Abstract
ADAMTS13 limits platelet-rich thrombosis by cleaving von Willebrand factor at the Tyr1605–Met1606 bond. Previous studies showed that ADAMTS13 truncated after spacer domain remains proteolytically active or hyper-active. However, the relative contribution of each domain within the proximal carboxyl terminus of AD-AMTS13 in substrate recognition and specificity is not known. We showed that a metalloprotease domain alone was unable to cleave the Tyr–Met bond of glutathione S-transferase (GST)-VWF73-H substrate in 3 h, but it did cleave the substrate at a site other than the Tyr–Met bond after 16–24 h of incubation. Remarkably, the addition of even one or several proximal carboxyl-terminal domains of ADAMTS13 restored substrate specificity. Full proteolytic activity, however, was not achieved until all of the proximal carboxyl-terminal domains were added. The addition of TSP1 2–8 repeats and two CUB domains did not further increase proteolytic activity. Furthermore, ADAMTS13 truncated after the spacer domain with or without metalloprotease domain bound GST-VWF73-H with a Kd of ≈7.0 or 13 nM, comparable with full-length ADAMTS13 (Kd = 4.6 nM). Metalloprotease domain did not bind GST-VWF73-H detectably, but the disintegrin domain, first TSP1 repeat, Cys-rich domain, and spacer domain bound GST-VWF73-H with Kd values of 489, 136, 121, and 108 nM, respectively. These proximal carboxyl-terminal domains dose-dependently inhibited cleavage of fluorescent resonance energy transfer (FRETS)-VWF73 by full-length ADAMTS13 and ADAMTS13 truncated after the spacer domain. These data demonstrated that the proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for recognition and cleavage of von Willebrand factor between amino acid residues Asp1595 and Arg1668.
ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeat) (1–6) cleaves von Willebrand factor (VWF)1 at the Tyr1605–Met1606 bond of the central A2 subunit (7, 8). Inability to cleave newly synthesized and released “unusually large” VWF multimers from endothelial cells or platelets due to deficiency of ADAMTS13 proteolytic activity may result in an accumulation of the unusually large VWF multimers (9), leading to formation of disseminated platelet-rich microthrombi in small arteries, a characteristic pathologic feature of thrombotic thrombocytopenic purpura.
For purposes of discussion, ADAMTS13 may be divided arbitrarily into four functionally distinct regions: the metalloprotease, the proximal, the middle, and the distal carboxyl-terminal regions. The metalloprotease domain is the catalytic center, containing a characteristic HEXXHXXGXXHD sequence that coordinates Zn2+ or Ca2+ binding (1–6, 10). The proximal carboxyl-terminal region consists of a disintegrin domain, a first thrombospondin type 1 (TSP1) repeat, a Cys-rich domain, and a spacer domain. The middle and distal carboxyl-terminal regions of ADAMTS13 have seven additional TSP1 repeats and two CUB (C1r/C1s, urinary epidermal growth factor, bone morphogenetic protein) domains (3, 6, 10, 11).
Studies have shown that ADAMTS13 truncated after the spacer domain remains proteolytically active toward plasma VWF in the presence of 1.5 M urea and low ionic strength (12, 13) and appears to be hyperactive toward unusually large VWF that are newly released from cultured endothelial cells under flow conditions (14). In addition, the autoantibodies identified in patients with idiopathic thrombotic thrombocytopenic purpura all react with the Cys-rich domain and spacer domain (15, 16). These data suggest a critical role of the proximal C terminus of ADAMTS13 in substrate recognition in vivo. However, the relative contribution of each domain within the proximal carboxyl-terminal region of ADAMTS13 to substrate recognition and enzymatic activity remains unclear, as the assays using plasma VWF in the presence of urea are extremely slow and lacking in sensitivity. For example, ADAMTS13 mutants truncated after the Cys-rich domain are essentially inactive toward plasma VWF (12, 13), making it impossible to assess the contribution of the shorter truncated ADAMTS13 mutants. In addition, denaturing reagents added into the assay system may partially inactivate ADAMTS13 protease while unfolding multimeric VWF substrate to expose the cleavage site.
To further determine the relative contribution of each domain within the proximal carboxyl-terminal domains of AD-AMTS13 in substrate recognition and specificity, we employed more sensitive peptide substrates, GST-VWF73-H and FRETS-VWF73. In each case, a 73-amino acid peptide (Asp1595–Arg1668) derived from the central A2 domain of VWF was either tagged by GST at the amino terminus (17) or labeled by a fluorescent dye and a quencher (18) on either side of the Tyr–Met bond. Kokame et al. (17) have shown that VWF73 is the minimal and sufficient peptide region of VWF to be specifically recognized and cleaved by ADAMTS13. The cleavage of the peptidyl substrates by ADAMTS13 and various carboxyl-terminal or internal deleted mutants of ADAMTS13 was determined and compared with the cleavage of plasma VWF. In addition, the direct binding interaction between GST-VWF73-H peptide and full-length ADAMTS13, various mutants, and proximal carboxyl-terminal domains of ADAMTS13 was determined. The data confirmed that each one of the proximal carboxyl-terminal domains of ADAMTS13 participates in substrate recognition and is required for cleavage of VWF peptide (Asp1595–Arg1668).
EXPERIMENTAL PROCEDURES
Plasmid Constructs
pDEST15-VWF73, consisting of 73 amino acid residues (Asp1595–Arg1668) of VWF, flanked by a GST tag at the amino terminus and His6 at the carboxyl terminus, was kindly provided by Dr. Han-Mou Tsai, Montefiore Medical Center, Bronx, NY (19). Plasmid pADAMTS13-V5-His containing full-length ADAMTS13 and V5-His epitope at the carboxyl terminus was described previously (3, 13). Using pADAMTS13-V5-His as a template, we created a variety of the carboxyl termini-deleted mutants of ADAMTS13 by PCR with a Hotstart Turbo Pfu DNA polymerase (Stratagene) and domain-specific primers as described previously (3, 13). The constructs corresponding to the specific domain or domains of ADAMTS13 are illustrated in Figs. 1 and 4. All cDNA fragments were ligated into a pcDNA3.1 V5-His TOPO vector according to manufacturer’s recommendation (Invitrogen). The accuracy of all constructs was confirmed by BigDye automatic sequencing at the Nuclear Acid Core Facility, Children’s Hospital of Philadelphia.
Fig. 1. The domain structure of ADAMTS13 and various carboxyl-terminal truncated mutants.
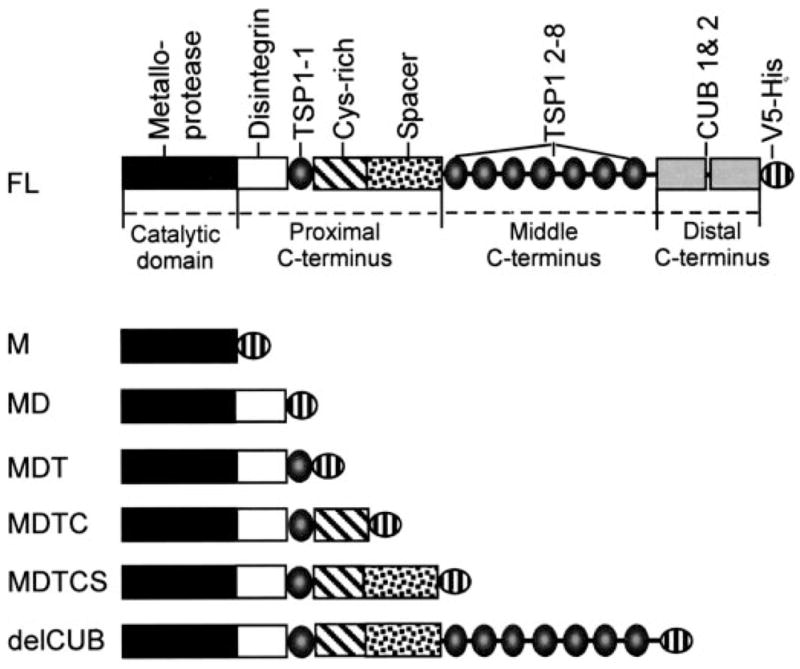
Full-length ADAMTS13 (FL) consists of a metalloprotease domain (M), a disintegrin domain (D), the first TSP1 repeat (T), a Cys-rich domain (C), and a spacer domain (S) followed by seven more TSP1 repeats (T2–8) and two CUB domains. The DTCS domains are located in the proximal C terminus of AD-AMTS13. The TSP1 2–8 and two CUB domains are located in the middle and the distal C termini of ADAMTS13, respectively. Each construct is tagged at its C terminus by a V5-His epitope derived from pcDNA3.1 V5-His TOPO.
Fig. 4. Relative proteolytic activity of ADAMTS13 and the carboxyl-terminal truncated mutants.
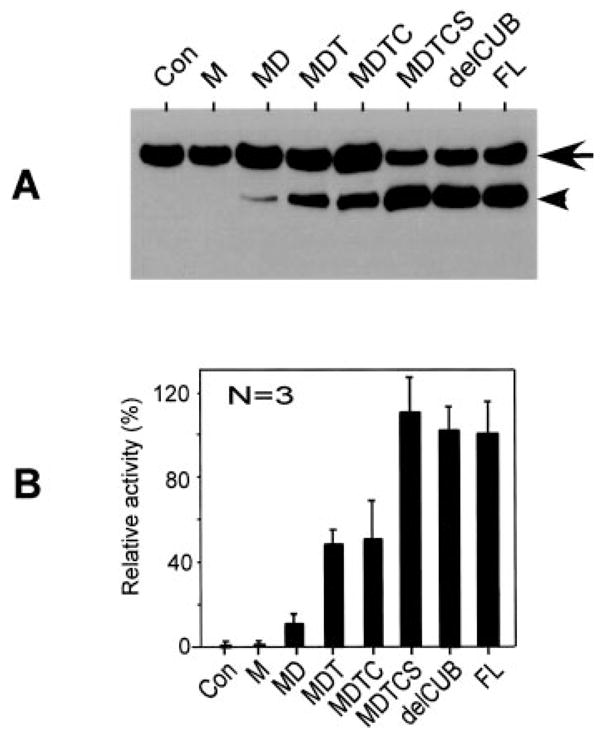
A, Western blot with anti-GST detects cleavage of GST-VWF73-H (200 ng) by 14 nM full-length AD-AMTS13 (FL) and the various carboxyl-terminal deleted mutants for 2 h. Lanes 1–7 correspond to the constructs negative control (Con), M, MD, MDT, MDTC, MDTCS, and delCUB, respectively, as illustrated in Fig. 1. The arrow indicates the intact substrate, and the arrowhead indicates the cleaved product. B, the signal on the luminogram was quantified by densitometry with ImageJ and is shown as the means ± S.D. from three independent experiments. Sequential addition of the proximal carboxyl-terminal domains gradually increases the proteolytic activity.
Constructs of the Proximal Carboxyl-terminal Domain or Domains of ADAMTS13
To express proximal carboxyl-terminal fragments of AD-AMTS13 in mammalian cells, a signal peptide and propeptide were fused directly with disintegrin domain, first TSP1 repeat, Cys-rich domain, and spacer domain (construct DTCS) by a PCR strategy. The products were gel-purified and ligated into pcDNA3.1 V5-His TOPO according to manufacturer’s protocol (Invitrogen). To express the individual domains of ADAMTS13 in bacteria, PCR with HotStart Turbo pfu DNA polymerase and the domain-specific primer pairs amplified the disintegrin domain, the first TSP1 repeat, the Cys-rich domain, and the spacer domain. The products were gel-purified and ligated into Champion™ pET151/D directional TOPO vector according to manufacturer’s protocol (Invitrogen). Each construct starts with the sequence MHHHHHHGKPIPNPLLGLDSTENLTFQ (the His6 tag and the AcTEV protease cleavage region are underlined, and the V5 epitope is bolded). A linker amino acid sequence (–IDPFTM–) immediately precedes the specific domain of ADAMTS13.
Preparation of Recombinant VWF73 Substrate
Bacterial strain BL21Star™ (DE3) (Stratagene) was transformed with 5 ng of pD-EST15-VWF73 incubated with SOC medium (Invitrogen; 2% tryptone, 2.5% yeast extract, 10 mM sodium chloride, 2.5 mM potassium chloride, 10 mM magnesium chloride, 10 mM magnesium sulfate, 20 mM glucose) at 37 °C for 1 h. After being selected overnight at 37 °C on an agar plate containing 50 μg/ml ampicillin, a single colony was selected and expanded to a 50-ml and then a 1-liter culture. After being induced by the addition of 1% L-arabinose (Sigma) at 37 °C for 4 h, the cells were harvested and lysed with 50 μg/ml lysozyme. The recombinant GST-VWF73-H was purified by affinity chromatography with 5 ml of HiTrap nickel-chelating column (Amersham Biosciences), followed by a 1-ml glutathione-Sepharose 4B column (Amersham Biosciences). The purified GST-VWF73-H was diluted (1:20) with phosphate-buffered saline (PBS), pH 7.4, and dialyzed against PBS overnight. The purity of GST-VWF73-H was determined by electrophoresis on a 15% Tris-HCl SDS-gel and Coomassie blue staining. The protein concentration was determined using a BCA kit (Pierce) according to the manufacturer’s protocol.
Expression and Preparation of Recombinant ADAMTS13 and Mutants
Full-length ADAMTS13 and its mutants were prepared from COS7 cells (13) or human embryonic kidney (HEK) 293 cells. Briefly, cells were seeded at 50–80% confluency 1 day prior to transfection in 10 cm-dishes in Dulbecco’s modified Eagle’s medium containing 10% FetalPlex (Gemini BioProducts). The cells were transfected with 5 μg of plasmid DNA each premixed for 15 min with 30 μl of Lipofectamine 2000 (Invitrogen). After 5 h of transfection, the transfectants were removed and replaced with serum-free Opti-MEM (Invitrogen). The conditioned media were collected and concentrated 10–20-fold with a Centricon-10 (Millipore). The cells were dissociated from the dish and transferred onto a 48-well plate after 1:20 dilution. The stable clones were selected with G418 (0.5 mg/ml) for 14 days. The clones expressing recombinant proteins were determined by Western blotting with anti-V5 (Invitrogen). The concentration of ADAMTS13 and the mutants in the conditioned medium were quantified by Western blotting with anti-V5 as described previously, using a purified protein (Positope™, Invitrogen) as a standard (13, 20, 21).
Recombinant ADAMTS13 and the mutants were also purified from the serum-free conditioned media of HEK293, which stably expressed AD-AMTS13 and various mutants by affinity chromatography with DEAE (Amersham Biosciences), ammonia sulfate precipitation, nickel-nitrilotriacetic acid-agarose (Invitrogen), and Superose-6 (Amersham Biosciences) gel filtration on an ÅKTA™ liquid chromatography system (Amersham Biosciences). The concentration of purified ADAMTS13 was determined by using a BCA kit (Pierce) with bovine serum albumin as reference.
Preparation of the Proximal Carboxyl-terminal Domains of AD-AMTS13 from Escherichia coli
The bacterial strain BL21Ai was transformed by 1 ng of plasmid encoding the disintegrin domain (His-V5-Dis), the first TSP1 repeat (His-V5-TSP1), the Cys-rich domain (His-V5-CysR), and the spacer domain (His-V5-Spa). A single colony was selected and expanded to 1 liter of culture. After being induced by 0.5 mM isopropyl-β-D-thiogalactoside (Fisher Scientific) for 4 h at 37 °C, the cells were harvested by centrifugation at 3,500 rpm for 15 min. The inclusion bodies of the E. coli cells were isolated with B-Per reagents according to the manufacturer’s recommendation (Pierce) and lysed with 8 M urea in 100 mM NaH2PO4, 10 mM Tris-HCl, pH 8.0. Then 10 ml of the cell lysate was added dropwise to 200 ml of refolding buffer (50 mM MES, pH 6.0, 240 mM NaCl, 10 mM KCl, 1 mM EDTA, 0.5 M arginine, 0.4 M sucrose, 0.5% Triton X-100, and 0.05% polyethylene glycol 16,000 the in presence of 1 mM fresh GSH and 0.1 mM fresh GSSH) (Sigma) according to the protocol from the Athena enzyme system (Athena Environmental Sciences, Inc., Baltimore, MD). The protein was added dropwise to the refolding buffer with gentle stirring at 22 °C for 72 h. After being dialyzed against 20 mM Tris-HCl, 150 mM NaCl, pH 8.0, the recombinant domains of ADAMTS13 were purified by affinity chromatography with nickel-nitrilotriacetic acid-agarose (Invitrogen). The imidazole in the eluate was removed by PD-10 gel filtration column per the manufacturer’s instruction (Amersham Biosciences). SDS-gel electrophoresis and Coomassie Blue staining determined the purity of the protein. The BCA reagents determined the protein concentration.
Cleavage of GST-VWF73-H by ADAMTS13 Its Mutants
Purified GST-VWF73-His (200 ng) was incubated with ~16 nM ADAMTS13 and the mutants in 50 mM Tris-HCl, 50 mM NaCl, pH 8.0, at 37 °C for 3 h and/or 24 h. Boiling for 5 min with sample buffer containing 20 mM Tris-HCl, pH 7.5, 0.5% SDS, and 5% β-mercaptoethanol stopped the reaction. One-fifth of the digested material was separated by 12% Tris-HCl, SDS-gel and transferred onto a polyvinylidene difluoride membrane (pore size 0.22 μm). After being blocked by 2.5% nonfat milk (Pathmark, Philadelphia, PA) in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20 (TBST) for 30 min, the membrane was incubated overnight at room temperature with rabbit anti-GST IgG (1:1,000) (Molecular Probes) (17) followed by anti-rabbit IgG, peroxidase-conjugated (1:25,000) (Amersham Biosciences) at room temperature for 2 h. After being washed three times with TBST, the bound secondary antibodies were detected by the SuperSignal™ chemiluminescent ECL detection system (Pierce) (13). The luminograms were scanned, and the relative amount of proteins was determined by densitometry using NIH ImageJ (developed at the National Institutes of Health and available on the Internet at rsb.info.nih.gov/nih-image/).
Cleavage of Plasma VWF by ADAMTS13 and Its Mutants
The proteolytic cleavage of plasma VWF at the Tyr–Met bond by ADAMTS13 and various mutants was determined by electrophoresis on a 5% Tris-HCl SDS-gel under denatured and reduced conditions followed by Western blotting with a peroxidase-conjugated anti-VWF IgG (DAKO) as described (13).
ADAMTS13-VWF73 Binding
A 96-well microtiter plate (Nunc) was coated with or without 50 nM GST-VWF73-H diluted in PBS overnight. After being blocked by 2.5% bovine serum albumin in PBS, ADAMTS13 and various mutants or domains were added and incubated at 37 °C for 2–3 h in 50 mM Tris-HCl, 50 mM NaCl, pH 7. After being washed three times with PBS, the bound ADAMTS13 and mutants were quantified by peroxidase-conjugated anti-V5 IgG (Invitrogen) followed by a chromogenic substrate, O-phenylenediamine dihydrochloride-hydrogen peroxide (OPD-H2O2, Sigma). After subtracting the nonspecific binding, the dissociation constants (Kd) were determined by fitting the data to a double-reciprocal version of the binding equation as described recently by Majerus et al. (21).
Alternatively, ADAMTS13 domain-VWF73 binding was determined in solution by a GST pull-down assay. Briefly, 250 nM GST-VWF73-H was incubated with various domains of ADAMTS13 (50 nM) in 50 mM Tris-HCl, 50 mM NaCl, pH 8.0 for 1 h. The ADAMTS13 domains bound to GST-VWF73-H were pulled down by the addition of 15 μl of glutathione-Sepharose 4B in the presence of 1% Triton X-100. After electrophoresis, Western blotting with anti-V5 IgG and chemiluminescent reagents detected the domains of ADAMTS13 bound to GST-VWF73-H.
Competition Inhibition Assay
The affinity-purified proximal carboxyl-terminal domains as a whole (construct DTCS) (0, 0.5, 1, 2, and 4 nM) or individually (constructs Dis, TSP1, CysR, and Spa) (0, 200, 500, and 1000 nM) were incubated with FRETS-VWF73 (1 μM) (Peptides International, Lexington, KY) for 15 min at 30 °C. ADAMTS13 (0.5 nM) or ADAMTS13 mutant (MDTCS) (1 nM) was added into the reaction containing 5 mM bis-Tris, 25 mM CaCl2, and 0.05% Tween 20, pH 6.0. The generation of fluorescence was determined by a fluorescence spectrophotometer (λex = 340 nm and λem = 450 nm) every 5 min for 2 h (Molecular Devices, Sunnyvale, CA). The residual proteolytic activity was calculated from the slope using normal human plasma as a standard. In the absence of competitive inhibitors, the proteolytic activity was defined as 100% of that of normal human plasma (18).
RESULTS
Metalloprotease Domain of ADAMTS13 Alone Did Not Cleave VWF73 Specifically
Consistent with previous studies using plasma VWF as a substrate (12, 13), a recombinant metalloprotease domain of ADAMTS13 alone at a concentration of 16 nM, 2–5 times that of plasma concentration (≈2–5 nM) (1, 13), did not cleave GST-VWF73-H (Fig. 2) or FRETS-VWF73 (data not shown) specifically at 37 °C in 3 h, during which time the substrate was completely cleaved by full-length recombinant ADAMTS13 at the same concentration (Fig. 2A, lanes 3 and 4; Figs. 3 and 4). However, upon a prolonged incubation (≈16–24 h), the metalloprotease domain did cleave GST-VWF73-H, but at a site other than the predicted Tyr–Met bond, generating an amino-terminal product approximately 3 kDa smaller than expected (Fig. 2B, lanes 3 and 4, and Fig. 3B). Such cleavage of GST-VWF73-H was not observed using the same amount of concentrated (10-fold) serum-free conditioned medium that was collected from COS7 cells transfected with vector alone (Fig. 2, A and B, lanes 1 and 2). The cleavage of GST-VWF73-H by the metalloprotease domain could be abrogated by the addition of 10 mM EDTA (Fig. 2B, lane 3), suggesting a metal ion-dependent proteolysis. These data demonstrated unexpectedly that recombinant metalloprotease domain of ADAMTS13 produced from transfected cells is an active enzyme but alone is not sufficient for recognition and specific cleavage of the Tyr–Met bond of VWF.
Fig. 2. Cleavage of GST-VWF73-H by ADAMTS13 and the carboxyl-terminal truncated mutants.
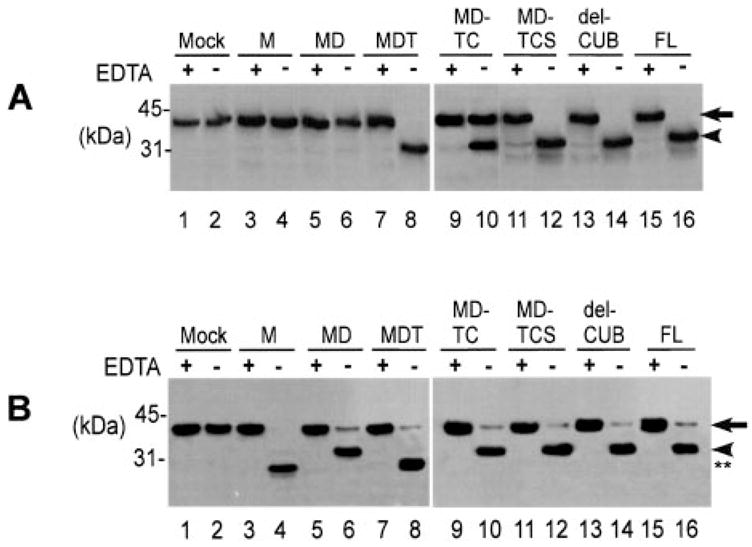
Purified GST-VWF73-H (200 ng) was incubated with 16 nM ADAMTS13 and the various carboxyl-terminal deleted mutants of ADAMTS13 in the absence (−) or presence (+) of 10 mM EDTA for 3 h (A) and 24 h (B), respectively, in a total volume of 40 μl. Western blotting with rabbit anti-GST IgG and chemiluminescent reagents detected the cleavage of GST-VWF73-H. Construct derivations are as in Fig. 1. The arrows indicate intact GST-VWF73-H (38.4 kDa), and the arrowheads indicate amino-terminal fragments of GST-VWF73-H (34.4 kDa) generated by cleavage of the Tyr–Met bond. The double stars (30 kDa) indicate amino-terminal fragments generated by cleavage of GST-VWF73-H at the site other than the Tyr–Met bond.
Fig. 3. Cleavage of GST-VWF73-H by ADAMTS13 mutants lacking one or several proximal carboxyl-terminal domains.
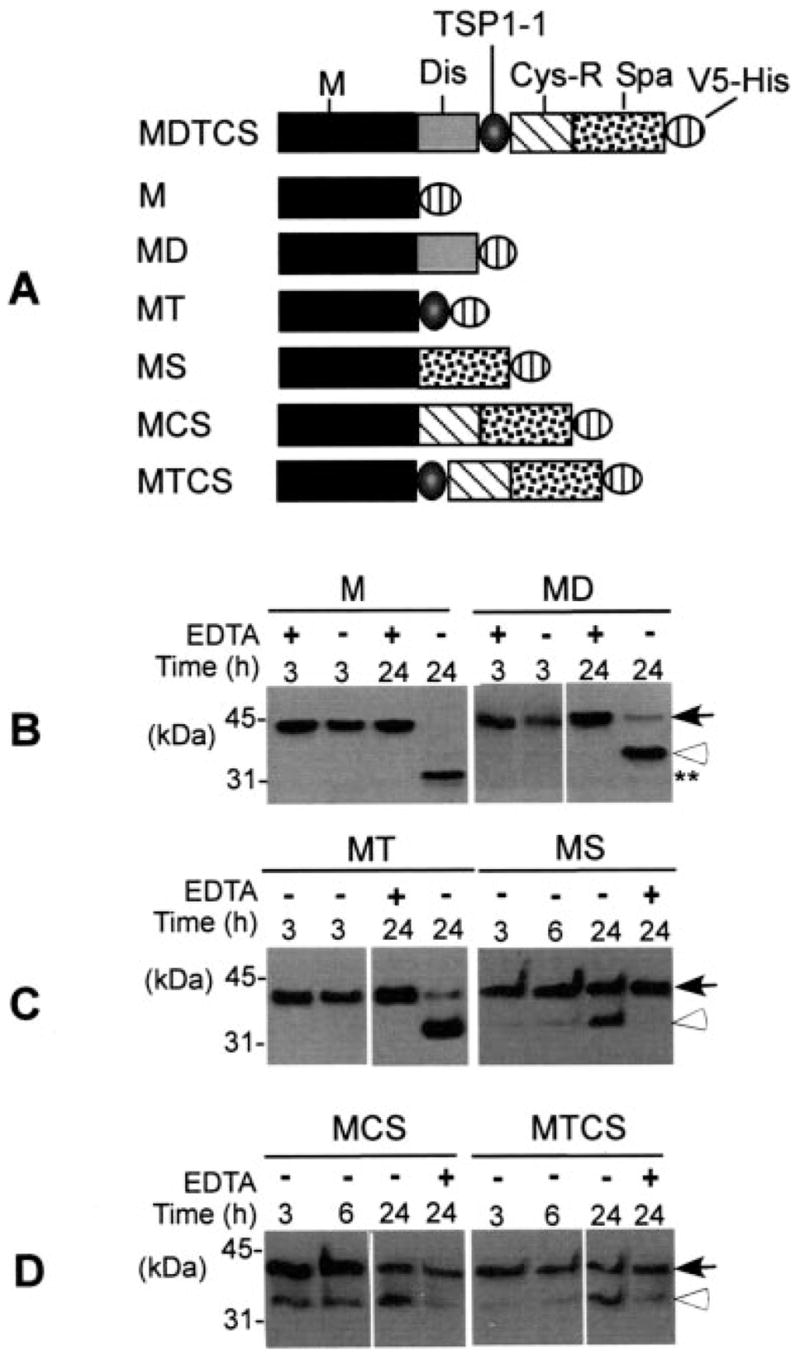
GST-VWF73-H (200 ng) was incubated with 14 nM ADAMTS13 mutants (constructs M, MD, MT, MS, MCS, and MTCS) as illustrated in A at 37 °C for 3 h and/or 6 and 24 h in the presence (+) or absence (−) of 20 mM EDTA as indicated in B and C. Western blotting with anti-GST IgG and chemiluminescent reagents determined the cleavage of GST-VWF73-H. The closed arrows indicate intact substrate (38.4 kDa), and the open arrowheads indicate the cleaved product specifically at the Tyr–Met bond (B–D).
Addition of Proximal Carboxyl-terminal Domains of AD-AMTS13 Restores Specificity
To assess the role of the proximal carboxyl-terminal domains of ADAMTS13 in substrate recognition, we determined the proteolytic cleavage of GST-VWF73-H by recombinant full-length ADAMTS13 and various truncation or deletion mutants. We showed that the addition of even one of the proximal carboxyl-terminal domains, such as the disintegrin domain (construct MD), the first TSP-1 repeat (construct MT), or the spacer domain (construct MS), immediately restores specific cleavage of GST-VWF73-H at the Tyr–Met bond (Fig. 3). The addition of two or more proximal carboxyl-terminal domains to the metalloprotease domain (constructs MCS and MTCS) was still not sufficient to restore full proteolytic activity (Fig. 3). The proteolytic activity of all of these mutants lacking one or several domains between the catalytic domain and the spacer domain was quite low. Only a minimal amount of GST-VWF73-H was cleaved in 3 h of incubation, but the cleavage occurred specifically at the Tyr–Met bond as judged by the molecular weight of cleaved products, even after a prolonged incubation for up to 24 h (Fig. 3). None of these constructs (MD, MT, MS, MCS, and MTCS) cleaved plasma VWF in the presence of 1.5 M urea and low ionic strength (Table I). These data suggested that each one of the proximal carboxyl-terminal domains of ADAMTS13 participates in substrate recognition or is required for conformation.
Table I. Cleavage of GST-VWF73-H and plasma VWF by ADAMTS13 and various mutants.
The signs +++, ++, and +/− indicate >50%, 10–50%, and <10% activity, respectively, compared with normal human plasma. S, indicates the predicted Tyr–Met bond was cleaved; NS, indicates the bond other than the Tyr–Met bond was cleaved. The domain structures of full-length ADAMTS13 (FL) and the mutants (from M to delCUB) are illustrated in detail in Fig. 1. The cleavage of GST-VWF73-H or plasma VWF was determined by 12% or 5% Tris-HCl SDS-gel and Western blotting with anti-GST IgG or anti-VWF IgG as described under “Experimental Procedures.”
| Constructs | Cleavage of VWF73
|
Cleavage of VWF,16 h | |
|---|---|---|---|
| 3 h | 16–24 h | ||
| M | − | + NS | − |
| MD | +/− | + S | − |
| MT | − | + S | − |
| MS | − | + S | − |
| MDT | + | ++ S and NS | − |
| MCS | +/− | ++ S | − |
| MTCS | +/− | ++ S | − |
| MDTC | ++ | ++ S | − |
| MDTCS | +++ | ++ S | ++ S |
| delCUB | +++ | ++ S | ++ S |
| FL | +++ | ++ S | ++ S |
When the disintegrin domain, the first TSP1 repeat, the Cys-rich, domain, and the spacer domain were sequentially added to the metalloprotease domain, proteolytic activity toward GST-VWF73-H (Fig. 4) or FRETS-VWF73 (data not shown) gradually increased while the specificity was maintained. ADAMTS13 truncated after the first TSP1 repeat (construct MDT), however, cleaved GST-VWF73-H, specifically at the Tyr–Met bond initially (3 h) (Fig. 2A, lane 8), but cleaved additional two peptidyl bonds (Phe–Asn and Lys–Lys) upon a prolonged incubation (24 h) (Fig. 2B, lane 8). The peptidyl bonds cleaved by construct MDT were confirmed by amino-terminal protein sequencing of the carboxyl-terminal portion of the cleaved product. Full proteolytic activity toward GST-VWF73-H (Fig. 4) or FRETS-VWF73 (data not shown) and plasma VWF (Table I) was not achieved until all of the proximal carboxyl-terminal domains were added to the metalloprotease domain. Notably, the proteolytic activity of ADAMTS13 truncated after the spacer domain (construct MDTCS) toward GST-VWF73-H (Fig. 4), FRETS-VWF73 (data not shown), or plasma VWF (Table I) is nearly equal to that of full-length ADAMTS13. The further addition of the 2–8 TSP1 repeats and the CUB domains did not alter the proteolytic activity and specificity (Fig. 4 and Table I). These data support a hypothesis that the proximal carboxyl-terminal domains of ADAMTS13 determine the substrate recognition and specificity, whereas the middle and distal carboxyl-terminal domains are dispensable, at least in vitro.
Binding of ADAMTS13 and Mutants to GST-VWF73-H
To determine the relative contribution of the proximal carboxyl-terminal domains of ADAMTS13 in direct substrate binding and recognition, we have determined the interaction between ADAMTS13 (or various domains) and GST-VWF73-H with an enzyme-linked immunosorbent assay. We showed that full-length ADAMTS13, MDTCS, and DTCS all bound GST-VWF73-H, but not GST alone, in a dose-dependent and saturable manner, with Kd values of 4.6 ± 1.3, 7.0 ± 1.9, and 13 ± 2.1 nM, respectively (Fig. 5A and Table II). However, the metalloprotease did not bind GST-VWF73-H detectably at a concentration of 100 nM, suggesting that the proximal carboxyl-terminal domains contain the major binding sites for the 73-amino acid peptide (Asp1595–Arg1668) of VWF.
Fig. 5. Binding of ADAMTS13 and mutants or the individual domains of ADAMTS13 to immobilized GST-VWF73-H.
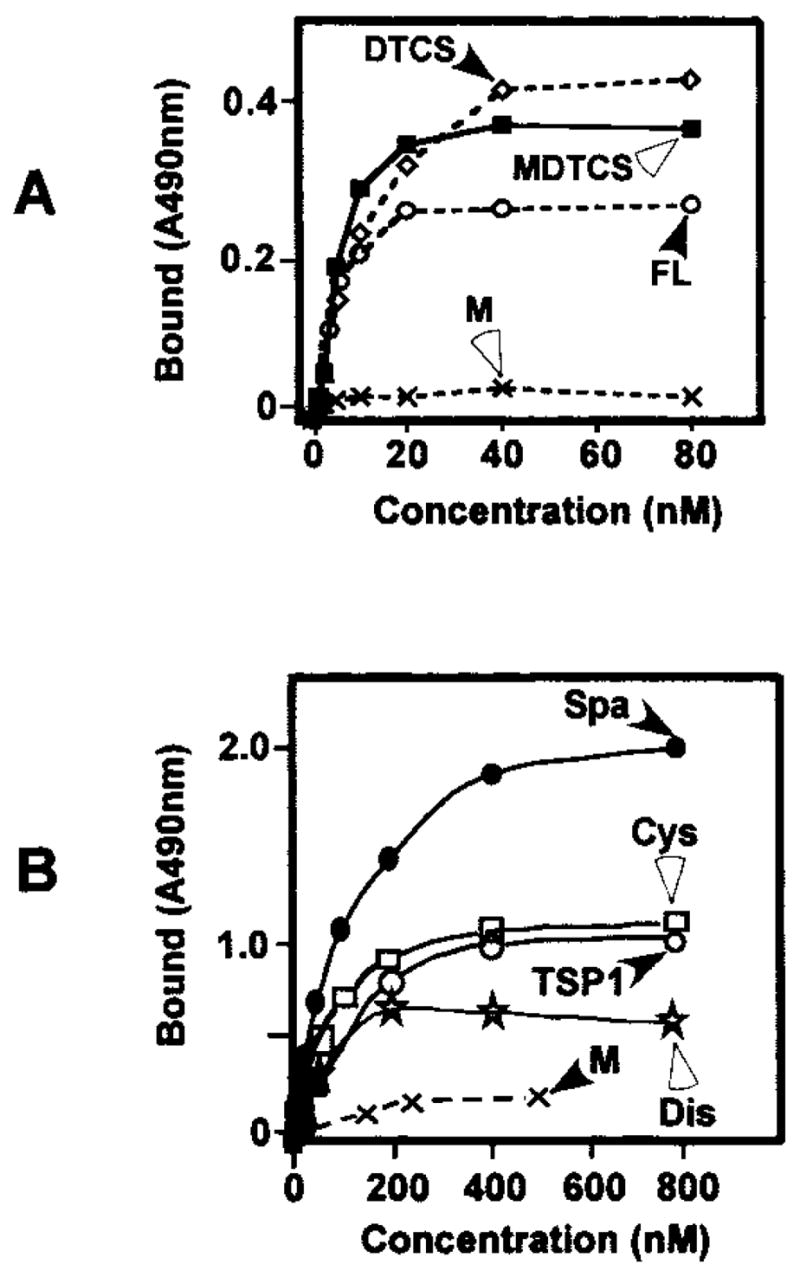
A, affinity-purified ADAMTS13 mutant deleted after the spacer domain (MDTCS) at concentrations of 0 to 50 nM was added to immobilized GST-VWF73-H (closed circle) or bovine serum albumin (open circle). B, full-length ADAMTS13 and ADAMTS13 truncated after the spacer domain with (MDTCS) or without the metalloprotease domain (DTCS) bound GST-VWF73 to saturation. The metalloprotease domain did not bind GST-VWF73-H detectably. Purified individual proximal carboxyl-terminal domains of ADAMTS13 including the disintegrin domain (Dis), the first TSP1 repeat (TSP1), the Cys-rich domain (CysR), and the spacer domain (Spa) also bound immobilized GST-VWF73-H to saturation.
Table II. Binding of ADAMTS13 and various mutants or the proximal C-terminal domains to GST-VWF73-H.
Binding of full-length ADAMTS13 (FL) and the mutants (MDTCS and DTCS) or individual domains (M, Dis, TSP1, CysR, and Spa) to immobilized GST-VWF73-H was determined on an enzyme-linked immunosorbent assay plate. Kd values were calculated as described under “Experimental Procedures.” ND, Kd for this construct was not determined. Entries represent the average ± S.E. (n = 3).
| Constructs | Kd |
|---|---|
| nm | |
| FL | 4.3 ± 1.3 |
| MDTCS | 7.0 ± 1.9 |
| DTCS | 13 ± 2.1 |
| M | ND |
| Dis | 489 ± 42 |
| TSP1 | 136 ± 4.0 |
| CysR | 121 ± 5.0 |
| Spa | 108 ± 14 |
Furthermore, an individual recombinant disintegrin domain, first TSP1 repeat, Cys-rich domain, and spacer domain, all tagged by His-V5 epitope at the N amino terminus, bound GST-VWF73-H dose-dependently to saturation with Kd values of 489 ± 42, 136 ± 4, 121 ± 5, and 108 ± 14 nM, respectively (Fig. 5B and Table II).
The binding interaction between the individual domains of the proximal carboxyl terminus of ADAMTS13 and GST-VWF73-H was further verified in solution by a GST pull-down assay. When GST-VWF73-H (0 or 250 nM) was incubated without or with 50 nM affinity-purified disintegrin domain, first TSP1 repeat, Cys-rich domain, and spacer domain for 2 h, each one of the proximal carboxyl-terminal domains of ADAMTS13 could be pulled down by glutathione-Sepharose 4B and detected by Western blotting with anti-V5 IgG (Fig. 6). As a negative control, GST protein alone at a concentration of 250 nM did not bind either one of the ADAMTS13 domains (data not shown). These data suggested that a specific binding interaction does occur between the proximal carboxyl-terminal domains of ADAMTS13 and the 73-amino acid peptide between Asp1595 and Arg1668 of VWF.
Fig. 6. Binding of ADAMTS13 domains to GST-VWF73-H in solution.
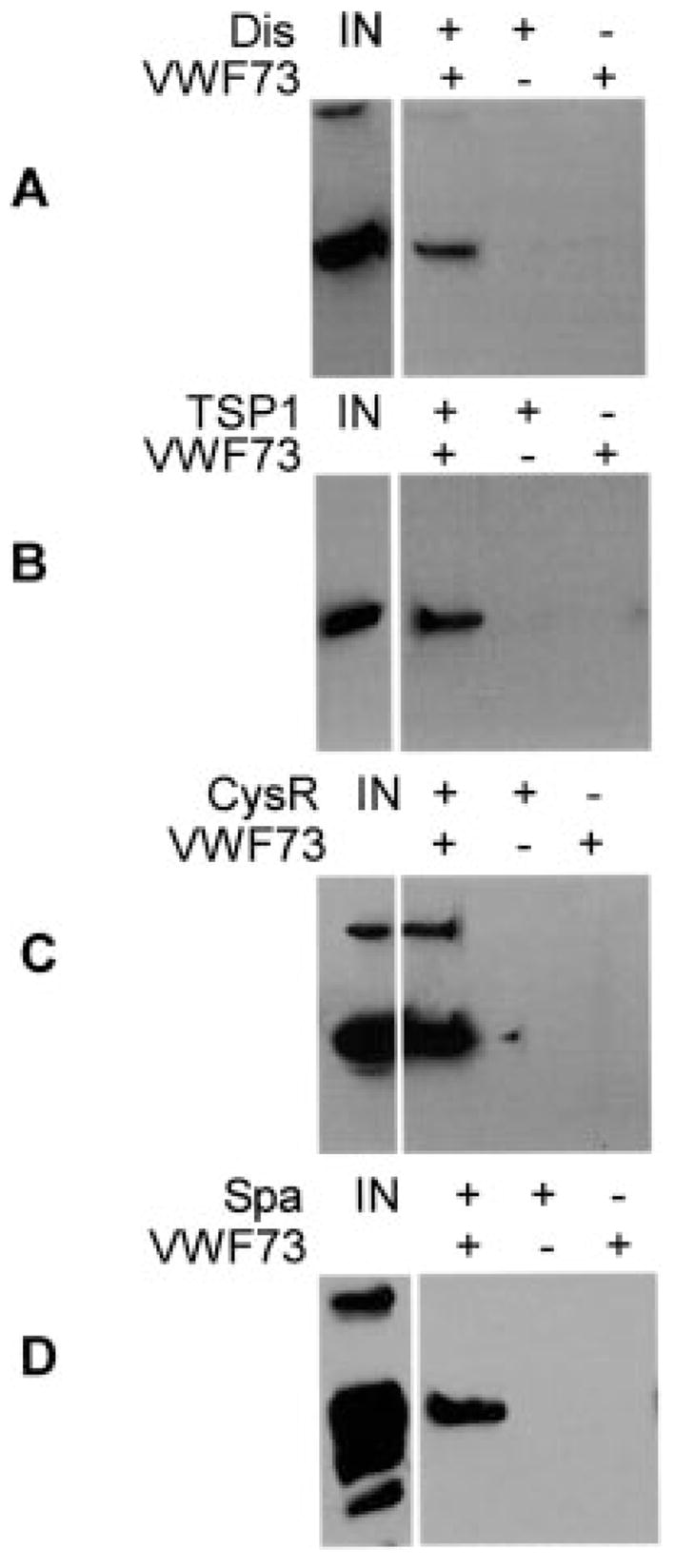
Purified disintegrin domain (Dis) (A), first TSP1 (TSP1) (B), Cys-rich domain (CysR) (C), and spacer domain (Spa) (D) bound to GST-VWF73-H (VWF73) in solution were pulled down by glutathione-Sepharose 4B and detected by Western blotting with anti-V5 IgG and chemiluminescent reagents. IN, amount of initial ADAMTS13 domain used for the assay. No ADAMTS13 domain was pulled down in the reactions omitting either GST-VWF73-H (−) or the V5-His-tagged AD-AMTS13 domain (−).
Competition of Cleavage of FRETS-VWF73 by the Proximal Carboxyl-terminal Domain or Domains
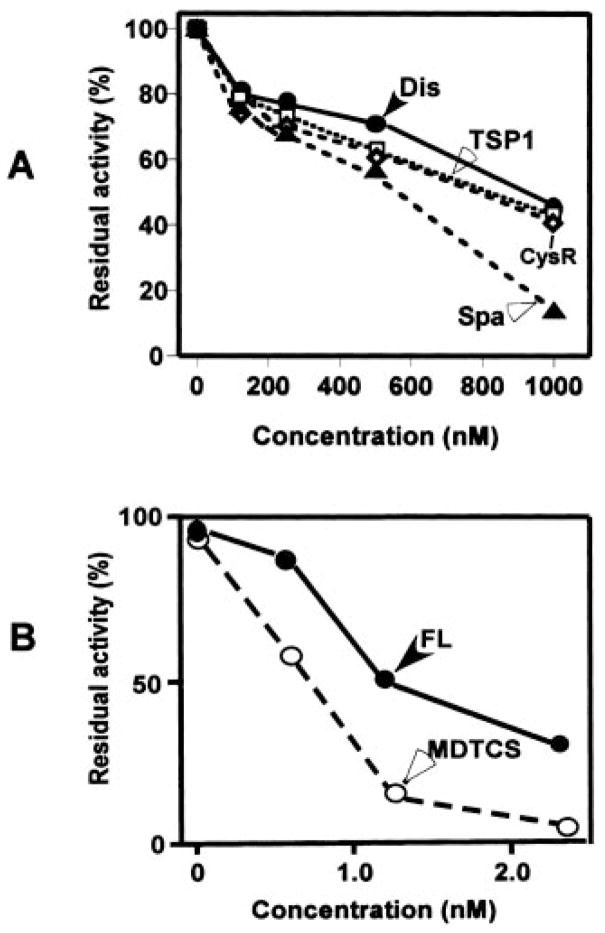
To demonstrate that the binding interaction described above is functionally relevant, a competition assays between MDTCS and the proximal carboxyl-terminal domains for cleavage of FRETS-VWF73 was performed. The FRETS-VWF73 has been shown to be by far the most sensitive and highly specific assay to detect ADAMTS13 activity and inhibition.2 When affinity-purified recombinant disintegrin, first TSP1 repeat, Cys-rich, and spacer domains at concentrations of 0 to 1000 nM were preincubated with FRETS-VWF73 (1 μM) for 15 min, the proteolytic cleavage of FRETS-VWF73 by MDTCS was inhibited dose-dependently (Fig. 7A) with concentrations to inhibit 50% of MDTCS activity (IC50) of 900, 800, 800, and 500 nM, respectively. However, when the proximal carboxyl terminus of ADAMTS13 expressed as a single fragment (DTCS) was added into the reaction, the cleavage of FRETS-VWF73 by ADAMTS13 and MDTCS was almost completely inhibited with IC50 values of 1.2 and 0.75 nM, respectively (Fig. 7B), suggesting a collaborative effort of all of these proximal carboxyl-terminal domains of ADAMTS13 in substrate binding.
Fig. 7. Inhibition of ADAMTS13 activity by recombinant proximal carboxyl-terminal domains of ADAMTS13.
A, affinity-purified proximal carboxyl-terminal domains of ADAMTS13 including the disintegrin domain (Dis), first TSP1, Cys-rich domain (CysR), and spacer domain (Spa) dose-dependently block cleavage of FRETS-VWF73 by ADAMTS13 truncated after the spacer domain (MDTCS). B, the proximal carboxyl-terminal domain as a single fragment (DTCS) dose-dependently blocks the cleavage of FRETS-VWF73 by full-length ADAMTS13 (FL) and MDTCS. The percentage of residual ADAMTS13 activity was plotted on the y axis, whereas the concentrations of the disintegrin, TSP1, Cys-rich, and spacer or DTCS domains were plotted on the x axis. The values entered represent the average of three independent experiments.
DISCUSSION
In this study, we have demonstrated that a metalloprotease domain of ADAMTS13 alone does not cleave GST-VWF73-H or FRETS-VWF73 in 3 h, but it does cleave a peptide bond ≈3 kDa upstream of the expected Tyr–Met bond (Fig. 2B). Remarkably, the addition of even one of the proximal carboxyl-terminal domains, such as the disintegrin domain (construct MD), the first TSP1 repeat (construct MT), or the spacer domain (construct MS), immediately restores specificity (Figs. 2–4). The addition of one or more proximal carboxyl-terminal domains to the growing constructs did not much improve the proteolytic activity (constructs MCS and MTCS) (Fig. 3). The proteolytic activity of all of these mutants, including MD, MT, MS, MCS, and MTCS lacking one or several proximal carboxyl-terminal domains, remained quite low compared with that of full-length ADAMTS13 and MDTCS (Figs. 3 and 4). However, the proteolytic activity of MDTCS was nearly equal to that of full-length ADAMTS13 as determined by GST-VWF73-H (Fig. 4), FRETS-VWF73 (data not shown), and plasma VWF (Table I), suggesting that each one of the proximal carboxyl-terminal domains participates in substrate recognition and is required for cleavage of VWF between amino acid residues Asp1595 and Arg1668.
This hypothesis was further supported by the binding experiments. The recombinant disintegrin, first TSP1 repeat, Cys-rich, and spacer domains, collectively or individually, bound GST-VWF73-H with a reasonable affinity. Metalloprotease domain did not bind immobilized GST-VWF73-H (Fig. 5) and plasma VWF (21) detectably. The Kd values for the disintegrin domain, the first TSP1 repeat, the Cys-rich domain, and the spacer domain to bind GST-VWF73-H were ≈489, 136, 121, and 108 nM, respectively (Fig. 5 and Table II), whereas the Kd values for MDTCS and DTCS to bind GST-VWF73-H were only ≈7.0 and 13 nM, respectively, nearly equal to that of full-length ADAMTS13 binding to same peptide (Kd ≈ 4.6 nM). These data suggested that the proximal carboxyl-terminal domains of AD-AMTS13 contain the major binding sites for VWF73 peptide and that they work collaboratively to confer an efficient recognition of the substrate.
Although the exact amino acid residues on the VWF73 peptide responsible for ADAMTS13 binding are not clear, it is reasonable to believe that ADAMTS13 may bind the very last nine amino acid residues of the VWF73 peptide, because deletion of the nine amino acid residues at the carboxyl terminus of VWF73 (i.e. VWF64) renders the peptide uncleavable (17). More interestingly, the Kd for ADAMTS13 and MDTCS to bind GST-VWF73-H (Table II) is ~3–4-fold smaller than for AD-AMTS13 and MDTCS to bind plasma VWF (Kd ≈ 16 and 24 nM, respectively), as determined by a similar approach (21); this suggests that the domains surrounding the VWF73 peptide, particularly around the cleavage site, such as domain A1 (22), part of domain A2, domain A3 (23), and the carboxyl terminus of VWF, may negatively regulate ADAMTS13-VWF binding. Even in the presence of denaturing reagents such as urea and guanidine, the cleavage site on VWF may not be fully exposed to or become freely accessible by ADAMTS13. The domain A1 appears in the way of ADAMTS13 to cleave the Tyr–Met bond, as the addition of soluble platelet glycoprotein 1b to denatured VWF (22) increases cleavage of VWF by ADAMTS13. The binding peptide or cleavage site may be better exposed by shear stress under flow or domain deletion than by denaturing reagents or immobilization of VWF to the plastic surface, as the cleavage of unusually large VWF multimers by ADAMTS13 under flow conditions occurs within minutes (14, 23, 24), rather than hours or days, in the presence of 1.5 M urea (13, 25–28).
The proteolytic activity of MDTCS and full-length AD-AMTS13 was nearly equal (Fig. 4), and so is the binding affinity toward GST-VWF73-H (Fig. 5 and Table II) and plasma VWF (21). These data further confirmed the notion that the middle and distal carboxyl-terminal domains of ADAMTS13 are not required for recognition and cleavage of VWF in vitro (12, 13). It, however, does not rule out the potential role of the middle and the distal carboxyl-terminal domains of AD-AMTS13 in the modulation of ADAMTS13-VWF binding and proteolytic activity in vivo. Tao et al. (14) have recently shown that the cleavage of unusually large VWF multimers secreted from cultured endothelial cells by a ADAMTS13 mutant lacking the distal CUB domains is in fact increased under flow. In addition, truncation after the seventh TSP1 repeat increases the affinity for VWF by about 2-fold (21). Mutations in the TSP1 2–8 repeats and/or CUB domains resulting in thrombotic microangiopathy (6, 29–32) suggest the biological importance of these domains in vivo. This finding is not contradictory to our results with carboxyl-terminal truncated mutants, as several point mutations in the TSP1 repeats 2–8 and the CUB domains resulted in intracellular retention of the mutant ADAMTS13, and therefore no ADAMTS13 activity in plasma could be detected (6, 29, 33, 34).
We conclude that the proximal carboxyl-terminal domains including the disintegrin domain, the first TSP1 repeat, the Cys-rich domain, and the spacer domain determine the substrate specificity and are all required for recognition and cleavage of VWF between amino acid residues Asp1595 and Arg1668.
Acknowledgments
We thank Dr. Han-Mou Tsai for providing the pDES15-VWF73 construct for generating GST-VWF73-H substrate. We also thank Dr. Sriram Krishnaswamy for helpful discussions.
Footnotes
This work was supported by Grant HL079027 from the National Institutes of Health, Grant 0465532U from the American Heart Association, and a grant from the National Blood Foundation.
The abbreviations used are: VWF, von Willebrand factor; TSP1, thrombospondin type 1; GST, glutathione S-transferase; PBS, phosphate-buffered saline; MES, 4-morpholineethanesulfonic acid; bis-Tris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; FRETS, fluorescent resonance energy transfer.
X. L. Zheng, P. Smith, J. Ai, and S. Shelat, unpublished data.
References
- 1.Gerritsen HE, Robles R, Lammle B, Furlan M. Blood. 2001;98:1654–1661. doi: 10.1182/blood.v98.6.1654. [DOI] [PubMed] [Google Scholar]
- 2.Fujikawa K, Suzuki H, McMullen B, Chung D. Blood. 2001;98:1662–1666. doi: 10.1182/blood.v98.6.1662. [DOI] [PubMed] [Google Scholar]
- 3.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 4.Cal S, Obaya AJ, Llamazares M, Garabaya C, Quesada V, Lopez-Otin C. Gene. 2002;283:49–62. doi: 10.1016/s0378-1119(01)00861-7. [DOI] [PubMed] [Google Scholar]
- 5.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, Furlan M, Gerritsen H, Lammle B, Schwarz HP, Scheiflinger F. Blood. 2002;100:3626–3632. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 6.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 7.Furlan M, Robles R, Lammle B. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 8.Tsai HM. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 9.Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, Azocar J, Seder RH, Hong SL, Deykin D. N Engl J Med. 1982;307:1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 10.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. J Biochem (Tokyo) 2001;130:475–480. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 11.Bork P, Beckmann G. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 12.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, Nozaki C, Miyata T, Fujimura Y, Nakagaki T. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Nishio K, Majerus EM, Sadler JE. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Blood. 2005;106:141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, Scheiflinger F. Blood. 2004;103:4514–4519. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 16.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. Thromb Haemostasis. 2005;93:267–274. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 17.Kokame K, Matsumoto M, Fujimura Y, Miyata T. Blood. 2003;103:607–612. doi: 10.1182/blood-2003-08-2861. [DOI] [PubMed] [Google Scholar]
- 18.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Tsai HM. Thromb Haemostasis. 2004;91:806–811. doi: 10.1160/TH03-11-0675. [DOI] [PubMed] [Google Scholar]
- 20.Majerus EM, Zheng X, Tuley EA, Sadler JE. J Biol Chem. 2003;278:46643–46648. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majerus EM, Anderson PJ, Sadler JE. J Biol Chem. 2005;280:71773–71778. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 22.Nishio K, Anderson PJ, Zheng XL, Sadler JE. Proc Natl Acad Sci U S A. 2004;101:10578–10583. doi: 10.1073/pnas.0402041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, Lopez JA, Cruz MA. J Biol Chem. 2003;278:29633–29639. doi: 10.1074/jbc.M301385200. [DOI] [PubMed] [Google Scholar]
- 24.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 25.Furlan M, Lammle B. Baillieres Clin Haematol. 1998;11:509–514. doi: 10.1016/s0950-3536(98)80064-4. [DOI] [PubMed] [Google Scholar]
- 26.Furlan M, Robles R, Solenthaler M, Lammle B. Blood. 1998;91:2839–2846. [PubMed] [Google Scholar]
- 27.Veyradier A, Obert B, Houllier A, Meyer D, Girma JP. Blood. 2001;98:1765–1772. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 28.Zheng XL, Richard KM, Goodnough LT, Sadler JE. Blood. 2004;103:4043–4049. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y. Proc Natl Acad Sci U S A. 2002;99:11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assink K, Schiphorst R, Allford S, Karpman D, Etzioni A, Brichard B, van de Kar N, Monnens L, van den Heuvel L. Kidney Int. 2003;63:1995–1999. doi: 10.1046/j.1523-1755.63.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 31.Schneppenheim R, Budde U, Oyen F, Angerhaus D, Aumann V, Drewke E, Hassenpflug W, Haberle J, Kentouche K, Kohne E, Kurnik K, Mueller-Wiefel D, Obser T, Santer R, Sykora KW. Blood. 2003;101:1845–1850. doi: 10.1182/blood-2002-08-2399. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto M, Kokame K, Soejima K, Miura M, Hayashi S, Fujii Y, Iwai A, Ito E, Tsuji Y, Takeda-Shitaka M, Iwadate M, Umeyama H, Yagi H, Ishizashi H, Banno F, Nakagaki T, Miyata T, Fujimura Y. Blood. 2003;103:1305–1310. doi: 10.1182/blood-2003-06-1796. [DOI] [PubMed] [Google Scholar]
- 33.Pimanda JE, Maekawa A, Wind T, Paxton J, Chesterman CN, Hogg PJ. Blood. 2004;103:627–629. doi: 10.1182/blood-2003-04-1346. [DOI] [PubMed] [Google Scholar]
- 34.Kokame K, Miyata T. Semin Hematol. 2004;41:34–40. doi: 10.1053/j.seminhematol.2003.10.002. [DOI] [PubMed] [Google Scholar]