medium collected 24h after treatment was analyzed by immunoblotting. Smooth muscle conditioned medium contains Ntm and its amount is increased by estrogen treatment (p=0.047, n=5 and 6 in control and estrogen, respectively).
Because several IgCAMs have secreted isoforms, we collected conditioned medium bathing smooth muscle cells following 24h incubation in the presence or absence of estrogen. Immunoblots showed the presence of Ntm protein in the culture medium (Fig. 3B), indicating that Ntm is secreted spontaneously from cultured smooth muscle cells. Estrogen treatment significantly increased the amount of Ntm in the culture medium, without affecting the appearance or vitality of the cultures.
To provide information on the subcellular distribution of Ntm, we co-labeled cultured smooth muscle cells with antibodies to Ntm and α-smooth muscle actin. Confocal microscopy showed that Ntm is expressed by all uterine smooth muscle cells in culture. Staining was most prominent in the perinuclear region, consistent with Ntm's localization within endoplasmic reticulum and secretory vesicles (Fig. 4.).
Figure 4. Localization of Ntm protein within cultured uterine smooth muscle cells.
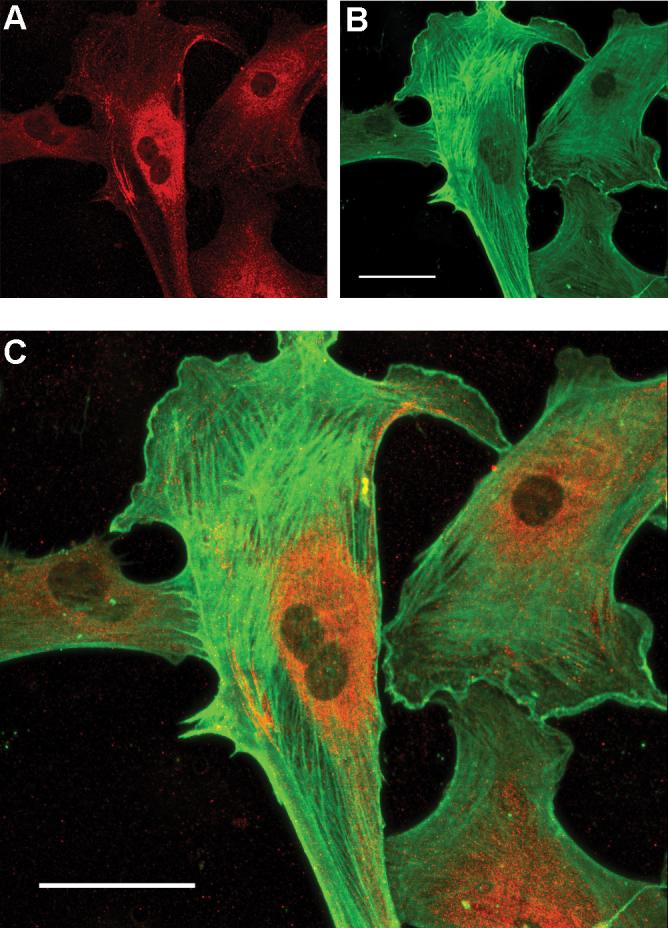
Uterine smooth muscle cells were immunostained for Ntm (red, A) and α-smooth muscle actin (green, B). Merged images (C) show Ntm localization in the perinuclear region of the cell. Bar = 50 μm.
Estrogen regulates sympathetic innervation of smooth muscle in vitro
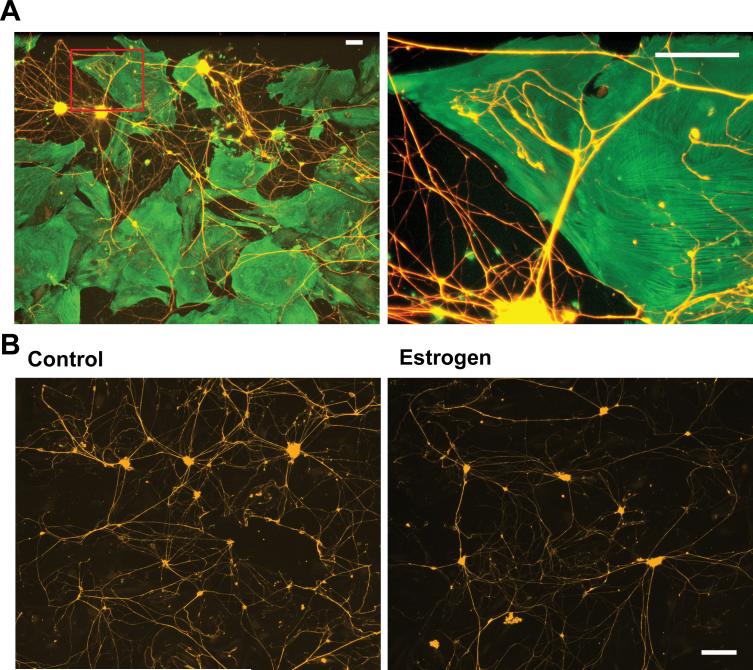
To determine if Ntm plays a functional role in regulating sympathetic innervation, we co-cultured sympathetic neurons with smooth muscle cells. In previous studies we used tissue explants of sympathetic ganglia and myometrium to show that estrogen induces smooth muscle to become inhospitable to sympathetic neurites, in part as a result of secretion of diffusible BDNF (Krizsan-Agbas and Smith 2002; Krizsan-Agbas et al. 2003). However, because Ntm is likely to act by way of surface contact as well as secretion, we used a system where both modes of action could influence sympathetic outgrowth. In this system freshly dissociated sympathetic neurons are seeded on top of a pre-plated monolayer of dissociated smooth muscle cells. Under these conditions, sympathetic neurons survive without NGF supplementation and elaborate neurites with varicosities that contact muscle cells (Fig. 5A).
Figure 5. Estrogen decreases neurite outgrowth in smooth muscle – neuron co-cultures.
Uterine myometrium smooth muscle cells were grown as a monolayer and sympathetic neurons seeded over the muscle cells and grown for 3d. Smooth muscle cells were immunostained for α-smooth muscle actin (Cy2) and neurons for peripherin (Cy3). (A) Low magnification image (left panel) shows sympathetic neurons extending neurites to smooth muscle cells. Box insert corresponds to the region depicted in the right panel. This higher magnification image shows sympathetic neurites branching and making terminal contacts with cultured smooth muscle cells. Bars in both panels = 100 μm.
(B) Sympathetic neurons were co-cultured with smooth muscle for 3 days and stained for peripherin. In cultures lacking estrogen, neurons elaborated many neurites (left panel). In the presence of 2×10−8 M 17 β-estradiol, neurite outgrowth was greatly reduced (right panel). Scale bar in the left panel = 200 μm for both panels.
We assessed neurite outgrowth of peripherin-immunoreactive (ir) neurites in control and estrogen treated cultures. In cultures lacking estrogen, outgrowth of sympathetic neurons was robust. The addition of estrogen to the culture media markedly decreased neurite outgrowth (Fig. 5B).
siRNA knockdown of Ntm synthesis in cultured smooth muscle cells
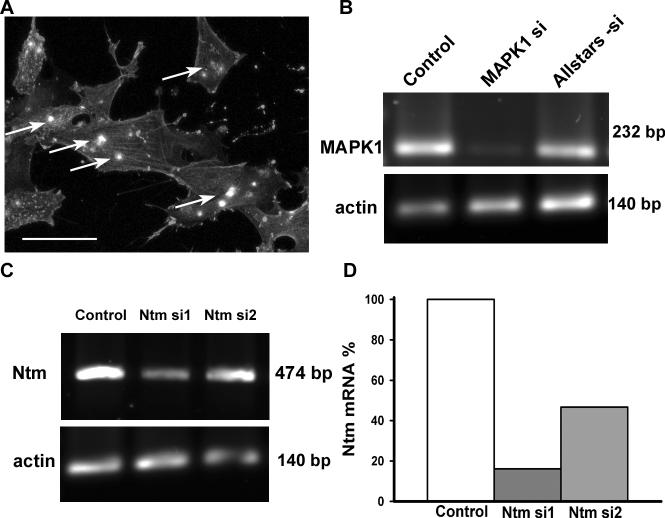
To determine if Ntm plays a role in estrogen-induced diminution of sympathetic outgrowth in co-culture, we downregulated Ntm synthesis using RNAi. Nearly all smooth muscle cells transfected for 3.5h with Alexa 488-conjugated Allstar-si negative control siRNA showed incorporation of fluorescence (Fig. 6A). To further confirm transfection efficiency, we used MAPK1 siRNA (Qiagen) as a positive control and obtained >90% reduction in MAPK1mRNA, indicating high transfection efficiency (Fig. 6B).
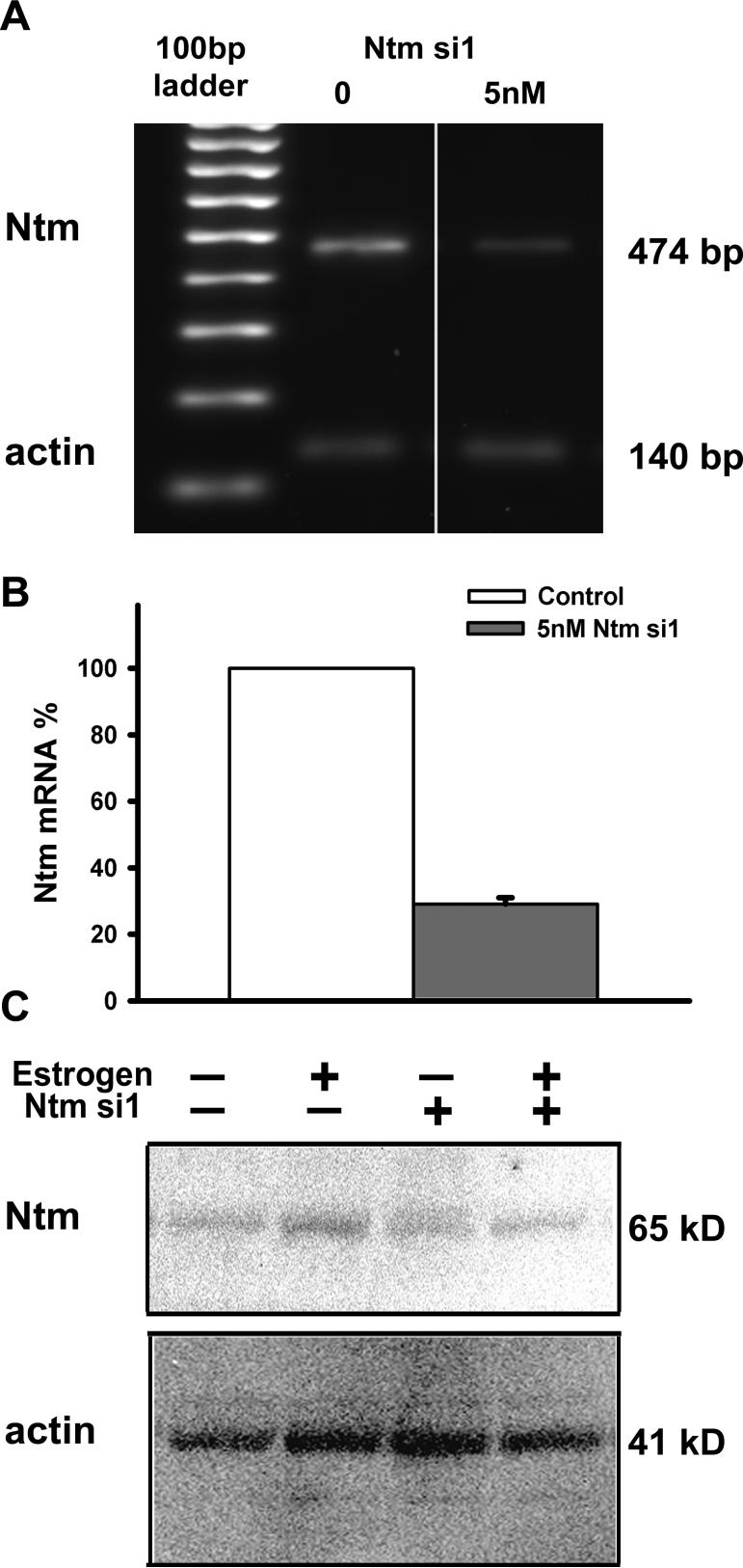
Figure 6. Evaluation of siRNA transfection efficiency.
(A) To confirm smooth muscle transfection, cells were incubated for 3.5h with 20nM Alexa488 labeled Allstars-si negative control siRNA, washed and fixed. Cells were stained with α-smooth muscle actin antibody and fluorescence incorporation was evaluated by confocal microscopy. The presence of intracellular material within inclusion bodies (arrow) confirms the incorporation of siRNA into the muscle cells. Scale bar = 50μm. (B) Smooth muscle cells that were treated only with HiPerfect transfection agent (control), transfected with MAPK1si positive control siRNA, or Allstars-si negative control siRNA were lysed after 24h in culture and MAPK1 mRNA was evaluated by RT-PCR. MAPK1 expression was suppressed by MAPK1 si but not by other treatments. (C) Two siRNA constructs targeted for Ntm (5nM) were tested and knockdown efficiency measured by RT-PCR. (D) Knockdown efficiency was assessed densometrically and the band intensity of the 474bp product obtained with Ntm primers was normalized to the band intensity of a 140bp actin amplicon, confirming that Ntm si1 siRNA does produce effective Ntm knockdown.
We initially tested 2 siRNAs designed for Ntm mRNA knockdown, one of which (Ntm si1) had good silencing efficiency (Fig. 6C&D). Two days after a 3.5 h transfection with siRNA, Ntm mRNA was reduced by 84%. Ntm si1 was used in all further transfection experiments.
To achieve extended downregulation synchronous with neurite outgrowth measured at 3d in culture, we transfected cells for 6 h with 5 nM Ntm si1 and evaluated Ntm mRNA and protein content after 3 days in culture (Fig. 7). Ntm mRNA was reduced by 71% at 3 days in culture (Fig. 7A&B). To confirm effective downregulation of Ntm protein, immunoblots were performed with lysates from cells transfected with 5nM Ntm si1 for 6 h and cultured for 3 d in control or estrogen treated medium. In control cultures, where Ntm expression is weak, Ntm si1 slightly decreased Ntm protein levels. In estrogen treated cultures siRNA transfection negated estrogen induced Ntm protein increase (Fig. 7C), therefore Ntm knockdown is sufficient for testing whether estrogen inducement of Ntm is involved in reduced sympathetic outgrowth.
Figure 7. Ntm si1 downregulates Ntm effectively for 72 h.
Uterine smooth muscle cells were transfected for 6h with 5nM Ntm si1 and cultured for 3d. (A) Total RNA prepared from cells 3 days after transfection was analyzed by RT-PCR for Ntm mRNA content. (B) Quantitative analysis by PCR shows that Ntm mRNA is still significantly downregulated in smooth muscle cultures 3 days after transfection (p<0.001). (C) Smooth muscle cells with or without transfection with 5nM Ntmsi1 were cultured in the presence or absence of estrogen (2×10−8M 17β-estradiol) and protein content was assessed by immunoblotting. Ntm si1 did not significantly reduce Ntm protein in cells cultured in control medium, but reduced the upregulated levels normally seen with estrogen.
Ntm knockdown impairs estrogen's ability to reduce sympathetic innervation
To determine whether Ntm si1 knockdown affects estrogen-induced modulation of sympathetic innervation, sympathetic neurons were plated for 3d on muscle cells with or without prior Ntm si1 transfection in the presence or absence of estrogen. Neither the transfection reagent alone nor negative control siRNA transfections influenced neurite outgrowth from sympathetic neurons co-cultured with transfected muscle cells (not shown).
Estrogen treatment resulted in significantly shorter neurites in cultures with non-transfected smooth muscle cells (Fig. 8 A, B&E). When neurons were cultured in control medium with Ntm si1-transfected smooth muscle cells, neurite outgrowth was similar to control cultures with non-transfected smooth muscle cells (Fig. 8 C&E). Ntm knockdown in control cultures had no effect in neurite outgrowth, indicating that Ntm does not play a significant role in modulating sympathetic innervation in low-estrogen conditions. However, when neurons were co-cultured with smooth muscle cells transfected with Ntm si1, estrogen failed to reduce neurite outgrowth (Fig. 8 D&E), indicating that elevated Ntm expression is required for estrogen to reduce smooth muscle cell innervation.
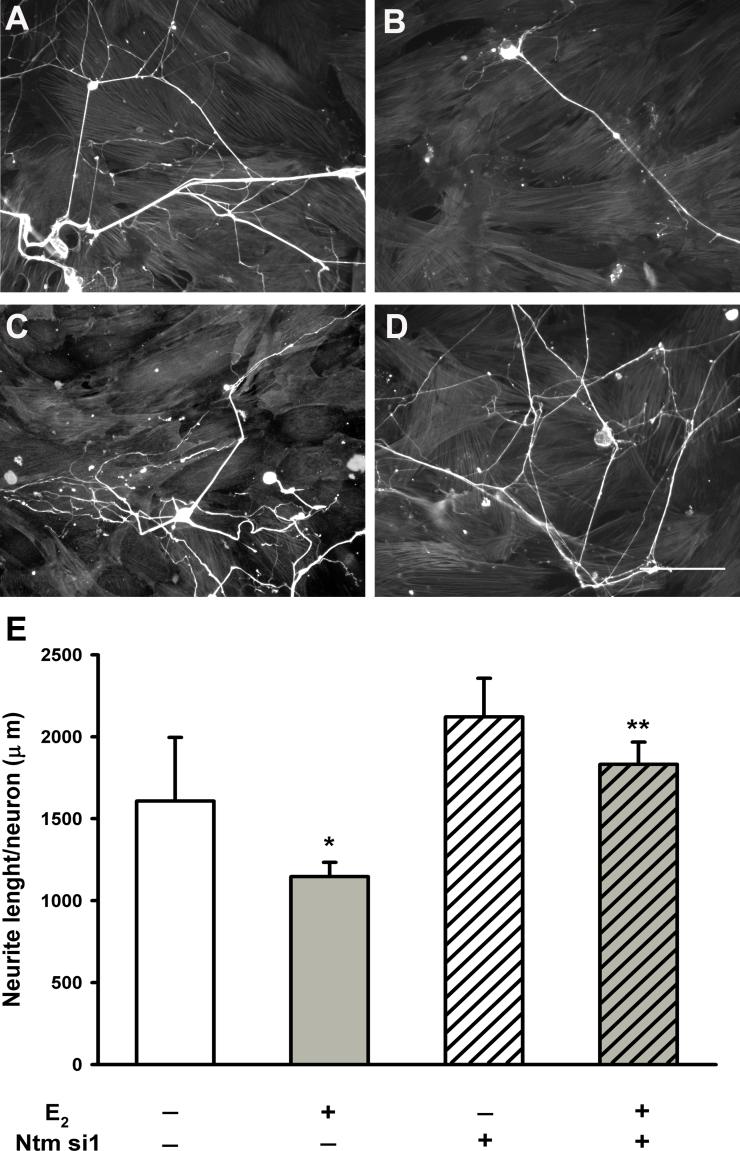
Figure 8. Ntm knockdown in smooth muscle attenuates estrogen-mediated inhibition of neurite outgrowth.
Sympathetic neurons were cultured on a smooth muscle monolayer for 3d and stained for peripherin. (A) Sympathetic neuron arborizations in co-culture with smooth muscle cells without transfection. (B) Sympathetic neuron outgrowth in a co-culture with non-transfected smooth muscle and in medium containing 2×10−8M 17 β-estradiol (E2). (C) Neurons plated onto transfected smooth muscle cells and cultured in control medium. (D) Sympathetic neurons cultured with transfected smooth muscle cell cultured in medium containing 2×10−8M E2. Scale bar = 200μm for all panels. (E)
Quantitative analysis of total neurite length per neuron. In non-transfected smooth muscle co-cultures, estrogen reduced neurite length (* p<0.05 vs. non-transfected control, 1-way anova). Ntm si1 transfection did not affect outgrowth in control cultures, but attenuated estrogen's ability to reduce neuritogenesis (** p<0.05 vs. non-transfected estrogen treated, 1-way anova). N=6 wells for each treatment group per culture, and cultures were repeated in triplicate.
Discussion
Ntm is a peripheral target protein
Ntm belongs to the IgLON family of adhesion proteins, which includes limbic-system associated protein, opioid-binding cell adhesion molecule and kilon. IgLONs have been identified primarily in the central nervous system, where they represent the most abundantly expressed GPI-anchored proteins (Salzer 1996). Their expression within specific areas of the central nervous system is well documented, and roles in axon fasciculation, pathfinding and synapse formation have been proposed (Struyk et al. 1995; Chen et al. 2001). Recently, it was reported that ovarian cells and renal carcinomas also express IgLONs, and their expression is altered in malignancy, suggesting that IgLONs may act as tumor suppressor genes (Chen et al. 2003; Ntougkos et al. 2005). However, the expression and function of these proteins in peripheral tissues have not been further characterized.
Our studies show that Ntm is expressed in myometrial smooth muscle cells. Ntm mRNA and protein are detectable in myometrium in vivo as well as in cultured myometrial cells. Ntm was also detected in culture medium, which could reflect either shedding of Ntm into the culture medium, possibly due to endogenous peptidases, or active secretion. Consistent with the latter, in cultured smooth muscle Ntm is localized mainly perinuclearly, possibly in secretory vesicles. Secretion of soluble forms of adhesion molecules is common within the Ig superfamily; for example, NCAM120, Thy-1 and F3/F11 exist in soluble forms in vivo (Salzer 1996), and within the IgLON family a secreted analog of CEPU was cloned from chicken brain (Kim et al. 1999). Secreted forms of these proteins appear to function similarly to the anchored forms; thus both soluble and GPI-anchored variants of Ntm and its chick orthologue CEPU exert similar effects on neurite outgrowth (Gil et al. 1998; Lodge et al. 2001). Accordingly, Ntm may influence sympathetic innervation by acting as both a diffusible protein as well as by contact-mediated actions.
Estrogen regulates Ntm expression
While Ntm and other members of the IgLON family of adhesion molecules are developmentally regulated, little is known regarding factors influencing their expression in the adult. Our studies show that Ntm is modulated in vivo under physiological conditions. Hence, very low levels of Ntm-ir are detectable at diestrus, but at estrus 2−3 d later, substantial protein is present. The increase at estrus is attributable to the spike in estrogen that immediately precedes estrus. Both microarray and PCR show marked in vivo upregulation of Ntm mRNA within 6h of a single estrogen injection, with levels returning to control or below by 24h. Ntm-ir protein is also upregulated but with a slower time course. Since estrogen alone is sufficient to induce increased Ntm synthesis in vivo and in vitro, this hormone acts directly on the myometrium to regulate Ntm expression.
Ntm as a mediator of peripheral nerve remodeling
The innervation of the adult rodent uterus is remarkably plastic. Estrous cycle-driven increases in serum estrogen produce a rapid and dramatic degenerative loss of sympathetic innervation in uterine myometrium (Zoubina and Smith, 2000). Degeneration is highly selective; sympathetic nerves undergo widespread and extensive degeneration while calcitonin gene-related peptide-ir sensory axons are spared (Zoubina et al., 1998).
We have shown previously that estrogen acts on the myometrium to make it repulsive to sympathetic axons (Krizsan-Agbas and Smith, 2002), and this occurs, at least in part, through release of diffusible factor(s). Prior research shows that BDNF fulfills criteria to qualify as an estrogen-regulated repellant protein. Thus, exogenous BDNF inhibits sympathetic neurite outgrowth and estrogen upregulates BDNF mRNA and protein in uterine smooth muscle cells (Kohn et al. 1999; Krizsan-Agbas et al. 2003). Furthermore BDNF function-blocking antibodies partially reverse estrogen's ability to make the myometrium inhospitable to sympathetic nerves, and estrogen's ability to repel sympathetic axons is also impaired in mouse mutants heterologous for a null mutation of the BDNF gene (Krizsan-Agbas et al. 2003). Collectively, this evidence strongly supports a role of BDNF in estrogen-driven modulation of sympathetic uterine innervation.
Evidence presented here supports the idea that estrogen acts multifactorially to induce sympathetic degeneration. Ntm is expressed and regulated in manner similar to that of BDNF. Like BDNF, Ntm is synthesized in the myometrial smooth muscle, its synthesis and release are increased by estrogen, and both exert repellent effects on sympathetic innervation. However, these 2 proteins apparently act through quite different mechanisms. BDNF exerts its effect via p75 neurotrophin receptors present on sympathetic axons, which activate sphingomyelinase to increase intracellular levels of ceramides, which in turn impede sympathetic outgrowth (Brann et al. 1999; Kohn et al. 1999; Song and Posse de Chaves 2003). However, unmyelinated sensory neurons also express p75 neurotrophin receptor (Mu et al. 1993) and, as is the case with sympathetic axons, BDNF inhibits axon outgrowth from small dorsal root ganglion neurons in culture (Gavazzi et al. 1999). It therefore is curious that, unlike sympathetic nerves, uterine sensory nerves don't degenerate in the presence of elevated estrogen.
In contrast to BDNF, culture experiments with hippocampal, dorsal root and superior cervical ganglion neurons show that Ntm can both inhibit and promote outgrowth. Ntm inhibits neurite outgrowth when acting through heterophilic interactions, as in the case of sympathetic axons. Conversely, it can promote outgrowth through homophilic effects, as occurs with hippocampal and sensory neurons (Gil et al. 1998).
Why is it important to have multiple mechanisms inducing uterine sympathetic axon degeneration when estrogen is elevated? One reason may be to provide redundancy. If a process is biologically important, as is pruning of uterine sympathetic nerves in the case of pregnancy, sperm transport, and embryo implantation, having 2 factors regulating degeneration may be prudent. A second reason may be differing time courses of expression. Thus Ntm is rapidly upregulated and may serve to initiate degeneration, which might be then sustained by BDNF which appears to have a longer time course; while Ntm mRNA peaks at 6h after estrogen, BDNF mRNA remains elevated at 24h (Krizsan-Agbas et al. 2003). However, a third reason for multifactorial regulation of pruning may be the conferral of selectivity to estrogen's actions. While inducing sympathetic repulsion in concert with BDNF, Ntm should be acting to promote outgrowth in resident sensory nerves; this in turn may act to stabilize sensory axons in the face of sympathetic degeneration (Fig. 9). Collectively, these studies are consistent with the idea that estrogen regulates multiple aspects of the uterine milieu to selectively induce sympathetic axon loss while ensuring the preservation of other axonal populations.
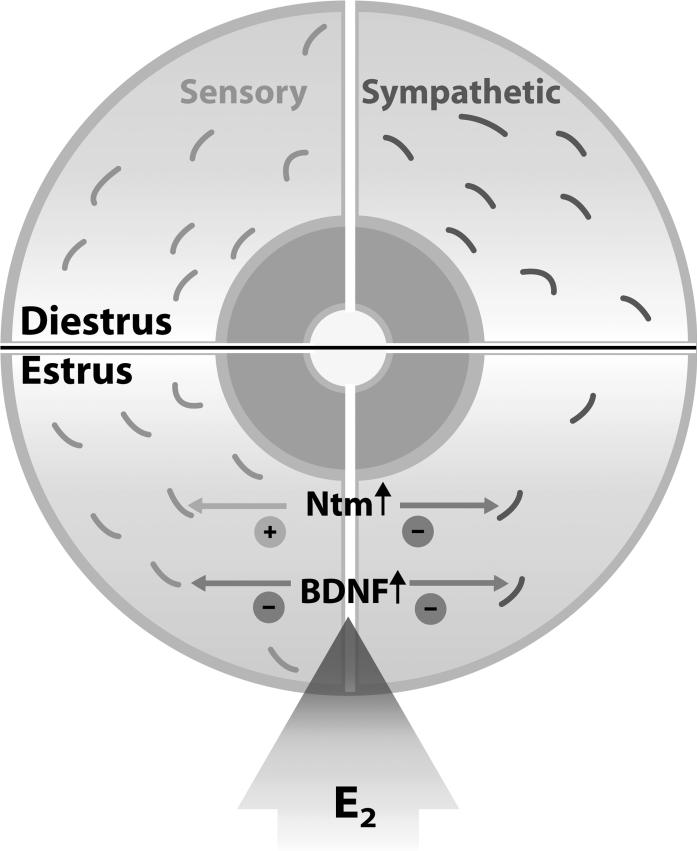
Figure 9. Schematic representation of estrogen's effects on uterine innervation.
During diestrus (low estrogen, top half), the myometrium enjoys rich sensory (grey) and sympathetic (black) innervation. When estrogen is elevated in estrus (bottom half), sympathetic axons degenerate whereas sensory axons are preserved (Zoubina et al., 1998). Previous studies show that estrogen (E2) increases levels of BDNF, which is repulsive to sympathetic innervation (Krizsan-Agbas et al., 2003). The present study shows that estrogen also upregulates Ntm, which is also repulsive to sympathetic axons and presumably works in concert with BDNF to promote sympathetic axon degeneration. However, Ntm is known to stimulate sensory fiber outgrowth. We propose that these differential effects of Ntm help stabilize the uterine sensory nerves while promoting sympathetic axon degeneration.
Acknowledgements
This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1 NS053796 to PGS, with research core support from P30 HD02528 and P20 RR016475. Special thanks to Phillip Shafer of the Kansas Intellectual and Developmental Disabilities Research Center Integrative Imaging Core for assistance with illustrations and to Sachin Mathur and Dr. Stanislav Svojanovsky of the Kansas IDeA Network for Biomedical Research Excellence Bioinformatics Core for assistance with microarray data analysis.
Supported by NIH grants RO1 NS053796, P30 HD02528 and P20 RR016475.
References
- Brann AB, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman AH. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19(19):8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. PNAS. 1970;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. NGF and the local control of nerve terminal growth. J Neurobiol. 1994;25(6):599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- Chen J, Lui WO, Vos MD, Clark GJ, Takahashi M, Schoumans J, Khoo SK, Petillo D, Lavery T, Sugimura J, Astuti D, Zhang C, Kagawa S, Maher ER, Larsson C, Alberts AS, Kanayama HO, Teh BT. The t(1;3) breakpoint-spanning genes LSAMP and NORE1 are involved in clear cell renal cell carcinomas. Cancer Cell. 2003;4(5):405–413. doi: 10.1016/s1535-6108(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Chen S, Gil O, Ren YQ, Zanazzi G, Salzer JL, Hillman DE. Neurotrimin expression during cerebellar development suggests roles in axon fasciculation and synaptogenesis. J Neurocytol. 2001;30(11):927–937. doi: 10.1023/a:1020673318536. [DOI] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nature Rev. 2005;6(11):889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20(4):1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. Autonomic peripheral neuropathy. Lancet. 2005;365(9466):1259–1270. doi: 10.1016/S0140-6736(05)74815-7. [DOI] [PubMed] [Google Scholar]
- Gavazzi I, Kumar RD, McMahon SB, Cohen J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur J Neurosci. 1999;11(10):3405–3414. doi: 10.1046/j.1460-9568.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- Gil OD, Zanazzi G, Struyk AF, Salzer JL. Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J Neurosci. 1998;18(22):9312–9325. doi: 10.1523/JNEUROSCI.18-22-09312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Ribchester RR. Compartmental neurodegeneration and synaptic plasticity in the Wld(s) mutant mouse. J Physiol. 2001;534(Pt 3):627–639. doi: 10.1111/j.1469-7793.2001.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Rhew TH, Moss DJ, Kim JY. cDNA cloning of the CEPUS, a secreted type of neural glycoprotein belonging to the immunoglobulin-like opioid binding cell adhesion molecule (OBCAM) subfamily. Molecules and cells. 1999;9(3):270–276. [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19(13):5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18(10):2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Smith PG. Estrogen modulates myometrium-induced sympathetic neurite formation through actions on target and ganglion. Neurosci. 2002;114(2):339–347. doi: 10.1016/s0306-4522(02)00262-2. [DOI] [PubMed] [Google Scholar]
- Lodge AP, McNamee CJ, Howard MR, Reed JE, Moss DJ. Identification and characterization of CEPU-Se-A secreted isoform of the IgLON family protein, CEPU-1. Mol Cell Neurosci. 2001;17(4):746–760. doi: 10.1006/mcne.2001.0964. [DOI] [PubMed] [Google Scholar]
- Long JA, Evans HM. The oestrus cycle in the rat and associated fenomena. Mem Univ Calif. 1922;6:1–148. [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur J Neurosci. 1989;1(1):27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Effects of ovarian steroids and pregnancy on andrenergenic nerves of uterus and oviduct. Am J Physiol. 1981;240:C165. doi: 10.1152/ajpcell.1981.240.5.C165. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci. 1993;13(9):4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntougkos E, Rush R, Scott D, Frankenberg T, Gabra H, Smyth JF, Sellar GC. The IgLON family in epithelial ovarian cancer: expression profiles and clinicopathologic correlates. Clin Cancer Res. 2005;11(16):5764–5768. doi: 10.1158/1078-0432.CCR-04-2388. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10(6):991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296(5569):868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Ross R, Klebanoff SJ. Fine structural changes in uterine smooth muscle and fibroblasts in response to estrogen. J Cell Biol. 1967;32(1):155–167. doi: 10.1083/jcb.32.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Rosen CL, Struyk AF. GPI-anchored proteins in neural cell adhesion. In: C DR, editor. Advences in Molecular and Cellular Biology. Cell adhesion Greenwich,CT: 1996. pp. 193–222. [Google Scholar]
- Sjoberg NO. The adrenergic transmitter of the female reproductive tract: distribution and functional changes. Acta Physiol Scand. 1967;305:1–32. [PubMed] [Google Scholar]
- Song MS, Posse de Chaves EI. Inhibition of rat sympathetic neuron apoptosis by ceramide. Role of p75NTR in ceramide generation. Neuropharmacol. 2003;45(8):1130–1150. doi: 10.1016/s0028-3908(03)00284-3. [DOI] [PubMed] [Google Scholar]
- Sporrong B, Alm P, Owman C, Sjoberg NO, Thorbert G. Ultrastructural evidence for adrenergic nerve degeneration in the guinea pig uterus during pregnancy. Cell Tissue Res. 1978;195(1):189. doi: 10.1007/BF00233686. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Canoll PD, Wolfgang MJ, Rosen CL, D'Eustachio P, Salzer JL. Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J Neurosci. 1995;15(3 Pt 2):2141–2156. doi: 10.1523/JNEUROSCI.15-03-02141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AY, Blacklock AD, Smith PG. Estrogen regulates vaginal sensory and autonomic nerve density in the rat. Biol Reprod. 2004;71:1397–1404. doi: 10.1095/biolreprod.104.030023. [DOI] [PubMed] [Google Scholar]
- Wokke JH, van Dijk GW. Sensory neuropathies including painful and toxic neuropathies. J Neurol. 1997;244(4):209–221. doi: 10.1007/s004150050075. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Fan Q, Smith PG. Variations in uterine innervation during the estrous cycle in rat. J Comp Neurol. 1998;397(4):561–571. [PubMed] [Google Scholar]
- Zoubina EV, Mize AL, Alper RH, Smith PG. Acute and chronic estrogen supplementation decreases uterine sympathetic innervation in ovariectomized adult virgin rats. Histol Histopathol. 2001;16(4):989–996. doi: 10.14670/HH-16.989. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Axonal degeneration and regeneration in rat uterus during the estrous cycle. Auton Neurosci. 2000;84(3):176–185. doi: 10.1016/S1566-0702(00)00209-5. [DOI] [PubMed] [Google Scholar]