Abstract
Background
Various authors have described conductive hearing loss (CHL), defined as an air-bone gap on audiometry, in patients without obvious middle ear pathologic findings. Recent investigations have suggested that many of these cases are due to disorders of the inner ear, resulting in pathologic third windows.
Objective
To provide an overview of lesions of the inner ear resulting in a CHL due to a third-window mechanism. The mechanism of the CHL is explained along with a classification scheme for these disorders. We also discuss methods for diagnosis of these disorders.
Data Sources
The data were compiled from a review of the literature and recent published research on middle and inner ear mechanics from our laboratory.
Conclusion
A number of disparate disorders affecting the labyrinth can produce CHL by acting as a pathologic third window in the inner ear. The common denominator is that these conditions result in a mobile window on the scala vestibuli side of the cochlear partition. The CHL results by the dual mechanism of worsening of air conduction thresholds and improvement of bone conduction thresholds. Such lesions may be anatomically discrete or diffuse. Anatomically discrete lesions may be classified by location: semicircular canals (superior, lateral, or posterior canal dehiscence), bony vestibule (large vestibular aqueduct syndrome, other inner ear malformations), or the cochlea (carotid-cochlear dehiscence, X-linked deafness with stapes gusher, etc.). An example of an anatomically diffuse lesion is Paget disease, which may behave as a distributed or diffuse third window. Third-window lesions should be considered in the differential diagnosis of CHL in patients with an intact tympanic membrane and an aerated, otherwise healthy, middle ear. Clues to suspect such a lesion include a low-frequency air-bone gap with supranormal thresholds for bone conduction, and presence of acoustic reflexes, vestibular evoked myogenic responses, or otoacoustic emission responses despite the CHL. Imaging studies can help confirm the diagnosis.
Keywords: Air, bone gap, Conductive hearing loss, Third window lesions
Various authors have described conductive hearing loss (CHL), defined as an air-bone gap measured by standard audiometry, in patients without obvious middle ear pathologic findings (1–3). The clinical presentation of these patients often mimics middle ear diseases such as otosclerosis. Many of these patients undergo negative middle ear exploration, whereas in some, stapedectomy or tympanoplasty is mistakenly performed without any benefit to the hearing. Bess et al. (1), who described 3 such patients, stated that H. House and C. Shea had observed at least 90 similar patients in their clinical practices. Schuknecht (4) speculated that the incidence of such “otologic mysteries” may be 1 in 3,000 procedures. The air-bone gaps in these patients with unexplained CHL are generally ascribed to putative lesions in the inner ear that are hypothesized to result in an “inner ear CHL” (1–3).
Recent investigations have provided insight into both specific lesions of the inner ear that result in CHL and possible mechanisms of the CHL. Snik et al. (5) studied 2 patients with mixed sensorineural and conductive loss due to X-linked stapes gusher syndrome (now called DFN-3) and proposed that the CHL was the result of improved bone-conduction thresholds. The improved bone thresholds were hypothesized to result from a larger-than-normal “third window” in the inner ear, which also caused the stapes gusher [third window is a term that Ranke et al. (6), Ranke (7), and Tonndorf and Tabor (8) used to describe secondary fluid paths between the inner ear and the cranial cavity]. Minor et al. (9) also hypothesized that a pathologic third window explained the air-bone gap in 4 patients with a dehiscence of the bone overlying the superior canal; they hypothesized that the superior canal dehiscence (SCD) window permitted “dissipation of acoustic energy” into the cranial cavity and was “acting as an amplifier for bone-conducted sounds.” The report of Minor et al. is noteworthy because these patients did not have any features of genetic deafness or apparent malformations of the inner ear, and their clinical presentation was similar to patients with middle ear disease such as otosclerosis. Several authors have now reported CHL as a manifestation of SCD (9–16). Our research group has conducted a series of studies to investigate the mechanisms by which an SCD results in a CHL (11,17–22). We have put forth theoretic and experimental evidence that an SCD does act as a pathologic third window between the vestibular portion of the inner ear and the cranial cavity, resulting in a CHL by the dual mechanism of worsening of air conduction thresholds and improvement of bone conduction thresholds.
Armed with our understanding of the mechanisms that produce an SCD-associated CHL, we now evaluate whether the CHL related to DFN-3 and a number of disparate disorders that affect the labyrinth and produce CHL can be explained by a similar third-window mechanism. In this report, we provide an overview of such disorders based on our own observations and a review of the literature. We propose a mechanism for the CHL and present a classification scheme for these disorders. We also discuss methods for the diagnosis of these disorders.
THE WINDOWS OF THE INNER EAR
The fluid spaces of the normal inner ear are nearly completely surrounded by the bone of the otic capsule. There are, however, several openings or windows that connect the inner ear fluid spaces to the cranial cavity or to the air-filled middle ear. The primary and secondary inner ear windows are the oval and round windows that serve as conduits for the transmission of sound from the middle ear into the inner ear. Other normal inner ear windows comprise the cochlear aqueduct (that courses through the otic capsule, opening into the posterior cranial fossa at one end and into the scala tympani of the cochlea adjacent to the round window membrane at the other), the vestibular aqueduct (that traverses the otic capsule between the posterior cranial fossa and an opening in the medial wall of the bony vestibule), and the foramina associated with the passage of blood vessels or nerve bundles; these other normal windows between the inner ear fluids and the cranial cavity are relatively thin and long.
The role of the oval and round windows in the transmission of sound to the inner ear is generally understood. These 2 windows are comparatively large in area and short in length, thereby minimizing the impedance of fluid flow through these windows and facilitating transmission of sound. The inner ear fluids are functionally incompressible, but the presence of the 2 mobile middle ear windows permits bulk motion of the fluid between the windows. The incompressible fluid entrains the motion of the 2 windows such that an inward displacement of the stapes in the oval window is accompanied by an outward displacement, of equal volume, of the round window (23,24). This fluid flow between the stimulating source (the stapes in the oval window) and the low-impedance round window produces a pressure gradient along the length of the cochlear partition that drives the motion of the basilar membrane and leads to the perception of sound (23,25,26).
The vestibular aqueduct, cochlear aqueduct, and foramina for blood vessels were collectively referred to as a normal third window by Ranke et al. (6), Ranke (7), and Tonndorf and Tabor (8). These smaller-caliber and longer third-window pathways are generally of such high impedance that they are functionally closed to sound flow [e.g., Gopen et al. (27)], and the auditory role of the normal third window is considered to be small. Békésy (23) investigated the acoustic role of these pathways comprising the normal third window and provided evidence that they may be of functional importance in certain pathologic situations. Ranke et al. (6), Ranke (7), and Tonndorf and Tabor (8) thought that the normal third window provided enough compliance to allow bone conduction hearing at near-normal levels when both the oval and round windows were closed.
In contrast to a normal third window, conditions such as SCD and others described in this article constitute “pathologic” third windows that direct air-conducted sound energy away from the cochlea in amounts consistent with a clinically appreciable hearing loss. At the same time, these pathologic third windows improve thresholds for bone-conducted sounds or leave the bone thresholds unchanged. Thus, the net audiometric effect of a pathologic third window is a CHL.
MECHANISM OF CONDUCTIVE HEARING LOSS
We have conducted a series of studies to investigate the mechanisms by which an air-bone gap arises in SCD, including theoretic model analyses (17,22), experimental measurements in a cadaveric human temporal bone preparation (21), experiments in a chinchilla model of SCD (17–19), and measurements of middle ear sound transmission in patients with SCD (11,17,21,28). These investigations have provided strong evidence to support the pathologic third-window hypothesis in SCD. We hypothesize that similar mechanisms are responsible for the CHL that is observed in various other disorders that are characterized by a pathologic window on the vestibular side of the cochlear partition (i.e., the window is within the bony vestibule or within one of the semicircular canals, or, if it is large enough, in the bony wall of the scala vestibuli of the cochlea).
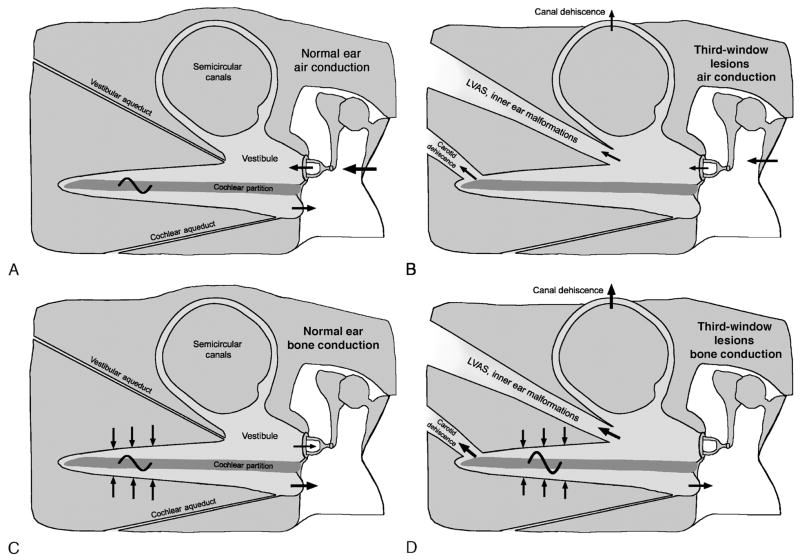
A schematic representation of our conceptual mechanism is shown in the Figure 1. In the normal ear, air-conducted sound stimuli enter the vestibule through motion of the stapes (panel A in the Figure). An inward motion of the stapes in the oval window is accompanied by an equal but outward motion of the round window membrane. The fluid flow between the windows produces a pressure difference between the scala vestibuli and the scala tympani, resulting in motion of the cochlear partition, activation of hair cells, and perception of sound. We hypothesize that a pathologic third window on the vestibular side of the cochlea (e.g., SCD, enlarged vestibular aqueduct, inner ear malformation, etc.) allows for a portion of this acoustic energy to be shunted away from the cochlear partition, producing a decrease in the sound pressure within the vestibule, thus resulting in a loss of hearing sensitivity to air-conducted sound (panel B in Fig. 1).
FIG. 1.
Schematic representations of mechanism of air-bone gap in third-window lesions. A, Normal ear, air conduction. Air-conducted sound stimuli enter the vestibule through motion of the stapes. There is a pressure difference between the scala vestibuli and the scala tympani, resulting in motion of the cochlear partition. The volume velocities of the oval and round windows are equal in magnitude but opposite in phase. B, Third-window lesions, air conduction. It is hypothesized that a third window (in one of the canals, the vestibule or the scala vestibuli) allows a portion of the acoustic energy entering the vestibule through motion of the stapes to be shunted away from the cochlea. The shunting occurs primarily at low frequencies, resulting in a hearing loss by air conduction. C, Normal ear, bone conduction. Compression of inner ear fluid by bone-conducted sound results in a hearing percept because of an inequality in the impedance between the scala vestibuli side and the scala tympani side of the cochlear partition. This inequality is primarily due to a difference in the impedance between the oval and windows. As a result, there is a pressure difference across the cochlear partition, resulting in motion of the basilar membrane that leads to perception of bone-conducted sound. D, Third-window lesions, bone conduction. A third window increases the difference between the impedance on the scala vestibuli side and the scala tympani side of the cochlear partition by lowering the impedance on the vestibuli side, thereby improving the cochlear response to bone conduction. In patients with healthy cochleae as in SCD, supranormal bone conduction thresholds may be evident. In other patients with an accompanying true sensorineural hearing as in DFN-3, LVAS, etc., the improved bone conduction due to the third-window mechanism may not result in supranormal thresholds. LVAS indicates large vestibular aqueduct syndrome.
The effect of a third window on bone conduction thresholds is less intuitive but can be understood based on the compressional mechanism of bone conduction (panel C in Fig. 1). In the normal ear, compression of inner ear fluids by bone-conducted sound results in a hearing percept because of an inequality in the impedance between the scala vestibuli side and the scala tympani side of the cochlear partition, which in turn, is due to a difference between the impedance of the oval and round windows, respectively. This inequality leads to a pressure difference across the cochlear partition, resulting in motion of the basilar membrane that leads to the perception of bone-conducted sound. A pathologic third window on the vestibular side of the cochlear partition increases the pressure difference between the 2 sides of the cochlear partition by lowering the impedance on the vestibuli side, thereby improving the cochlear response to bone conduction (panel D in Fig. 1). Therefore, supranormal thresholds for bone conduction may be evident, as in many patients with SCD (11,13–16). Such improvements of bone-conducted thresholds produced by the third window may be masked by an accompanying true sensorineural hearing loss.
We stress that according to the concept of sound transmission described in Figure 1, a pathologic third window must be on the scala vestibuli side of the cochlear partition to produce a CHL. A third window on the scala tympani side (such as an enlarged cochlear aqueduct) would not be predicted to result in a CHL and might even produce some small improvement in low-frequency air-conducted hearing performance.
The disorders discussed in this article are also interesting from a basic science perspective. These lesions are unique in that they have differing effects on air conduction versus bone conduction. In a sense, they can be considered experiments of nature, and research into their mechanics has provided insight into pathways of sound transmission in the middle and inner ears (18–20).
Pathologic third windows also have the potential to result in vestibular manifestations by making the vestibular sense organs sensitive to sound or to mechanical stimulation. For example, patients with SCD may exhibit sound- and/or pressure-induced vertigo or demonstrate low thresholds for air-conducted vestibular evoked myogenic potential (VEMP) responses (10,11,29). Similarly, patients with an enlarged vestibular aqueduct may have low thresholds on air-conducted VEMP testing (30). It is not known why some individuals with third-window lesions have solely auditory or vestibular manifestations, whereas others have both.
CLASSIFICATION AND CLINICAL SYNDROMES
Third-window lesions may be anatomically discrete or diffuse (Table 1). Anatomically discrete lesions may be classified by location: semicircular canals, bony vestibule, or the cochlea. An example of a diffuse lesion is Paget disease affecting the temporal bone that, we think, behaves as a distributed or diffuse third window.
TABLE 1.
Third-window lesions of the inner ear causing conductive hearing loss
| Anatomic third window |
| Semicircular canal |
| Superior canal dehiscence |
| Posterior canal dehiscence |
| Lateral canal dehiscence |
| Vestibule |
| Large vestibular aqueduct syndrome |
| Inner ear malformations causing a dehiscence between internal auditory canal and vestibule |
| Cochlea |
| Dehiscence between carotid canal and scala vestibuli |
| Inner ear malformations causing a dehiscence between internal auditory canal and scala vestibuli, e.g., DFN-3 (X-linked deafness with stapes gusher) |
| Diffuse or distributed third window |
| Paget disease of the temporal bone |
Superior Canal Dehiscence
An SCD is characterized by loss of bone covering the superior canal, so that there is a potential communication between the canal and the cranial cavity. The SCD syndrome is the best documented and most investigated third-window lesion of the inner ear. Patients with SCD may present with CHL, vestibular manifestations, or both. More than 60 patients with documented CHL have been described in the literature (9–16). The typical manifestation is an air-bone gap in the low and middle frequencies (≤2,000 Hz) with no gap or only a small gap at higher frequencies. Low-frequency (<2,000 Hz) bone conduction thresholds are sometimes at supranormal levels, 0 to −20 dB, or better. The lack of middle ear pathologic findings as a cause for the CHL in SCD has been well documented by a variety of diagnostic tests such as tympanometry, acoustic reflexes, laser Doppler vibrometry, air-conducted VEMP testing, otoacoustic emission (OAE) testing, and by exploration of the middle ear (9–16). Definitive evidence that the SCD can cause a CHL is demonstrated by resolution of the air-bone gap upon patching or plugging the dehiscence, as has been reported by various investigators (9,12,20,21). The mechanism of CHL in an SCD is a combination of an increase in air conduction thresholds combined with an improvement in bone conduction thresholds (11,17–22), as described above.
Posterior Canal Dehiscence
A CHL due to a posterior canal dehiscence with no apparent middle ear pathologic findings has been described in at least 3 patients (31–33). An additional case of CHL due to combined SCD and posterior canal dehiscence has also been described (34). These patients have included a dehiscence between the canal and cranial cavity or between the canal and jugular bulb. The mechanism of the CHL is probably similar to that of SCD.
Lateral Canal Dehiscence
Dehiscence of the lateral canal may be the result of middle ear disease such as cholesteatoma or chronic otitis media with granulation tissue causing erosive osteitis of the otic capsule and a fenestration procedure for otosclerosis. These conditions are usually associated with abnormalities of the middle ear sound transmission system, which act as confounding variables, making it difficult to ascertain precisely how much of the observed CHL is due to a third-window effect of the lateral canal dehiscence (31). Of interest to this discussion is a report by Juers (35) showing improved thresholds for bone conduction after fenestration for otosclerosis in 28 patients; these improved thresholds may be the result of the fenestration acting as a third window. Ribaric et al. (36,37) fenestrated the lateral semicircular canal in 5 bilaterally profound deaf individuals and found that all had improved hearing by bone conduction testing, although the air-conducted thresholds remained unchanged. Again, the observed effects on bone conduction may be due to the fenestration acting as a third window.
Large Vestibular Aqueduct Syndrome
Various authors (reviewed by Merchant et al. [30]) have reported a low-frequency air-bone gap in patients with an enlarged vestibular aqueduct. These patients often present with a mixed hearing loss. The lack of pathologic findings in the middle ear in these patients has been shown by a number of studies using tests such as tympanometry, acoustic reflexes, laser Doppler vibrometry, air-conducted VEMP and OAE testing, and middle ear explorations (30). The enlarged vestibular aqueduct probably provides a pathologically large communication between the bony vestibule and the cranial cavity, resulting in an air-bone gap, similar to that observed in SCD.
DFN-3 (X-Linked Deafness With Stapes Gusher)
DFN-3 is characterized by a mixed hearing loss and occurrence of a perilymph gusher upon attempted fenestration of the stapes (5,38–41). The air-bone gap is greater in the lower frequencies, and the stapedial reflex may be preserved (5,41). Radiologic studies typically demonstrate dilation of the internal auditory canal, often with deficiency of bone between the internal auditory canal and cochlea (40–43). Other studies have suggested deficiency of bone between the internal auditory canal and the vestibule (39) and enlargement of the vestibular aqueduct (42). At middle ear surgery, the stapes has been reported to be congenitally fixed in some patients but not in others (5,38–41). In patients with a mobile stapes, we believe that the CHL is caused by an abnormal communication between the internal auditory canal and the inner ear (either the scala vestibuli of the cochlea or the vestibule), resulting in a pathologic third window that worsens air-conducted thresholds and improves bone-conducted thresholds. The hypothesized improvement in bone conduction sensitivity may be masked by a true sensorineural hearing loss associated with this disorder.
Dehiscence Between the Cochlea and Carotid Canal
Kim and Wilson (44) described a patient with an air-bone gap that persisted after uneventful stapedectomy who, on further evaluation, was found to have a dehiscence between the cochlea and carotid canal. They hypothesized that this dehiscence acted as a third window, dissipating the acoustic energy away from the cochlear partition. Our examination of the CT images in the article by Kim and Wilson suggests that the dehiscence was on the scala vestibuli side of the basilar membrane. The presence of a large dehiscence in the scala vestibuli can act to decrease cochlear input impedance and reduce the sound pressure within scala vestibuli produced by air-conducted sound.
Other Inner Ear Malformations
Karlberg et al. (45) described a patient with a predominantly low-frequency CHL characterized by supranormal bone conduction thresholds, presence of acoustic reflexes, and presence of air-conducted VEMPs despite the CHL. Radiologic studies showed a Mondini-like malformation of the cochlea, a deficient modiolus, and a communication between the basal turn of the cochlea and the internal auditory canal. The authors hypothesized that the inner ear dysplasia was the cause of the CHL. Assuming that the CHL in this patient was due to a pathologic third window, we would predict that the abnormal communication between the internal auditory canal and the basal turn was at the level of the scala vestibuli. Abnormal communication between the internal auditory canal and scala vestibuli has been documented by histologic studies in individuals with labyrinthine malformations (e.g., Fig. 4.31 in Schuknecht [46]).
Huang et al. (47) described a patient with Apert syndrome who showed a mixed hearing loss with an air-bone gap of 20 to 60 dB. Exploratory tympanotomy showed no evidence of middle ear pathologic findings. Computed tomographic scan demonstrated a dilated bony vestibule, slight dilation of the internal auditory canal, and enlargement of the lateral semicircular canal. The authors concluded that the inner ear malformations were responsible for the CHL. Although the precise mechanism of the CHL in this patient is unclear, it is possible that it also represents a third-window effect if one hypothesizes an abnormal communication between the dilated internal auditory canal and the vestibule.
Paget Disease of the Temporal Bone
Patients with extensive Pagetic involvement of the otic capsule typically present with a mixed hearing loss with an air-bone gap in the lower frequencies. Histopathologic temporal bone evaluation in 26 ears from 16 patients by Khetarpal and Schuknecht (48) and clinical studies presented by Monsell (49) in 66 ears from 33 patients failed to find evidence for lesions within the middle ear that can account for the air-bone gap. We measured umbo velocity by laser Doppler vibrometry in 3 patients with Paget disease affecting the temporal bone and a mixed hearing loss and found relative hypermobility of the umbo similar to what is observed in SCD (unpublished data). Our examination of 8 temporal bones with Paget disease and a CHL revealed multiple microfractures within the otic capsule on the scala vestibuli side of the cochlear partition and extensive Pagetic involvement of the otic capsule surrounding the labyrinth (unpublished data). We hypothesize that the microfractures and/or Pagetic bone act as a distributed third window, dissipating sound energy transmitted through the stapes footplate away from the cochlea.
Other Lesions of the Inner Ear
We point out that there are lesions of the inner ear that can also present with a CHL but are not obvious examples of third-window defects. Examples include some patients of Méniére’s syndrome (50) and intralabyrinthine schwannoma (authors’ observations). We cannot explain the air-bone gaps observed in these conditions on the basis of the third-window hypothesis. The mechanism responsible for the CHL in these patients is unknown and needs further investigation.
DIAGNOSIS
Third-window lesions should be considered in the differential diagnosis of CHL especially when a patient presents with an intact tympanic membrane and an aerated, otherwise healthy middle ear. Individuals with third-window lesions can present with a CHL that can mimic otosclerosis or other middle ear disease. In some patients, exploratory tympanotomy and even stapedectomy or tympanoplasty has been mistakenly performed. A heightened awareness of the diagnostic possibility of third-window lesions should serve to reduce the number of patients who undergo negative middle ear explorations.
A numbers of clues and functional tests are available to the clinician for making an accurate diagnosis (Table 2). A low-frequency air-bone gap with bone conduction thresholds that are better than 0 dB (−5 to −20 dB) can be a clue especially in dehiscence of the semicircular canals (11,13–16,32). Therefore, it is important to accurately assess audiometric bone conduction thresholds to levels less than 0 dB hearing level. Acoustic reflexes are typically absent in true middle ear disease but are generally present in third-window lesions (9–11,13). Vestibular evoked myogenic potential testing with air-conducted sound and measurement of OAEs is also helpful. Vestibular evoked myogenic potential and OAE responses are typically absent in patients with true middle ear disease but may be present in third-window lesions (9–11,13,15). Measurement of umbo velocity by laser Doppler vibrometry can also help to differentiate lesions of the middle ear from the inner ear in patients with CHL (11,21,28). Vestibular manifestations comprising sound-induced or pressure-induced vertigo and eye movements may be evident in third-window lesions caused by canal dehiscences. When audiometric and other tests suggest that an inner ear lesion may be responsible for the CHL, an appropriate imaging study such as a CT scan will help to make a definitive diagnosis.
TABLE 2.
Differentiating middle ear from third-window lesions
| Lesion | Middle ear | Third-window lesion |
|---|---|---|
| Air-bone gap | 0–60 dB, may involve all frequencies | 0–60 dB, greatest at frequencies <2,000 Hz |
| Bone conduction thresholds | Rarely < 0 dB | May be negative (−5 to −20 dB or better) for frequencies <2,000 Hz |
| Acoustic reflex | Absent | Present |
| VEMP response | Absent | Present, thresholds lower than normal |
| OAEs | Absent | May be present |
| Umbo velocity on laser Doppler vibrometry | Variable: low normal-stapes fixation; abnormally low-malleus fixation; abnormally high-ossicular discontinuity | High normal |
| Sound- and/or pressure-induced vertigo | Absent | May be present |
| CT/MRI scan | May show middle ear abnormality | Inner ear lesion |
| Exploratory tympanotomy | Ossicular lesion, fixation or discontinuity | Normal ossicular mobility |
CT indicates computed tomography; MRI, magnetic resonance imaging; OAE, otoacoustic emission; VEMP, vestibular evoked myogenic potential.
Of the various functional tests described above, testing for the acoustic (stapedial) reflex is perhaps the most widely available and also relatively simple to administer. We recommend inclusion of acoustic reflex testing as part of the diagnostic work-up before middle ear exploration in a patient with CHL, an intact tympanic membrane, and an aerated middle ear.
NOMENCLATURE
The nomenclature used in the literature to describe hearing losses caused by third-window lesions is confusing. The term “air-bone gap” is appropriate because it accurately conveys the audiometric finding of a difference between air and bone conduction thresholds regardless of the source of the air-bone gap. One can further characterize an air-bone gap depending on the location of the problem, for example, in the external, middle, or inner ear. The term “pseudoconductive” hearing loss is ambiguous. It implies that the observed hearing loss is not conductive in nature. By default then, such a loss must be sensorineural. However, in patients with third-window lesions of the inner ear, the air-bone gap is clearly not the result of a lesion of the sensory or neural structures of the cochlea. Similarly, the term “cochlear conductive loss” is also ambiguous. The term was originally used to refer to a pure sensorineural hearing loss as determined by an audiogram wherein no abnormalities were observed in the inner ear at light microscopy that could explain the hearing loss (46). However, cochlear-conductive loss has also been used to refer to an air-bone gap as determined by audiometry that is thought to result from a mechanical problem within the cochlea (51).
We recommend avoidance of the terms “pseudo-conductive loss” and “cochlear conductive loss.” We propose that hearing losses should be classified as sensorineural hearing (no air-bone gap on audiometry) or conductive (presence of an air-bone gap on audiometry). Conductive hearing losses should be subclassified according to the site of lesion, if known, such as the external, middle, or inner ear.
Acknowledgments
The authors thank Axel Eliasen and Lakshmi Mittal for their support.
This study was supported by Grants R01 DC04798 and R01 DC000194 from the National Institutes of Health.
References
- 1.Bess FH, Miller GW, Glasscock ME, Bratt GW. Unexplained conductive hearing loss. South Med J. 1980;73:335–8. doi: 10.1097/00007611-198003000-00018. [DOI] [PubMed] [Google Scholar]
- 2.House JW, Sheehy JL, Antunez JC. Stapedectomy in children. Laryngoscope. 1980;90:1804–9. doi: 10.1288/00005537-198011000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Al Muhaimeed H, El Sayed Y, Rabah A, Al-Essa A. Conductive hearing loss: investigation of possible inner ear origin in three cases studies. J Laryngol Otol. 2002;116:942–5. doi: 10.1258/00222150260369507. [DOI] [PubMed] [Google Scholar]
- 4.Schuknecht HF. Otologic mystery. Am J Otol. 1987;8:182–3. [PubMed] [Google Scholar]
- 5.Snik AF, Hombergen GC, Mylanus EA, Cremers CW. Air-bone gap in patients with X-linked stapes gusher syndrome. Am J Otol. 1995;16:241–6. [PubMed] [Google Scholar]
- 6.Ranke OF, Keidel WD, Weschke H. Das Höeren bei Verschluss des Runden Fensters. Z Laryng. 1952;31:467–75. [PubMed] [Google Scholar]
- 7.Ranke O. Discussion remark to Von a. Meyer zum Gottesberg: Die Schalleitung im Mittelohr in klinischer Sicht. Z Laryng. 1958;37:366–7. [PubMed] [Google Scholar]
- 8.Tonndorf J, Tabor JR. Closure of the cochlear windows: its effect upon air- and bone-conduction. Ann Otol Rhinol Laryngol. 1962;71:5–29. doi: 10.1177/000348946207100101. [DOI] [PubMed] [Google Scholar]
- 9.Minor LB, Carey JP, Cremer PD, Lustig LR, Streubel SO, Ruckenstein MJ. Dehiscence of bone overlying the superior canal as a cause of apparent conductive hearing loss. Otol Neurotol. 2003;24:270–8. doi: 10.1097/00129492-200303000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Halmagyi GM, Aw ST, McGarvie LA, et al. Superior semicircular canal dehiscence simulating otosclerosis. J Laryngol Otol. 2003;117:553–7. doi: 10.1258/002221503322113003. [DOI] [PubMed] [Google Scholar]
- 11.Mikulec AA, McKenna MJ, Ramsey MJ, et al. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004;25:121–9. doi: 10.1097/00129492-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Mikulec AA, Poe DS, McKenna MJ. Operative management of superior semicircular canal dehiscence. Laryngoscope. 2005;115:501–7. doi: 10.1097/01.mlg.0000157844.48036.e7. [DOI] [PubMed] [Google Scholar]
- 13.Modugno G, Brandolini C, Savastio G, Ceroni AR, Pirodda A. Superior semicircular canal dehiscence: a series of 13 cases. ORL J Otorhinolaryngol Relat Spec. 2005;67:180–4. doi: 10.1159/000086573. [DOI] [PubMed] [Google Scholar]
- 14.Hillman TA, Kertesz TR, Hadley K, Shelton C. Reversible peripheral vestibulopathy: the treatment of superior canal dehiscence. Otolaryngol Head Neck Surg. 2006;134:431–6. doi: 10.1016/j.otohns.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Schmuziger N, Allum J, Buitrago-Téllez C, Probst R. Incapacitating hypersensitivity to one’s own body sounds due to a dehiscence of bone overlying the superior semicircular canal. A case report Eur Arch Otorhinolaryngol. 2006;263:69–74. doi: 10.1007/s00405-005-0939-9. [DOI] [PubMed] [Google Scholar]
- 16.Limb CJ, Carey JP, Srireddy S, Minor LB. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969–80. doi: 10.1097/01.mao.0000235376.70492.8e. [DOI] [PubMed] [Google Scholar]
- 17.Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN. Clinical, experimental and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25:323–32. doi: 10.1097/00129492-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Songer JE, Rosowski JJ. The effect of superior canal dehiscence on cochlear potential in response to air-conducted stimuli in chinchilla. Hear Res. 2005;210:53–62. doi: 10.1016/j.heares.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Songer JE, Rosowski JJ. The effect of superior-canal opening on middle-ear input admittance and air-conducted stapes velocity in chinchilla. J Acoust Soc Am. 2006;120:258–69. doi: 10.1121/1.2204356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchant SN, Rosowski JJ, McKenna MJ. Superior semicircular canal dehiscence mimicking otosclerotic hearing loss. Adv Otorhinolaryngol. 2007;65:137–145. doi: 10.1159/000098790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien W, Ravicz ME, Merchant SN, Rosowski JJ. Measurements of human middle and inner ear mechanics with dehiscence of the superior semicircular canal. Otol Neurotol. 2007;28:250–7. doi: 10.1097/01.mao.0000244370.47320.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Songer JE, Rosowski JJ. A mechano-acoustic model of the effect of superior canal dehiscence on hearing in chinchilla. J Acoust Soc Am. 2007;122:943–51. doi: 10.1121/1.2747158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Békésy GV. Akust Z. Vol. 1. 1936. Zur Physik des Mittelohres und über das Hören bei fehlerhaftem Trommelfell; pp. 13–23. [Google Scholar]; Békésy GV. In: Experiments in Hearing. Wever EG, editor. New York, NY: McGraw-Hill; 1960. pp. 104–115. [Google Scholar]
- 24.Stenfelt S, Goode RL. Bone-conducted sound: physiological and clinical aspects. Otol Neurotol. 2005;26:1245–61. doi: 10.1097/01.mao.0000187236.10842.d5. [DOI] [PubMed] [Google Scholar]
- 25.Wever EG, Lawrence M. Physiological Acoustics. Princeton, NJ: Princeton University Press; 1954. [Google Scholar]
- 26.Voss SE, Rosowski JJ, Peake WT. Is the pressure difference between the oval and round windows the effective acoustic stimulus for the cochlea? J Acoust Soc Am. 1996;100:1602–16. doi: 10.1121/1.416062. [DOI] [PubMed] [Google Scholar]
- 27.Gopen Q, Rosowski JJ, Merchant SN. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 28.Rosowski JJ, Nakijima HH, Merchant SN. Clinical utility of laser-Doppler vibrometer measurements in live normal and pathologic human ears. Ear Hear. doi: 10.1097/AUD.0b013e31815d63a5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–58. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 30.Merchant SN, Nakajima HH, Halpin C, et al. Clinical investigation and mechanism of air-bone gaps in large vestibular aqueduct syndrome. Ann Otol Rhinol Laryngol. 2007;116:532–41. doi: 10.1177/000348940711600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bance M. When is a conductive hearing loss not a conductive hearing loss? Causes of a mismatch in air-bone threshold measurements or a “pseudoconductive” hearing loss. J Otolaryngol. 2004;33:135–8. doi: 10.2310/7070.2004.00135. [DOI] [PubMed] [Google Scholar]
- 32.Mikulec AA, Poe DS. Operative management of a posterior semicircular canal dehiscence. Laryngoscope. 2006;116:375–8. doi: 10.1097/01.mlg.0000200358.93385.5c. [DOI] [PubMed] [Google Scholar]
- 33.Brantberg K, Bagger-Sjoback D, Mathiesen T, Witt H, Pansell T. Posterior canal dehiscence syndrome caused by an apex cholesteatoma. Otol Neurotol. 2006;27:531–4. doi: 10.1097/01.mao.0000201433.50122.62. [DOI] [PubMed] [Google Scholar]
- 34.Rajenderkumar D, Farrell KL, Alles RM, Savy L. Multiple dehiscence of semicircular canals. J Laryngol Otol. 2007;121:80–2. doi: 10.1017/S0022215106003537. [DOI] [PubMed] [Google Scholar]
- 35.Juers AL. Observations on bone conduction in fenestration cases. Ann Otol. 1948;57:28–40. doi: 10.1177/000348944805700102. [DOI] [PubMed] [Google Scholar]
- 36.Ribaric K, Bleeker JD, Wit HP. Perception of audio-frequency vibrations by profoundly deaf subjects after fenestration of the vestibular system. Acta Otolaryngol. 1992;112:45–9. doi: 10.3109/00016489209100781. [DOI] [PubMed] [Google Scholar]
- 37.Ribaric K, Kekic B, Dergenc R. On the capability of the vestibular apparatus to perceive sound stimuli. Acta Otolaryngol. 1992;112:221–4. doi: 10.1080/00016489.1992.11665408. [DOI] [PubMed] [Google Scholar]
- 38.Nance WE, Setleff R, McLeod A, Sweeney A, Cooper C, McConnell F. X-Linked mixed deafness with congenital fixation of the stapedial footplate and perilymphatic gusher. Birth Def. 1971;4:64–9. [PubMed] [Google Scholar]
- 39.Glasscock ME. The stapes gusher. Arch Otolaryngol. 1973;98:82–91. doi: 10.1001/archotol.1973.00780020088004. [DOI] [PubMed] [Google Scholar]
- 40.Phelps PD, Reardon W, Pembrey M, Bellman S, Luxom L. X-Linked deafness, stapes gushers and a distinctive defect of the inner ear. Neuroradiology. 1991;33:326–30. doi: 10.1007/BF00587816. [DOI] [PubMed] [Google Scholar]
- 41.Cremers CW, Snik AF, Huygen PL, Joosten FB, Cremers FP. X-linked mixed deafness syndrome with congenital fixation of the stapedial footplate and perilymphatic gusher (DFN3) Adv Otorhinolaryngol. 2002;61:161–7. doi: 10.1159/000066826. [DOI] [PubMed] [Google Scholar]
- 42.Talbot JM, Wilson DF. Computed tomographic diagnosis of X-linked congenital mixed deafness, fixation of the stapedial footplate, and perilymphatic gusher. Am J Otol. 1994;15:177–82. [PubMed] [Google Scholar]
- 43.Tang A, Parnes LS. X-linked progressive mixed hearing loss: computed tomography findings. Ann Otol Rhinol Laryngol. 1994;103:655–7. doi: 10.1177/000348949410300814. [DOI] [PubMed] [Google Scholar]
- 44.Kim HH, Wilson DF. A third mobile window at the cochlear apex. Otolaryngol Head Neck Surg. 2006;135:965–6. doi: 10.1016/j.otohns.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Karlberg M, Annertz M, Magnusson M. Mondini-like malformation mimicking otosclerosis and superior semicircular canal dehiscence. J Laryngol Otol. 2006;120:419–22. doi: 10.1017/S0022215106000934. [DOI] [PubMed] [Google Scholar]
- 46.Schuknecht HF. Pathology of the Ear. 2. Philadelphia, PA: Lea and Febiger; 1993. [Google Scholar]
- 47.Huang F, Sweet R, Tewfik TL. Apert syndrome and hearing loss with ear anomalies: a case report and literature review. Int J Pediatr Otorhinolaryngol. 2004;68:495–501. doi: 10.1016/j.ijporl.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Khetarpal U, Schuknecht HF. In search of pathologic correlates for hearing loss and vertigo in Paget’s disease. A clinical and histopathologic study of 26 temporal bones. Ann Otol Rhinol Laryngol. 1990;145:1–16. [PubMed] [Google Scholar]
- 49.Monsell EM. The mechanism of hearing loss in Paget’s disease of bone. Laryngoscope. 2004;114:598–606. doi: 10.1097/00005537-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muchnik C, Hildesheimer M, Rubinstein M, Arenberg IK. Low frequency air-bone gap in Ménière’s disease without middle ear pathology. A preliminary report. Am J Otol. 1989;10:1–4. [PubMed] [Google Scholar]
- 51.Govaerts PJ, Casselman J, Daemers K, De Ceulaer G, Somers Th, Offeciers FE. Audiological findings in large vestibular aqueduct syndrome. Int J Pediatr Otorhinolaryngol. 1999;51:157–64. doi: 10.1016/s0165-5876(99)00268-2. [DOI] [PubMed] [Google Scholar]