SUMMARY
Fetal stem cells differ phenotypically and functionally from adult stem cells in diverse tissues. However, little is known about how these differences are regulated. To address this we compared the gene expression profiles of fetal versus adult hematopoietic stem cells (HSCs) and discovered that the Sox17 transcriptional regulator is specifically expressed in fetal and neonatal but not adult HSCs. Germline deletion of Sox17 led to severe fetal hematopoietic defects, including a lack of detectable definitive HSCs. Conditional deletion of Sox17 from hematopoietic cells led to the loss of fetal and neonatal but not adult HSCs. HSCs stopped expressing Sox17 approximately 4 weeks after birth. During this transition, loss of Sox17 expression correlated with slower proliferation and the acquisition of an adult phenotype by individual HSCs. Sox17 is thus required for the maintenance of fetal and neonatal HSCs and distinguishes their transcriptional regulation from adult HSCs.
INTRODUCTION
Stem cells undergo discrete developmental changes throughout life but little is known about how these transitions are regulated. A particularly profound transition occurs between fetal development and adulthood, when stem cells from diverse tissues undergo changes in phenotype, function, and regulation (Molofsky et al., 2004). This raises the possibility that transcriptional programs that maintain stem cell identity and function change between fetal and adult life.
One cardinal feature of stem cells that changes with development is self renewal. Even self-renewal mechanisms that are broadly conserved among tissues fail to be maintained across developmental time (Molofsky et al., 2004). Oct4 and Nanog are required for the self renewal of embryonic stem cells but not fetal or adult somatic stem cells (Nichols et al., 1998; Chambers et al., 2003; Mitsui et al., 2003). Bmi-1 is required for the self renewal of every post-natal stem cell yet examined, including HSCs and neural stem cells, but it is not required in vivo for the self renewal of fetal stem cells in the same tissues (Lessard and Sauvageau, 2003; Molofsky et al., 2003; Park et al., 2003). Ink4a expression cannot be detected in fetal or young adult stem cells but increases with age in stem cells from diverse tissues, reducing self renewal potential and regenerative capacity (Janzen et al., 2006; Krishnamurthy et al., 2006; Molofsky et al., 2006). While a great deal has been learned about embryonic and adult stem cell self renewal, comparatively less is known about mechanisms that specifically maintain fetal stem cells.
Developmental changes in the properties of stem cells have been best described in hematopoiesis (Mikkola and Orkin, 2006). Fetal HSCs differ from adult HSCs in gene expression (Phillips et al., 2000; Ivanova et al., 2002), marker expression (Morrison et al., 1995; Kim et al., 2005), developmental potential (Ikuta et al., 1990; Kantor et al., 1992), self-renewal potential (Morrison et al., 1995; Harrison et al., 1997), and regulation (Park et al., 2003; Hock et al., 2004a, 2004b). HSCs transition from fetal to adult properties 3–4 weeks after birth, when HSCs suddenly become quiescent (Bowie et al., 2006).
It is not clear what regulates the unique properties of fetal HSCs. A number of genes including Scl (Porcher et al., 1996), Aml-1/Runx1 (Okuda et al., 1996; Wang et al., 1996), Lmo2 (Warren et al., 1994), Gata-2 (Tsai et al., 1994), cdx4 (Davidson et al., 2003), and mixed-lineage leukemia (Ernst et al., 2004) are required embryonically for the formation of HSCs. However, with the exception of Gata-2, there is little evidence that these genes play any ongoing role in the maintenance of HSCs after formation (Mikkola and Orkin, 2006). For example, Scl and Aml-1/Runx1 are dispensable for the maintenance of HSCs, at least in adulthood (Mikkola et al., 2003; Ichikawa et al., 2004). Other genes appear to regulate the maintenance of HSCs throughout fetal and adult life including Rae28 (Ohta et al., 2002; Kim et al., 2004), Meis1 (Hisa et al., 2004; Kirito et al., 2004; Azcoitia et al., 2005), c-myb (Mucenski et al., 1991; Sandberg et al., 2005), and Cbp (Rebel et al., 2002). However, to our knowledge no gene is known to regulate the maintenance of fetal but not adult HSCs. In contrast, a number of transcriptional regulators maintain adult but not fetal HSCs, including Gfi-1 (Hock et al., 2004a), Tel/Etv6 (Hock et al., 2004b), and Bmi-1 (Park et al., 2003). This raises the question of what transcriptional regulators act downstream of Scl and Aml-1/Runx1 to regulate fetal HSC identity and maintenance prior to the onset of the adult HSC self-renewal program.
Sry-related high mobility group box (Sox) transcription factors contain a DNA-binding domain (the HMG box) and regulate stem cell identity and function in multiple tissues (Schepers et al., 2002). Sox17 has been used as a marker of endodermal identity (Yasunaga et al., 2005) and is required for the formation and maintenance of endoderm (Hudson et al., 1997; Kanai-Azuma et al., 2002) and vascular endothelium (Matsui et al., 2006). But Sox family members have never been implicated in the regulation of HSCs. Here we report that within the hematopoietic system Sox17 is highly restricted in its expression to fetal and neonatal HSCs and is required for the maintenance of fetal and neonatal, but not adult HSCs. Sox17 is thus a marker of fetal identity in HSCs and distinguishes their transcriptional regulation from adult HSCs.
RESULTS
In this study we reexamined previously published gene expression profiles of highly purified fetal (Kiel et al., 2005a) and adult (Kiel et al., 2005b) mouse HSCs to identify genes that were more highly expressed in fetal as compared to adult HSCs. Sox17 was clearly detected in embryonic day (E)14.5 fetal liver HSCs, but not in CD45+ fetal liver hematopoietic cells, young adult bone marrow HSCs, young adult bone marrow CD45+ cells, or old adult bone marrow HSCs (Figure S1). We confirmed this by quantitative real-time RT-PCR (Figure S1). We hypothesized that Sox17 regulates fetal HSC identity or function.
Generation of Sox17-Deficient Mice
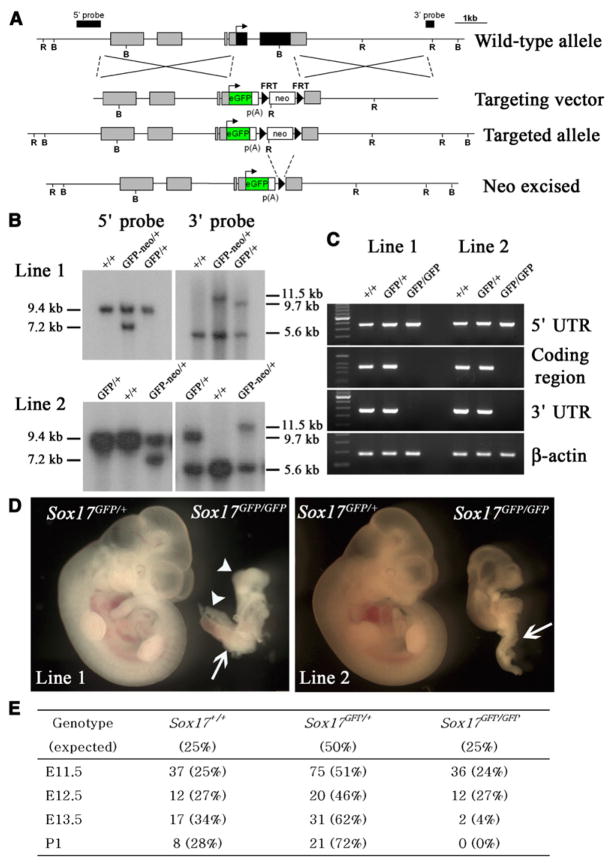
To test whether Sox17 regulates fetal HSCs we generated Sox17 knockout mice. The entire coding sequence of Sox17 was replaced with the gene encoding enhanced green fluorescence protein (eGFP), in frame with the Sox17 start codon, by homologous recombination (Figures 1A and 1B). Two mouse lines were generated using independently targeted ES cell lines, both of which gave germline transmission of Sox17GFP alleles. We could not detect mRNA that contained the Sox17 coding sequence in Sox17GFP/GFP mice by RT-PCR (Figure 1C). Sox17GFP/GFP mice from both lines showed severe growth retardation relative to littermate controls by E11.5, as well as posterior patterning and body axis rotation defects (Figure 1D). In both lines, Sox17GFP/GFP mice were observed in expected ratios through E12.5 but died by E13.5 (Figure 1E). This phenotype is consistent with that of independently targeted Sox17-deficient mice that also died embryonically with growth retardation and posterior patterning defects (Kanai-Azuma et al., 2002).
Figure 1. Generation of Sox17GFP/GFP Mice.
(A) Schematic representation of the Sox17 targeted allele. The genomic structure after neo-cassette removal is shown on the fourth line. B and R indicate BamHI and EcoRV digestion sites, respectively.
(B) Genotypes of heterozygous F1 mice (before neo excision) and F2 mice (after neo excision) from two independent lines were confirmed by Southern blot. Tail genomic DNA was digested with EcoRV and BamHI for 5′ and 3′ probe hybridization, respectively.
(C) The lack of Sox17 expression in Sox17GFP/GFP embryos from both lines of mice was confirmed by RT-PCR using primers that amplified the Sox17 5′ untranslated region (which was expressed from the targeted allele), the coding sequence (not expressed), and the Sox17 3′ untranslated region (not expressed).
(D) At E11.5, Sox17GFP/GFP embryos were much smaller than Sox17GFP/+ embryos and exhibited severe defects in posterior patterning (arrow) and body axis rotation (arrowheads).
(E) Genotypes of progeny from Sox17GFP/+ intercrosses revealed Mendelian inheritance up to E12.5 but loss of Sox17-deficienct embryos by E13.5.
Sox17 Deficiency Severely Reduces Fetal Hematopoiesis
In addition to recapitulating the previously described gross phenotypes associated with Sox17 deficiency, we observed a severe defect in fetal hematopoiesis that was not reported previously. Sox17GFP/GFP mice had some blood cells and hemorrhaging but did not develop visible hematopoiesis as observed in control littermates (Figures 1D and 2B). This failure of hematopoiesis affected the yolk sac (Figure 2A) as well as the fetal liver (Figure 2B) as both structures were pale in Sox17GFP/GFP mice. Sox17GFP/GFP mice had significantly (p < 0.05) fewer CD45+ blood cells and Ter119+ erythroid cells in both the yolk sac and whole embryo as compared to littermate controls (Figure 2C). Sox17GFP/GFP mice also exhibited significantly fewer colony-forming progenitors in yolk sac and whole embryo (CFU-C) (Figure 2D). All types of CFU-C were similarly reduced in number (data not shown). The fact that these defects were most severe intraembryonically suggests that Sox17 is particularly important for definitive hematopoiesis.
Figure 2. Sox17 Is Required for Hematopoiesis in the Fetal Liver and Yolk Sac.
The yolk sac (A) and fetal liver (B) of E11.5 embryos contained obvious blood cells in Sox17GFP/+ embryos but not in Sox17GFP/GFP littermates. The numbers of CD45+ hematopoietic cells and Ter119+ erythroid cells in E11.5 yolk sac and whole embryo were significantly (*, p < 0.05) reduced in Sox17GFP/GFP embryos as compared to Sox17GFP/+ and Sox17+/+ embryos (C, three independent experiments). The numbers of colony-forming progenitors (CFU-C) in E11.5 yolk sac and whole embryo were also significantly (#, p < 0.01) reduced in Sox17GFP/GFP embryos as compared to Sox17GFP/+ and Sox17+/+ embryos (D, three independent experiments).
In the Hematopoietic System Sox17 Is Mainly Expressed by Fetal and Neonatal HSCs
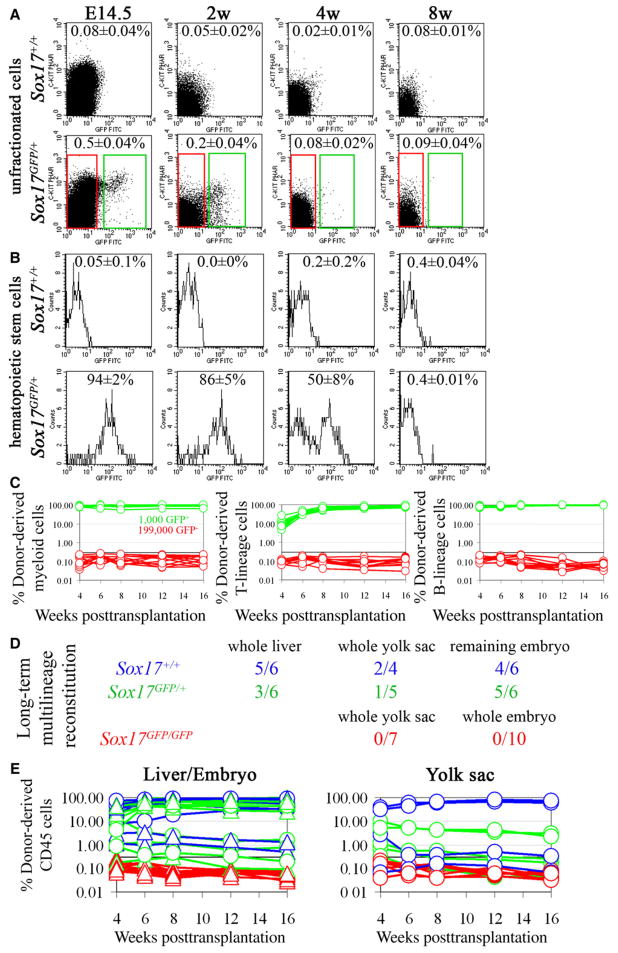
To determine how broadly Sox17 is expressed we examined GFP fluorescence in Sox17GFP/+ mice. Only 0.5% of E14.5 fetal liver cells were GFP+, and this percentage declined over time, with only 0.2% GFP+ cells in bone marrow from 2-week-old mice and levels similar to background in bone marrow from 4- and 8-week-old mice (Figure 3A).
Figure 3. Sox17 Is Expressed by, and Required for the Maintenance of, Fetal and Neonatal But Not Adult HSCs.
(A) Sox17 expression in fetal liver or bone marrow cells was analyzed by flow cytometry in Sox17GFP/+ mice (five independent experiments). Cells from Sox17+/+ mice established background. Less than 1% of fetal liver and neonatal bone marrow cells expressed Sox17 (green boxes).
(B) HSCs were isolated from fetal liver as Sca-1+lineage−Mac-1+CD48− cells and from bone marrow as Sca-1+lineage−c-kit+CD48− cells. Most fetal liver and neonatal bone marrow HSCs expressed Sox17, but expression began to decline by 4 weeks of age and was no longer evident at 8 weeks of age (five independent experiments).
(C) Irradiated mice were transplanted with 1,000 GFP+ fetal liver cells (green lines) or 199,000 GFP− fetal liver cells (red lines) from E14.5 Sox17GFP/+ donor mice. All mice transplanted with GFP+ cells were long-term multilineage reconstituted by donor cells (n = 12; each line represents a single mouse) but none of the mice transplanted with GFP− cells showed detectable reconstitution (n = 11 mice; the black line at 0.3% represents background).
(D) Irradiated recipient mice were transplanted with unfractionated fetal liver, yolk sac, or remaining embryo cells from single E12.5 Sox17+/+, Sox17GFP/+, or Sox17GFP/GFP embryos. Numbers indicate the fraction of recipient mice that were long-term multilineage reconstituted (>16 weeks) by donor cells.
(E) While control cells usually gave long-term multilineage reconstitution (blue and green lines), we never detected any reconstitution by Sox17GFP/GFP cells (red lines).
To examine GFP expression in fetal HSCs we examined Sca-1+Lineage−Mac-1+CD48− cells from E14.5 fetal liver, which represented 0.030 ± 0.006% of fetal liver cells in these experiments. These cells include all fetal liver HSC activity and are highly enriched for HSCs (Kim et al., 2006). Ninety-four percent (two percent standard deviation) of Sca-1+Lineage−Mac-1+CD48− cells were GFP+ in Sox17GFP/+ mice (Figure 3B). To functionally test whether all E14.5 fetal liver HSCs express Sox17, we transplanted 1,000 GFP+ fetal liver cells or 199,000 GFP− fetal liver cells from Sox17GFP/+ mice into irradiated recipient mice in a competitive reconstitution assay. All 12 of the mice transplanted with GFP+ cells showed long-term multilineage reconstitution by donor cells, while none of 11 mice transplanted with GFP− cells showed any donor cell reconstitution (Figure 3C). These data demonstrate that all, or virtually all, fetal liver HSCs express Sox17.
To examine GFP expression in postnatal bone marrow HSCs, we examined Sca-1+Lineage−c-kit+CD48− cells from the bone marrow of 2-, 4-, and 8 week-old mice. These cells represented 0.019% ± 0.004% of bone marrow cells at 2 weeks but fell to 0.004% ± 0.0004% of bone marrow cells by 8 weeks of age. This population contains all of the detectable HSC activity in adult bone marrow and is highly enriched for HSCs (Kiel et al., 2005b; Yilmaz et al., 2005). While 86% ± 5% of bone marrow Sca-1+Lineage−c-kit+CD48− cells in 2-week-old mice expressed GFP, this percentage dropped to 50% ± 8% in 4-week-old mice and to undetectable levels in 8-week-old mice (Figure 3B). Sox17 thus continues to be expressed by neonatal, but not adult, HSCs.
Some CFU-C also expressed Sox17 in the fetal liver. All cells that formed CFU-GEMM colonies (containing granulocytes, erythrocytes, macrophages, and megakaryocytes) in culture arose from GFP+ fetal liver cells, as did 65% ± 2% of all colony-forming progenitors (CFU-C) from fetal liver (data not shown). By 2 weeks after birth, only 18% ± 4% of CFU-GEMM and 9% ± 3% of all CFU-C arose from GFP+ bone marrow cells. We have not been able to detect GFP+ bone marrow cells in adults (Figure 3A). Sox17 is thus expressed by fetal and neonatal HSCs as well as some downstream progenitors.
To test whether Sox17 expression is induced in proliferating adult HSCs we treated adult Sox17GFP/+ mice with cyclophosphamide/G-CSF (Morrison et al., 1997). Although HSCs were driven into cycle, expanded in number, and mobilized after cyclophosphamide/G-CSF treatment, GFP was not expressed by HSCs in the bone marrow or the spleen (Figure S2). This indicates that Sox17 is not simply expressed by dividing HSCs and cannot be induced in adult HSCs by proliferation or mobilization.
Sox17 Is Required for the Generation or Maintenance of Fetal HSCs
To test whether Sox17 is required for HSC function during fetal development we generated E12.5 Sox17+/+, Sox17GFP/+, or Sox17GFP/GFP embryos and transplanted yolk sac, fetal liver, or dissociated cells from the rest of the embryo into irradiated recipients in competitive reconstitution assays. Each CD45.1+ recipient mouse was transplanted with all of the yolk sac, liver cells, or remaining embryo cells from a single CD45.2+ donor embryo. The livers of Sox17GFP/GFP embryos were so hypocellular and pale that the entire embryos were dissociated and transplanted, rather than just fetal liver. Most of the recipients that were transplanted with liver, yolk sac, or other cells from Sox17+/+ or Sox17GFP/+ embryos became long-term multilineage reconstituted by donor cells (Figures 3D and 3E). In contrast, none of the recipients transplanted with cells from Sox17GFP/GFP embryos ever became reconstituted by donor cells. Sox17 is thus required for the generation or maintenance of definitive HSCs.
Sox17 Is Required within the Hematopoietic/Endothelial Lineages for HSCs
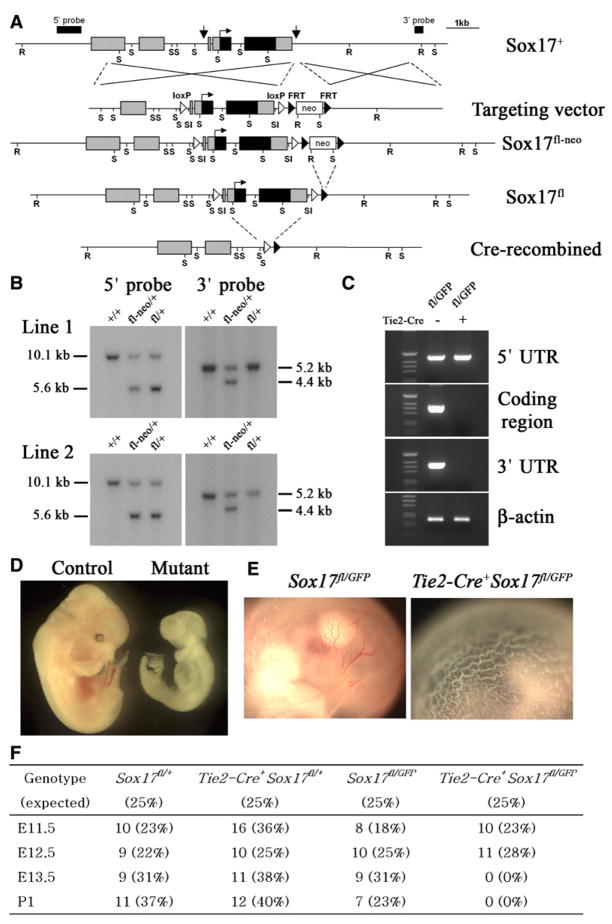
To test whether Sox17 is required autonomously for the generation or maintenance of HSCs we generated a floxed allele (Sox17fl) for the conditional deletion of Sox17 (Figures 4A–4C). The Sox17fl allele provided normal Sox17 function prior to recombination as Sox17fl/GFP mice (lacking Cre) were born in expected numbers and appeared developmentally normal (Figure 4F). Moreover, intercrosses between Sox17fl/+ mice led to the birth of Sox17fl/fl mice in expected numbers (Figure S3). Sox17fl/fl mice were developmentally normal, had normal blood cell counts, and were fertile (Figure S3).
Figure 4. Conditional Deletion of Sox17 in Hematopoietic/Endothelial Cells Leads to Severe Hematopoietic Defects and Lethality by E13.5.
(A) Targeting of Sox17 to generate a floxed allele. Arrows indicate the sites in the wild-type allele (Sox17+) where loxP elements were inserted. These sites were selected to avoid disrupting conserved sequences (potential regulatory elements). Note that Cre-mediated recombination removes the entire Sox17 coding sequence.
(B)The genotypes of heterozygous F1(Sox17fl-neo) and F2 (Sox17fl) mice from two independent lines were verified by Southern blot. Genomic tail DNA was digested with EcoRV (R) and SalI (Sl) for the 5′ probe and with SacI (S) for the 3′ probe.
(C) The lack of Sox17 expression in CD144+ endothelial cells sorted from E10.5 Tie2-Cre+Sox17fl/GFP embryos was confirmed by RT-PCR using primers that amplified the Sox17 5′ untranslated region (expressed from the targeted allele), the coding sequence (not expressed) and the Sox17 3′ untranslated region (not expressed).
(D) E12.5 Tie2-Cre+Sox17fl/GFP embryos were pale and growth retarded and lacked visible hematopoiesis.
(E) Unlike control embryos, the yolk sac from E12.5 Tie2-Cre+Sox17fl/GFP embryos lacked visible hematopoiesis.
(F) Progeny derived from mating Tie2-Cre+Sox17GFP/+ males with Sox17fl/fl females: conditional deletion of Sox17 using Tie2-Cre was lethal by E13.5.
To test whether Sox17 was required within the hematopoietic/endothelial lineages for the generation of HSCs we conditionally deleted Sox17 in endothelial and hematopoietic progenitors using Tie2-Cre (Koni et al., 2001). Tie2-Cre mice express Cre recombinase in endothelial cells and HSCs during embryonic development (Schlaeger et al., 2005; Li et al., 2006). E12.5 Tie2-Cre+Sox17fl/GFP embryos were growth retarded and lacked visible hematopoiesis in the liver and yolk sac, but did not have the obvious posterior patterning or axial turning defects observed in Sox17GFP/GFP embryos (Figures 4D and 4E). Like Sox17GFP/GFP embryos, Tie2-Cre+Sox17fl/GFP embryos were observed in expected numbers through E12.5 but were dead by E13.5 (Figure 4F).
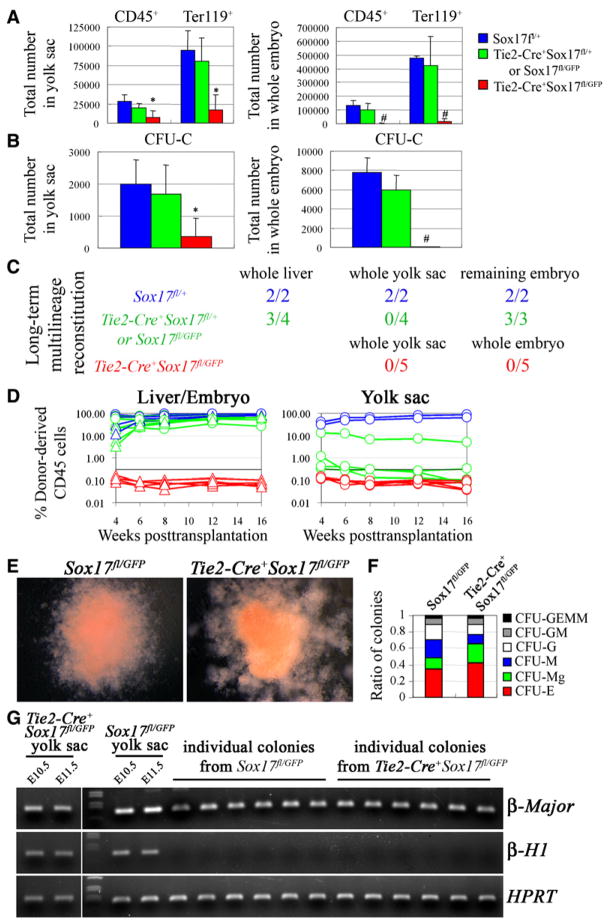
The numbers of CD45+, Ter119+ cells (Figure 5A), and CFU-C (Figure 5B) were also dramatically reduced in the yolk sac and embryo of E11.5 Tie2-Cre+Sox17fl/GFP mice as compared to controls. As in germline knockouts, these defects were most severe intraembryonically. Tie2-Cre+Sox17fl/GFP embryos also lacked any reconstituting activity when yolk sac or embryo cells were transplanted into irradiated mice, in contrast to littermate controls (Figure 5C). Sox17 is thus required in endothelial/hematopoietic cells for the generation or maintenance of definitive HSCs.
Figure 5. Conditional Deletion of Sox17 Using Tie2-Cre Leads to a Failure to Generate or Maintain HSCs.
The numbers of CD45+ or Ter119+ cells (A) and colony-forming progenitors (CFU-C; B) were dramatically reduced in the yolk sac and embryo of E11.5 Tie2-Cre+Sox17fl/GFP mice as compared to littermate controls (*, p < 0.05; #, p < 0.01; four independent experiments).
(C) Irradiated wild-type mice were transplanted with unfractionated fetal liver, yolk sac, or remaining embryo cells from single E12.5 Tie2-Cre+Sox17fl/GFP embryos or littermate controls. The livers of Tie2-Cre+Sox17fl/GFP embryos were so hypocellular and pale that the entire embryos were dissociated and transplanted, rather than just fetal liver. Numbers indicate the fraction of recipient mice that were long-term multilineage reconstituted by donor cells.
(D) While control cells usually gave long-term multilineage reconstitution (blue and green lines), we never detected any reconstitution above background (0.3%) from Tie2-Cre+Sox17fl/GFP cells (red lines).
(E) CFU-GEMM colonies from E11.5 Sox17fl/GFP and Tie2-Cre+Sox17fl/GFP yolk sac were similar in size and appearance.
(F) Sox17 deletion also did not affect the proportions of colony types, though the absolute number of all colonies was reduced (three independent experiments).
(G) E11.5 Tie2-Cre+Sox17fl/GFP and Sox17fl/GFP yolk sac cells expressed embryonic (β-H1) and adult (β-major) hemoglobin in vivo. In culture, all colonies from Sox17fl/GFP yolk sac (19/19) and Tie2-Cre+Sox17fl/GFP yolk sac (20/20) expressed adult hemoglobin (β-major). PCR on genomic DNA from individual colonies demonstrated that 95% of Tie2-Cre+Sox17fl/GFP colonies (19/20) had completely recombined Sox17 (data not shown).
Sox17-Deficient Progenitors Can Differentiate Normally and Form Definitive Erythrocytes
To test whether Sox17-deficient hematopoietic progenitors were blocked in their ability to differentiate or to transition from primitive to definitive hematopoiesis, we examined the rare colonies that arose in culture from E11.5 Tie2-Cre+Sox17fl/GFP yolk sac cells. Although the number of colonies was significantly reduced as compared to littermate controls (Figure 5B), the types of colonies that formed, the size of colonies, their appearance, and their composition did not differ between Tie2-Cre+Sox17fl/GFP mice and controls (Figures 5E and 5F). Flow-cytometric analysis revealed normal numbers of Ter119+ erythrocytes and Mac-1+ myeloid cells within colonies from Tie2-Cre+Sox17fl/GFP mice (data not shown). RT-PCR on freshly dissected, uncultured yolk sac cells revealed both embryonic (β-H1) and adult (β-major) hemoglobin expression from wild-type and Tie2-Cre+Sox17fl/GFP yolk sac cells in vivo (Figure 5G). All colonies from Tie2-Cre+Sox17fl/GFP mice and controls expressed adult but not embryonic hemoglobin (Figure 5G). Thus, hematopoietic progenitors are greatly reduced in number in Tie2-Cre+Sox17fl/GFP mice but appear to differentiate normally, consistent with a defect in HSC formation or maintenance.
Sox17 Is Cell-Autonomously Required for the Maintenance of Definitive HSCs
To test whether Sox17 is required for the ongoing maintenance of HSCs, or just for their initial formation, we generated Mx-1-Cre+Sox17fl/GFP mice. We treated newborn Mx-1-Cre+Sox17fl/GFP mice and littermate controls (that included both Mx-1-Cre−Sox17fl/GFP and Mx-1-Cre+Sox17fl/+ mice) with three injections of pIpC on days 2, 4, and 6 after birth to induce Cre expression (Hock et al., 2004b). This led to the death of all Mx-1-Cre+ Sox17fl/GFP mice by 14 days after birth, while all littermate controls survived (Figure 6A). In separate experiments, neonatal Mx-1-Cre+Sox17fl/GFP mice and littermate controls were sacrificed 4–5 days after ending pIpC treatment to test whether there was any effect on hematopoiesis. Mx-1-Cre+Sox17fl/GFP mice all exhibited significant (p < 0.05) reductions relative to littermate controls in bone marrow cellularity, spleen cellularity, numbers of Flk2−Sca-1+Lineage−c-kit+CD48− HSCs, and numbers of CFU-C in the bone marrow and spleen (Figures 6B–6D). These mice also had severely reduced thymus cellularity and significantly reduced white blood cell and platelet counts (data not shown). While CFU-C were substantially depleted after Sox17 deletion, the types of colonies they formed and the appearance and composition of the colonies did not differ from control cells (data not shown). The depletion of Flk2−Sca-1+Lineage−c-kit+CD48− HSCs after Sox17 deletion indicates that Sox17 is required for the maintenance of HSCs.
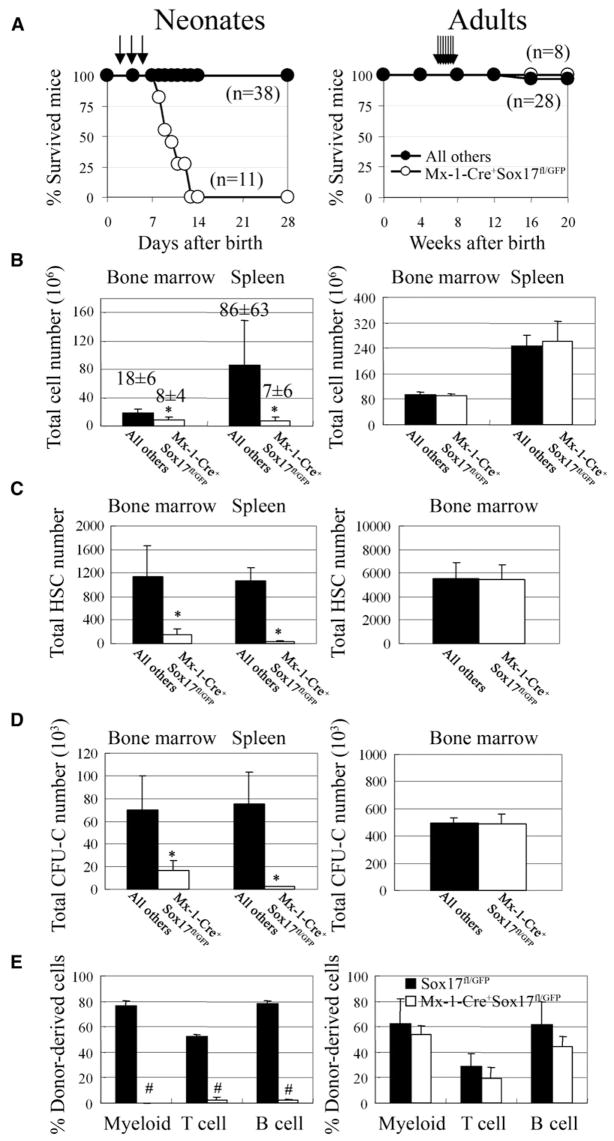
Figure 6. Sox17 Is Autonomously Required for the Maintenance of Neonatal But Not Adult HSCs.
(A) Mx-1-Cre+Sox17fl/GFP mice and littermate controls were administered pIpC 2, 4, and 6 days after birth (arrows). All Mx-1-Cre+ Sox17fl/GFP mice died by 14 days after birth, but no littermate controls died. Adult Mx-1-Cre+Sox17fl/GFP mice that were administered seven doses of pIpC over a 14 day period beginning 6 weeks after birth all survived.
(B–C) Four to five days after ending pIpC treatment in neonates and seven days after ending pIpC treatment in adults, bone marrow cellularity in the tibias and femurs and spleen cellularity were significantly (*, p < 0.05) reduced in neonatal Mx-1-Cre+Sox17fl/GFP mice (n = 5–7) as compared to littermate controls (n = 4–6) but were not affected in adult Mx-1-Cre+Sox17fl/GFP mice (n = 4–5) as compared to littermate controls (n = 4–5). The absolute numbers of Flk2−Sca-1+Lineage−c-kit+CD48− HSCs (C) and colony-forming progenitors (CFU-C; D) in the bone marrow (from tibias and femurs) and spleen were also significantly (p < 0.05) reduced in the same neonatal Mx-1-Cre+Sox17fl/GFP mice but were not affected in the adult Mx-1-Cre+Sox17fl/GFP mice.
(E) Bone marrow cells from pIpC-treated neonatal Mx-1-Cre+Sox17fl/GFP mice (CD45.2+) failed to give long-term multilineage reconstitution in irradiated CD45.1+ wild-type recipients (n = 5), in contrast to the same dose of cells from littermates or adult mice (n = 4 or 5; differences in myeloid, B, and T cell chimerism were highly statistically significant: #, p < 0.001; 6 week time point is shown). In each case, 200,000 donor bone marrow cells were competed against 200,000 recipient bone marrow cells.
To further test if Sox17 is autonomously required for the maintenance of HSCs, whole bone marrow cells were transplanted from pIpC-treated neonatal Mx-1-Cre+ Sox17fl/GFP mice or littermate controls into irradiated wild-type recipients along with wild-type whole bone marrow cells. Bone marrow cells taken from Mx-1-Cre+Sox17fl/GFP mice 4–5 days after pIpC treatment initially gave low levels of multilineage reconstitution, but multilineage reconstitution could no longer be detected by 6 weeks after transplantation (Figure 6E). In contrast, the same dose of bone marrow cells from littermate controls gave high levels of multilineage reconstitution in all recipients (Figure 6E). By performing PCR on genomic DNA extracted from individual CFU-GEMM or CFU-GM colonies that arose from the bone marrow of the donor mice used in these experiments we determined that 81% of early hematopoietic progenitors from the neonatal Mx-1-Cre+Sox17fl/GFP mice had deleted Sox17 (Figure S4). The failure of Sox17-deficient bone marrow cells to long-term multilineage reconstitute wild-type recipients indicates that Sox17 is autonomously required for the maintenance of neonatal HSCs.
We also tested whether fetal HSCs require Sox17 for their maintenance in a wild-type environment in a way that is independent of their ability to home to the bone marrow after transplantation. We transplanted 200,000 unfractionated fetal liver cells from E14.5 Sox17fl/+ or Mx-1-Cre+Sox17fl/GFP embryos into irradiated wild-type adult recipients along with a radioprotective dose of wild-type bone marrow cells. pIpC was administered to all recipient mice 5, 7, and 9 days after transplantation; then, the peripheral blood was analyzed 4 weeks after transplantation. High levels of multilineage reconstitution were observed from the control (Sox17fl/+) donor cells in all eight recipient mice: 80% ± 9% of all CD45+ cells were donor derived in these mice 6 weeks after transplantation (Figure S5). In contrast, none of seven recipient mice transplanted with Mx-1-Cre+Sox17fl/GFP cells were stably multilineage reconstituted: only 5% ± 10% of all CD45+ cells were donor derived 6 weeks after transplantation (Figure S5). Deletion of Sox17 from fetal HSCs that have already engrafted in a wild-type environment leads to a near complete loss of reconstituting potential, indicating that Sox17 is autonomously required for the maintenance of fetal HSCs.
Since Sox17 expression is extinguished in HSCs around 4 weeks after birth (Figure 3B) we also administered pIpC to 6-week-old mice to test whether adult HSCs are no longer Sox17 dependent. None of eight Mx-1-Cre+Sox17fl/GFP mice died, though one of twenty-eight control mice died (Figure 6A). In separate experiments, Mx-1-Cre+Sox17fl/GFP mice and littermate controls were sacrificed 1 week after ending pIpC. We observed no difference between Mx-1-Cre+Sox17fl/GFP mice and littermate controls in bone marrow cellularity, spleen cellularity (Figure 6B), number of Flk2−Sca-1+Lineage−c-kit+CD48− HSCs in the bone marrow (Figure 6C), numbers of CFU-C in the bone marrow (Fig. 6D), or ability of bone marrow cells to reconstitute irradiated mice (Figure 6E). We also treated adult Mx-1-Cre+Sox17fl/GFP mice and littermate controls (after pIpC treatment) with cyclophosphamide/G-CSF to mobilize HSCs but again detected no effect of Sox17 deficiency on bone marrow cellularity, spleen cellularity, HSC frequency, or HSC cell cycle status (Figure S2). By performing PCR on genomic DNA extracted from colonies that arose in culture from sorted Flk2−Sca-1+Lineage−c-kit+CD48− HSCs we confirmed that 96% of the HSCs from adult Mx-1-Cre+Sox17fl/GFP mice had deleted Sox17 (Figure S4). Sox17 is no longer required for adult HSC maintenance, proliferation, mobilization, or hematopoiesis.
Sox17 Deletion Leads to the Death of Neonatal HSCs
To further investigate the mechanism by which Sox17 deletion leads to the loss of HSCs, we examined Sca-1+Lineage−c-kit+CD48− HSCs isolated from the bone marrow of neonatal Mx-1-Cre+Sox17fl/GFP mice or littermate controls 5 days after pIpC treatment. Sox17 deletion had no immediate effect on the cell cycle distribution of HSCs (Figure 7A); however, Sox17 deletion did significantly (p < 0.05) increase the rate of cell death among HSCs (Figure 7B).
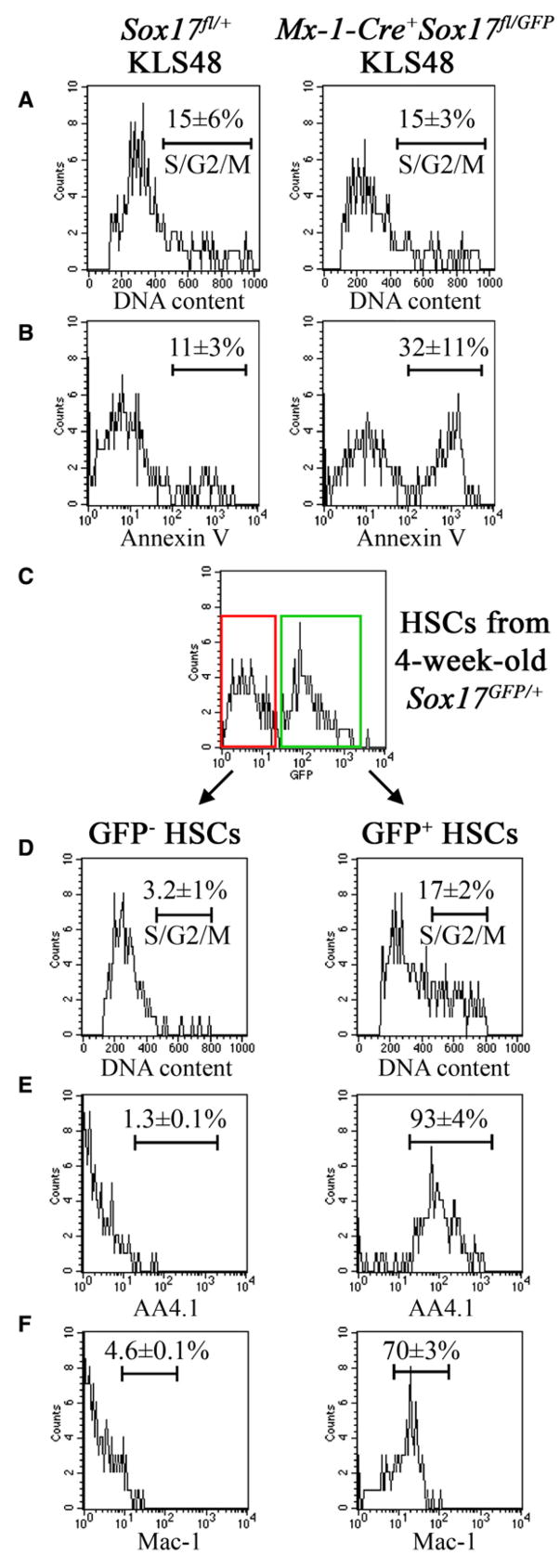
Figure 7. Sox17 Deletion from Neonatal HSCs Induces Cell Death, and the Postnatal Decline in Sox17 Expression by Wild-Type HSCs Is Associated with a Transition to an Adult Phenotype.
(A and B) Sca-1+Lineage−c-kit+CD48− HSCs isolated from the bone marrow of neonatal Mx-1-Cre+Sox17fl/GFP mice 5 days after pIpC treatment exhibited normal cell-cycle distribution relative to littermate controls (A) but a 3-fold increased frequency of cells undergoing cell death (Annexin V+; B).
(C–F) GFP+ (Sox17-expressing) and GFP− (Sox17-non-expressing) Sca-1+Lineage−c-kit+CD48− HSCs were isolated from the bone marrow of 3.5 to 4 week-old Sox17GFP/+ mice. GFP− Sca-1+Lineage−c-kit+CD48− HSCs were dividing less rapidly (D), and failed to express AA4.1 (E) or Mac-1 (F), consistent with an adult HSC phenotype. In contrast, GFP+ Sca-1+Lineage−c-kit+CD48− HSCs were more rapidly dividing (D), and expressed AA4.1 (E) and Mac-1 (F), consistent with a fetal HSC phenotype.
To begin to address the molecular mechanisms by which Sox17 promotes the maintenance of HSCs, we compared the expression of candidate genes by hematopoietic progenitors isolated from Sox17-deficient or control mice. We first tested whether Sox17 was required for the expression of Runx1, Scl, Gata-2, or Lmo2. However, all of these genes were expressed at approximately wild-type levels in both CD45+c-kit+ progenitors and CD45+c-kit− hematopoietic cells from E11.5 Tie2-Cre+Sox17fl/GFP embryos and yolk sacs (Figure S6). Sox17 was therefore not required for the expression of genes involved in the formation of HSCs, nor for the expression of other critical regulators of HSC function including c-Myb and Gfi-1 (Figure S6). In striking contrast, Dickkopf1 (Dkk1) expression was dramatically elevated, by 142-fold in CD45+c-kit+ progenitors from E11.5 Tie2-Cre+Sox17fl/GFP embryos (Figure S6). Dkk1 expression was also elevated 3.4-fold in the same population from yolk sac. Since Dkk1 is a powerful negative regulator of Wnt pathway activation (Glinka et al., 1998) these data raise the possibility that Sox17 is required to maintain Wnt signaling in HSCs.
To further explore this possibility we examined gene expression in c-kit+Lineage−CD48− hematopoietic progenitors from the bone marrow of neonatal Mx-1-Cre+Sox17fl/GFP mice or littermate controls 3 days after pIpC treatment. Out of 15 genes examined, including those listed above as well as Bmi-1, Etv6, Rae28, Meis1, and Cpb, the only gene that showed more than a 2-fold change in expression after Sox17 deletion was Dkk1 (3.3 ± 0.4-fold increased expression). Thus Dkk1 expression was elevated in every context in which Sox17 deficiency led to a loss of HSCs. These data raise the possibility that Sox17 may promote the maintenance of fetal and neonatal HSCs by promoting Wnt signaling.
Sox17 Expression Is Associated with the Maintenance of Fetal HSC Properties
To test if the decline in Sox17 expression in wild-type postnatal HSCs is associated with the acquisition of adult properties we examined 4-week-old Sox17GFP/+ mice. At this time HSCs are making the transition from a rapidly proliferating fetal phenotype to a quiescent adult phenotype (Bowie et al., 2006), and approximately 50% of Sca-1+Lineage−c-kit+CD48− HSCs express Sox17 (Figure 3B). To test whether the loss of Sox17 expression is associated with the loss of the rapidly proliferating phenotype, we compared the cell-cycle status of GFP+ and GFP− Sca-1+Lineage−c-kit+CD48− cells from Sox17GFP/+ mice. While GFP+ Sca-1+Lineage−c-kit+CD48− cells remained rapidly proliferating, with 17% ± 2% of cells in S/G2/M phase of the cell cycle, GFP− Sca-1+Lineage−c-kit+CD48− cells were dividing significantly more slowly, with only 3% ± 1% of cells in S/G2/M (Figure 7D). This demonstrates that the loss of Sox17 expression is associated with the acquisition of a more slowly dividing adult phenotype.
To test whether the loss of Sox17 expression is also associated with the acquisition of an adult surface marker phenotype, we compared the expression of AA4.1 and Mac-1 between the GFP+ and GFP− fractions of Sca-1+Lineage−c-kit+CD48− cells from Sox17GFP/+ mice. Mac-1 and AA4.1 are expressed by fetal but not adult HSCs (Morrison and Weissman, 1994; Morrison et al., 1995; Phillips et al., 2000). Note that when sorting Sca-1+Lineage−c-kit+CD48− cells from neonatal mice we did not include Mac-1 in the lineage cocktail. While GFP+ Sca-1+Lineage−c-kit+CD48− cells were mainly Mac-1+ and AA4.1+, GFP− Sca-1+Lineage−c-kit+CD48− cells were mainly Mac-1− and AA4.1− (Figures 7E and 7F). This demonstrates that the loss of Sox17 expression is associated with the acquisition of an adult surface marker phenotype by HSCs.
DISCUSSION
The discovery that Sox17 maintains fetal but not adult HSCs fills a critical void in our understanding of HSC regulation. Prior studies identified genes like Scl, Aml-1/Runx1, Lmo2, and Gata-2 that are required for the formation of fetal HSCs (Warren et al., 1994; Okuda et al., 1996; Porcher et al., 1996; Wang et al., 1996; Mikkola et al., 2003; Ichikawa et al., 2004), and genes like Gfi-1, Tel/Etv6, and Bmi-1 that are required for the maintenance of adult but not fetal HSCs (Park et al., 2003; Hock et al., 2004a; Hock et al., 2004b). However, we are not aware of other genes that are required to maintain fetal HSCs prior to the acquisition of adult HSC properties.
Our data suggest that Sox17 acts downstream of Scl, Aml-1/Runx1, Lmo2, and Gata-2 to maintain fetal HSCs prior to the acquisition of an adult phenotype. Quantitative RT-PCR on RNA from CD45+c-kit+ hematopoietic progenitors from E11.5 Sox17-deficient embryos showed normal or slightly elevated levels of Scl, Aml-1/Runx1, Lmo2, and Gata-2 transcripts (Figure S6). This demonstrates that Sox17 is not required for the expression of Scl, Aml-1/Runx1, Lmo2, or Gata-2 in embryonic hematopoietic cells around the time that definitive HSCs are emerging. Sox17 likely acts downstream of these genes to maintain fetal HSCs after they have formed. Our data do not rule out a role for Sox17 in the formation of definitive HSCs, but there are no data that directly support such a possibility so far.
Sox17 may be required to maintain fetal and neonatal HSCs by promoting Wnt signaling. Neonatal HSCs underwent cell death within days of Sox17 deletion (Figure 7B), and Sox17 deletion dramatically increased Dkk1 expression in neonatal and embryonic hematopoietic progenitors (Figure S6). Dkk1 negatively regulates Wnt pathway activation (Glinka et al., 1998), suggesting that Sox17 deletion may lead to reduced Wnt signaling in HSCs. Consistent with this possibility, Sox17 can negatively regulate the expression of β-catenin target genes in some contexts (Zorn et al., 1999; Sinner et al., 2004). Moreover, Wnt3a-deficient mice (Takada et al., 1994) and Tcf4/Tcf1-deficient mice (Gregorieff et al., 2004) have posterior patterning defects that are similar to the posterior patterning defect we observed in Sox17 deficient mice (Figure 1). Wnt pathway activation has been implicated in the regulation of adult HSC self renewal (Reya et al., 2003; Willert et al., 2003), though it may not be necessary (Cobas et al., 2004), and it is unknown whether Wnt signaling is important for fetal HSC maintenance. Additional studies will be required to test whether Sox17 promotes the maintenance of fetal/neonatal HSCs by promoting Wnt signaling. We also do not know whether overexpression of Sox17 in adult HSCs would be sufficient to induce a fetal phenotype.
The identification of Sox17 as a critical regulator of fetal/neonatal HSCs demonstrates that the transcriptional programs that maintain HSCs change over time. This implies that the regulation of stem cell identity may change with developmental time, perhaps in concert with functional changes. The discovery that Sox17 is autonomously required for the maintenance of fetal and neonatal but not adult HSCs identifies a new stage-specific mechanism for the maintenance of HSCs that fills a gap between previously identified mechanisms for the embryonic formation of HSCs and the maintenance of adult HSCs.
EXPERIMENTAL PROCEDURES
See Supplemental Data for details regarding the generation of Sox17 mutant mice.
Flow Cytometry and Isolation of HSCs
Fetal liver or bone marrow cells (flushed from tibias and femurs of adult mice) were triturated with Hank’s Buffered Salt Solution without calcium or magnesium, supplemented with 2% heat-inactivated calf serum (Gibco, Grand Island, NY; HBSS+) and filtered through nylon screen (45 μm, Sefar America; Kansas City, MO) to obtain a single cell suspension. To examine the expression of GFP in fetal liver HSCs (Sca-1+Lin−Mac-1+CD48−) and adult bone marrow HSCs (Sca-1+Lin−c-kit+CD48−), fetal liver cells and bone marrow cells were stained as previously described (Kiel et al., 2005b; Kim et al., 2006). Whole fetal liver cells were incubated with unconjugated mono-clonal antibodies to lineage markers including B220 (6B2), CD3 (KT31.1), CD5 (53-7.3), CD8 (53-6.7), Gr-1 (8C5), and Ter119 (Ter-119). Pelleted cells were resuspended in anti-rat IgG specific F(ab)2 conjugated to phycoerythrin (PE; Jackson ImmunoResearch; West Grove, PA). Cells were then stained with directly conjugated antibodies to Mac-1 (M1/70-allophycocyanin (APC)), Sca-1 (Ly6A/E-biotin), CD48 (HM48-1-PE), and CD4 (GK1.5-PE), followed by staining with streptavidin conjugated to APC-Cy7 (PharRed; PR) (BD Biosciences; San Jose, CA). Adult whole bone marrow cells were stained in the same manner as whole fetal liver cells except that different fluorochromes were used for Mac-1 (M1/70-PE) and Sca-1 (Ly6A/E-APC), and c-kit (2B8-biotin) was also included as a marker.
To analyze the expression of GFP in E10.5 or E11.5 embryos, embryos and yolk sacs were separated carefully and digested with collagenase type I (1 mg/ml) in the presence of 10% FBS at 37°C for 1.5 hr as previously described (Gekas et al., 2005). Isolated cells were stained with unconjugated anti-CD144 (clone 11D4.1; BD BioSciences), which was detected by anti-rat IgG-APC (Jackson Immuno-Research), anti-CD45-biotin (clone 30-F11; eBioscience, San Diego, CA), and Ter119-PE.
Cells were resuspended in 2μg/ml 7-AAD (Molecular Probes; Eugene, OR) to discriminate live from dead cells. All flow cytometry was performed on a FACSVantage SE-dual laser, three-line flow cytometer (Becton-Dickinson).
Long-Term Competitive Reconstitution Assays
Recipients in reconstitution assays were adult C57Bl/Ka-CD45.1:Thy-1.2 mice. Adult recipient mice were irradiated with an Orthovoltage x-ray source delivering approximately 300 rads/min in two doses of 550–570 rad each, delivered at least 2 hr apart. Transplanted cells were injected into the retro-orbital venous sinus of individual lethally irradiated recipients along with 200,000 recipient bone marrow cells for radioprotection. For at least 16 weeks after transplantation, blood was obtained from the tail veins of recipient mice, subjected to ammonium-chloride/potassium bicarbonate red cell lysis, and stained with directly conjugated antibodies to CD45.2 (104), B220 (6B2), Mac-1 (M1/70), CD3 (KT31.1) and Gr-1 (8C5) to monitor donor cell engraftment.
Methylcellulose Culture
Cells were plated in wells of 96-well plates (Corning; Corning, NY) containing 100 μl of 1.0% MethoCult GFM3434 (Stem Cell Technologies; Vancouver, Canada).
Supplementary Material
Supplemental Data include six figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at http://www.cell.com/cgi/content/full/130/3/470/DC1/.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and the U.S. Army Research Laboratory/Office under grant number DAAD19-03-1-0168. Flow cytometry was partially supported by the University of Michigan Comprehensive Cancer NIH CA46592, and the University of Michigan Multipurpose Arthritis Center NIH AR20557. Antibody production was partially supported by the Rheumatic Core Disease Center (1 P30 AR48310). I.K. was supported by a postdoctoral fellowship from the University of Michigan Cancer Biology Training Program. The authors are grateful to Elizabeth Hughes for generation of gene-targeted mice, to David Adams and Martin White for flow cytometry, and to Elizabeth Smith (UM Hybridoma Core) for antibody production.
References
- Azcoitia V, Aracil M, Martinez AC, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280:307–320. doi: 10.1016/j.ydbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. {beta}-Catenin Is Dispensable for Hematopoiesis and Lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Ernst P, Wang Y, Dekens MP, Kingsley PD, Palis J, Korsmeyer SJ, Daley GQ, Zon LI. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- Ernst P, Fisher JK, Avery W, Wade S, Foy D, Korsmeyer SJ. Definitive hematopoiesis requires the mixed-lineage leukemia gene. Dev Cell. 2004;6:437–443. doi: 10.1016/s1534-5807(04)00061-9. [DOI] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Grosschedl R, Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. EMBO J. 2004;23:1825–1833. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Zhong RK, Jordan CT, Lemischka IR, Astle CM. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp Hematol. 1997;25:293–297. [PubMed] [Google Scholar]
- Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J. 2004;23:450–459. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004a;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, Orkin SH. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004b;18:2336–2341. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Asai T, Saito T, Yamamoto G, Seo S, Yamazaki I, Yamagata T, Mitani K, Chiba S, Hirai H, et al. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med. 2004;10:299–304. doi: 10.1038/nm997. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Kina T, Macneil I, Uchida N, Peault B, Chien YH, Weissman IL. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Iwashita T, Yilmaz OH, Morrison SJ. Spatial differences in hematopoiesis but not in stem cells indicate a lack of regional patterning in definitive hematopoietic stem cells. Dev Biol. 2005a;283:29–39. doi: 10.1016/j.ydbio.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005b;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kim JY, Sawada A, Tokimasa S, Endo H, Ozono K, Hara J, Takihara Y. Defective long-term repopulating ability in hematopoietic stem cells lacking the Polycomb-group gene rae28. Eur J Haematol. 2004;73:75–84. doi: 10.1111/j.1600-0609.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106:903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirito K, Fox N, Kaushansky K. Thrombopoietin induces HOXA9 nuclear transport in immature hematopoietic cells: potential mechanism by which the hormone favorably affects hematopoietic stem cells. Mol Cell Biol. 2004;24:6751–6762. doi: 10.1128/MCB.24.15.6751-6762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen MJ, Stacy T, Speck NA. Runx1 function in hematopoiesis is required in cells that express Tek. Blood. 2006;107:106–110. doi: 10.1182/blood-2005-05-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–551. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wright D, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, Pietryga DW, Scott WJ, Potter SS. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Ohta H, Sawada A, Kim JY, Tokimasa S, Nishiguchi S, Humphries RK, Hara J, Takihara Y. Polycomb group gene rae28 is required for sustaining activity of hematopoietic stem cells. J Exp Med. 2002;195:759–770. doi: 10.1084/jem.20011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Vandeursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker M, Pihalja M, Weissman IL, Morrison SJ, Clarke M. Bmi-1 is required for the maintenance of adult self-renewing hematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Phillips RL, Ernst RE, Brunk B, Ivanova N, Mahan MA, Deanehan JK, Moore KA, Overton GC, Lemischka IR. The genetic program of hematopoietic stem cells. Science. 2000;288:1635–1640. doi: 10.1126/science.288.5471.1635. [DOI] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Rebel VI, Kung AL, Tanner EA, Yang H, Bronson RT, Livingston DM. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc Natl Acad Sci USA. 2002;99:14789–14794. doi: 10.1073/pnas.232568499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Sandberg ML, Sutton SE, Pletcher MT, Wiltshire T, Tarantino LM, Hogenesch JB, Cooke MP. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell. 2005;8:153–166. doi: 10.1016/j.devcel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Mikkola HK, Gekas C, Helgadottir HB, Orkin SH. Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood. 2005;105:3871–3874. doi: 10.1182/blood-2004-11-4467. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2005;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4:487–498. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data include six figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at http://www.cell.com/cgi/content/full/130/3/470/DC1/.