Abstract
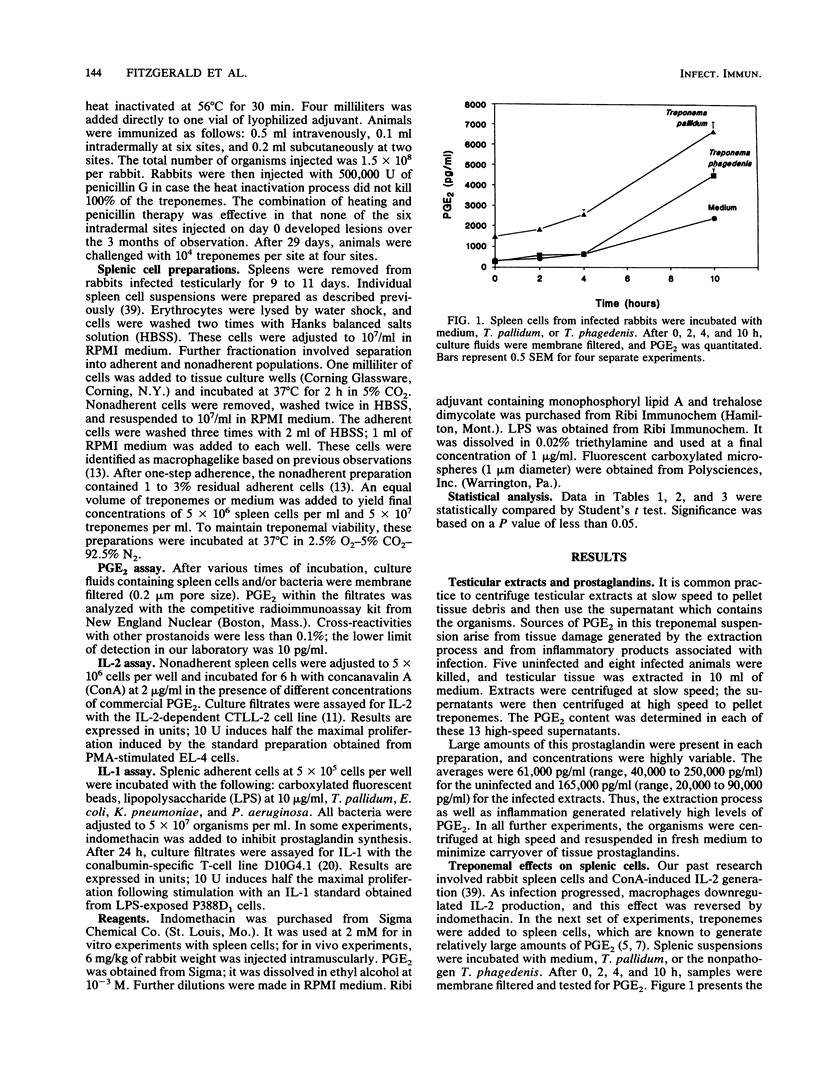
Incubation of microorganisms with macrophages enhances the production of prostaglandin E2 (PGE2). Previous research had indicated that macrophages from syphilitic rabbits suppressed spleen cell synthesis of interleukin-2 (IL-2); this suppressive activity was reversed by indomethacin. Experiments were designed to further characterize the involvement of prostaglandins in immune processing. When Treponema pallidum was incubated with unfractionated spleen preparations, PGE2 production was accelerated, and within 24 h, pharmacologic concentrations of the prostaglandin were detected. When cytochalasin B was used to block phagocytosis, decreased levels of PGE2 were apparent. Commercial preparations of PGE2, in the range generated by macrophage-treponeme interaction, inhibited concanavalin A-induced IL-2 secretion by splenic cells. T. pallidum stimulated IL-1 production by adherent cells, and indomethacin markedly enhanced this effect. In vivo, indomethacin upregulated immune function. Two groups of rabbits were infected, and one was given daily injections of indomethacin for 18 days. Both groups were treated with penicillin to terminate infections. One week later, rabbits were challenged with viable organisms to determine their immune status. The indomethacin-treated group was more resistant to reinfection. In further research, indomethacin enhanced the immunogenicity of vaccine preparations containing heat-killed T. pallidum. Results are discussed in terms of the role of PGE2 as it impinges on immune functions involving macrophage activation (IL-1 production) and T lymphocyte activation (IL-2 production).
Full text
PDF
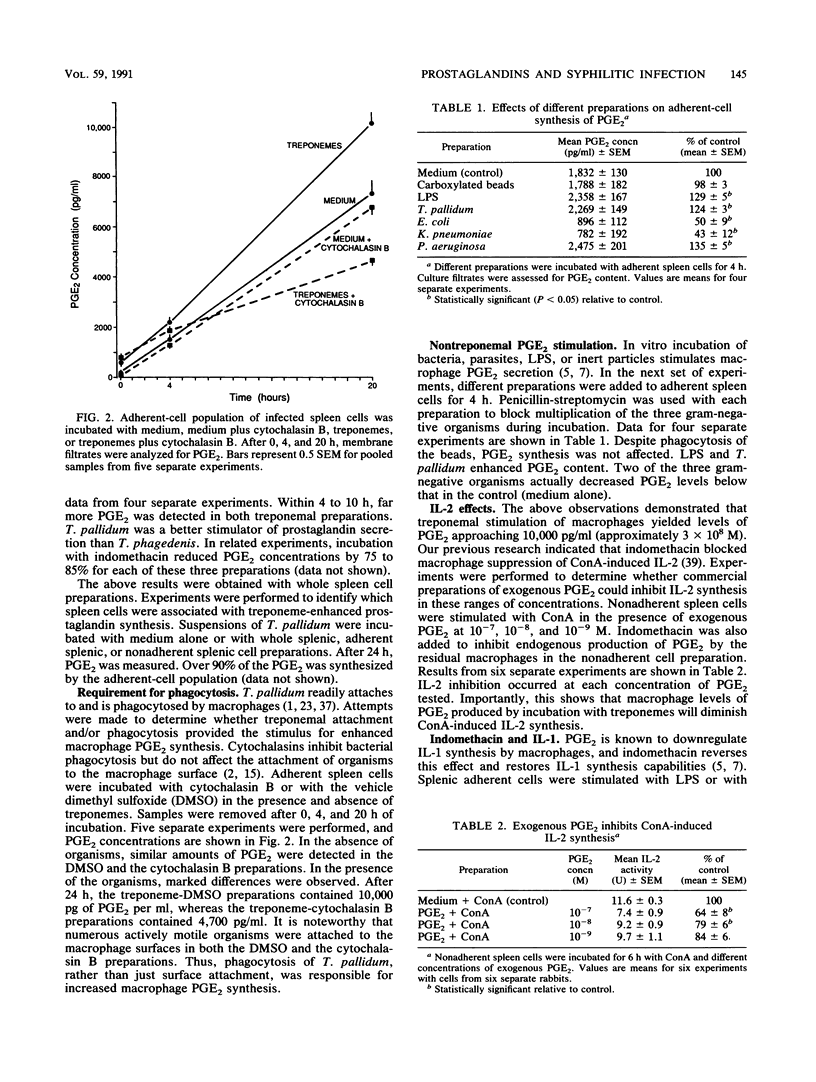




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alder J. D., Daugherty N., Harris O. N., Liu H., Steiner B. M., Schell R. F. Phagocytosis of Treponema pallidum pertenue by hamster macrophages on membrane filters. J Infect Dis. 1989 Aug;160(2):289–297. doi: 10.1093/infdis/160.2.289. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Cillari E., Liew F. Y., Lelchuk R. Suppression of interleukin-2 production by macrophages in genetically susceptible mice infected with Leishmania major. Infect Immun. 1986 Nov;54(2):386–394. doi: 10.1128/iai.54.2.386-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Bailey P. J., Goldenberg M. M., Ford-Hutchinson A. W. The role of arachidonic acid oxygenation products in pain and inflammation. Annu Rev Immunol. 1984;2:335–357. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Farrell J. P., Kirkpatrick C. E. Experimental cutaneous leishmaniasis. II. A possible role for prostaglandins in exacerbation of disease in Leishmania major-infected BALB/c mice. J Immunol. 1987 Feb 1;138(3):902–907. [PubMed] [Google Scholar]
- Ferreri N. R., Howland W. C., Spiegelberg H. L. Release of leukotrienes C4 and B4 and prostaglandin E2 from human monocytes stimulated with aggregated IgG, IgA, and IgE. J Immunol. 1986 Jun 1;136(11):4188–4193. [PubMed] [Google Scholar]
- Fitzgerald T. J., Elmquist B. J. Soluble factors from rabbit spleen cells kill and lyse Treponema pallidum in vitro. Can J Microbiol. 1990 Oct;36(10):711–717. doi: 10.1139/m90-120. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- Jerrells T. R. Immunosuppression associated with the development of chronic infections with Rickettsia tsutsugamushi: adherent suppressor cell activity and macrophage activation. Infect Immun. 1985 Oct;50(1):175–182. doi: 10.1128/iai.50.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F. Immunologic deficiency during experimental Chagas' disease (Trypanosoma cruzi infection): role of adherent, nonspecific esterase-positive splenic cells. J Immunol. 1982 Nov;129(5):2202–2205. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Moore R. N., Urbaschek R., Wahl L. M., Mergenhagen S. E. Prostaglandin regulation of colony-stimulating factor production by lipopolysaccharide-stimulated murine leukocytes. Infect Immun. 1979 Nov;26(2):408–414. doi: 10.1128/iai.26.2.408-414.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Barral A., Correa-Coronas R., Bogaert-Diaz H., Martinez D., Ward F. E. Monocyte suppression of antigen-specific lymphocyte responses in diffuse cutaneous leishmaniasis patients from the Dominican Republic. J Immunol. 1984 May;132(5):2603–2606. [PubMed] [Google Scholar]
- Petit J. C., Richard G., Burghoffer B., Daguet G. L. Suppression of cellular immunity to Listeria monocytogenes by activated macrophages: mediation by prostaglandins. Infect Immun. 1985 Aug;49(2):383–388. doi: 10.1128/iai.49.2.383-388.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., McGarry B. M., Loeffler K. M., Kunkel S. L. Regulation of macrophage-derived fibroblast growth factor release by arachidonate metabolites. J Leukoc Biol. 1987 Aug;42(2):106–113. doi: 10.1002/jlb.42.2.106. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Ratiwayanto S., Tuti S., Palmieri J. H., Piessens P. W., Koiman I., Dennis D. T. Antigen-specific suppressor cells and suppressor factors in human filariasis with Brugia malayi. N Engl J Med. 1980 Apr 10;302(15):833–837. doi: 10.1056/NEJM198004103021503. [DOI] [PubMed] [Google Scholar]
- Podwińska J. Identification of cells producing anti-treponemal lymphotoxin (ATL). Arch Immunol Ther Exp (Warsz) 1987;35(1):63–70. [PubMed] [Google Scholar]
- Podwińska J., Metzger M. Treponemicidal activity of supernatants from cultures of sensitized lymphocytes. Arch Immunol Ther Exp (Warsz) 1981;29(5):671–677. [PubMed] [Google Scholar]
- Podwińska J., Waśniowska K. Characterization of anti-treponemal lymphotoxin from lymphocytes of syphilitic rabbits. Arch Immunol Ther Exp (Warsz) 1984;32(1):29–36. [PubMed] [Google Scholar]
- Reiner N. E., Malemud C. J. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: in vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. J Immunol. 1985 Jan;134(1):556–563. [PubMed] [Google Scholar]
- Rice M., Fitzgerald T. J. Detection and functional characterization of early appearing antibodies in rabbits with experimental syphilis. Can J Microbiol. 1985 Jan;31(1):62–67. doi: 10.1139/m85-013. [DOI] [PubMed] [Google Scholar]
- Rutherford R. B. C3-mediated release of prostaglandin from human monocytes: evidence for independent regulation of thromboxane and prostaglandin synthesis. J Leukoc Biol. 1987 Nov;42(5):524–531. doi: 10.1002/jlb.42.5.524. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Pawlowski N. A., Murray H. W., Andreach M., Zrike J., Cohn Z. A. Regulation of arachidonic acid metabolism by macrophage activation. J Exp Med. 1982 Apr 1;155(4):1148–1160. doi: 10.1084/jem.155.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S., Powell H. C. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- Todd C. W., Goodgame R. W., Colley D. G. Immune responses during human schistosomiasis mansoni. V. Suppression of schistosome antigen-specific lymphocyte blastogenesis by adherent/phagocytic cells. J Immunol. 1979 Apr;122(4):1440–1446. [PubMed] [Google Scholar]
- Tomai M. A., Elmquist B. J., Warmka S. M., Fitzgerald T. J. Macrophage-mediated suppression of con A-induced IL-2 production in spleen cells from syphilitic rabbits. J Immunol. 1989 Jul 1;143(1):309–314. [PubMed] [Google Scholar]
- Tripp C. S., Wyche A., Unanue E. R., Needleman P. The functional significance of the regulation of macrophage Ia expression by endogenous arachidonate metabolites in vitro. J Immunol. 1986 Dec 15;137(12):3915–3920. [PubMed] [Google Scholar]



