Summary
Introduction
ADAMTS-13 is a member of A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats (ADAMTS) family, primarily synthesized in hepatic stellate cells (HSCs), one of the major cell types transdifferentiating into myofibroblasts during liver fibrosis. However, the association between ADAMTS-13 expression and HSC activation or liver fibrosis is not known.
Methods
In this study, we determined the ADAMTS-13 mRNA, protein, and activity in isolated primary HSCs upon activation on a plastic dish and in liver after administration of carbon tetrachloride (CCl4) in rats.
Results
We showed that ADAMTS-13 antigen and proteolytic activity in the activated rat HSCs were dramatically increased, whereas ADAMTS-13 mRNA in these cells was only minimally altered. Similarly, the ADAMTS-13 antigen and proteolytic activity in rat liver after CCl4 injury were also significantly increased, whereas the ADAMTS-13 mRNAs in these liver tissues were only slightly increased compared with normal. Surprisingly, despite the dramatic up-regulation of ADAMTS-13 protein synthesis in the activated HSCs after CCl4 administration, the plasma levels of ADAMTS-13 protease in rats did not increase concordantly.
Conclusion
We conclude that the up-regulation of ADAMTS-13 protein expression in rat HSCs during activation in vitro and in vivo suggests the possibility of ADAMTS-13 proteolysis, an important part of function of the activated HSCs, perhaps through modulation of liver regeneration or formation of liver fibrosis after various injuries. The data also suggest the minimal contribution of the activated HSCs in regulation of plasma levels of ADAMTS-13 protease.
Keywords: gene regulation, liver fibrosis, matrix metallo-protease, von Willebrand factor-cleaving protease
Introduction
von Willebrand factor (VWF)-cleaving metalloprotease (ADAMTS-13) is a 195 kDa glycoprotein present in plasma at concentration of approximately 0.5–1.2 mg L−1 [1–4]. It cleaves VWF at the Tyr1605-Met1606 bond [5]. Inability to cleave VWF multimers into smaller forms may result in an accumulation of hyperactive [6] or ‘unusually large’ VWF multimers [7–9], leading to platelet-rich thrombi in small arteries, a characteristic pathological feature of thrombotic thrombocytopenic purpura.
ADAMTS-13 biosynthesis appears to occur primarily in the liver [10–12], particularly in activated hepatic stellate cells (HSCs) located in the space of Disse, which normally store retinoids [13–15]. However, how such a big molecule of ADAMTS-13 protease synthesized and secreted from the activated HSCs gets into the blood stream is not known. We hypothesize that the ADAMTS-13 protease produced in the activated HSCs may function locally to regulate deposition of matrix proteins or to involve in the processes of liver regeneration or formation of liver fibrosis after various acute and chronic injuries. In the setting of chronic liver disease, HSCs transdifferentiate, adopting a myofibroblast-like phenotype characterized in part by proliferation and the deposition of abnormal matrix. This phenotypic change, termed ‘activation’, has been modeled in vitro by culturing HSCs on uncoated plastic. Freshly isolated HSCs appear small, round and undifferentiated, traditionally termed ‘quiescent’, whereas cells grown on uncoated plastic for 5–7 days become elongated and dendritic in shape, termed ‘activated’ [16]. In response to TGF-β, culture-activated cells produce extracellular matrix that is similar in composition with that seen in fibrotic liver [17–20]. In addition to type I collagen, activated HSCs produce type IV collagen, laminin, and proteoglycans, which accumulate in the fibrotic liver [21], despite increased expression of several matrix metalloproteases (MMPs) and tissue inhibitors of matrix metalloproteases (TIMPs). Studies have shown that MMP-2 and membrane type 1 (MT-1)-MMP, as well as TIMP-1 and TIMP-2 [22–25], are significantly up-regulated. These and perhaps many other proteases are involved in regulating the remodeling of both normal and fibrotic liver matrix [21].
To understand the biological roles of ADAMTS-13 proteolysis in liver fibrosis, we first determined the levels of ADAMTS-13 mRNA, protein, and proteolytic activity in isolated rat primary HSCs during activation in vitro. We then determined the ADAMTS-13 expression in rat livers after administration of carbon tetrachloride (CCl4). Rats have been shown to be an excellent animal species for induction of liver fibrosis by CCl4 [16,19,23,26–29]. In this study, we found that ADAMTS-13 protein and proteolytic activity, but not ADAMTS-13 mRNA, were dramatically increased in rat primary HSCs during in vitro activation and after CCl4-induced liver injury or fibrosis. However, the plasma levels of ADAMTS-13 in rats after CCl4 injection did not increase concordantly. These data suggest that the dramatically increased ADAMTS-13 protein and proteolytic activity in the activated rat HSCs in vitro and in vivo may be important part of functions of activated HSCs, perhaps by modulating the processes of liver regeneration or formation of liver fibrosis after various insults.
Materials and methods
Isolation and culture of HSCs
Primary rat HSCs were isolated from Sprague–Dawley rats (Charles River) by in situ perfusion of pronase and collagenase, followed by a single-step density gradient centrifugation as described previously [16,29]. The purity of HSCs was determined microscopically by autofluorescence of the stored retinoids in HSC lipid droplets. The cell viability was determined by trypan blue staining (Invitrogen, Carlsbad, CA, USA). The isolated rat HSCs were consistently 95–99% pure and greater than 95% viable. The cells were grown on uncoated plastic dishes with M199 medium (Invitrogen) in the presence of 10% fetal bovine serum (FBS). Under such culture condition, the HSCs start to change shape, lose their lipid droplets and become positive in α-smooth muscle actin (α-SMA). This process was termed activation.
CCl4 treatment
Animal study protocol was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Liver fibrosis was induced in 250–300 g male Sprague–Dawley rats by twice weekly subcutaneous injection of CCl4 (0.2 mL 100 g−1 of body weight for 2 weeks, then 0.1 mL 100 g−1 of body weight, thereafter). Animals were sacrificed at 10 and 70 days after the first injection. The animals prior to CCl4 injection were used as normal control. The whole blood and liver tissues were collected. The liver tissues were used for isolation of total RNA, proteins, and activity. A fraction of tissue was frozen in Tissue-Tek® optimum cutting temperature (OCT) compound for cryostat sectioning for histological examination after being stained with hematoxylin and eosin.
RT-PCR
Total RNA was isolated from cultured cells or tissues by TRIzol reagents according to manufacturer’s recommendation (Invitrogen). One microgram of purified total RNA was transcribed using random primers and SuperScriptFirst-Strand cDNA kit (Invitrogen). The routine PCR was performed with rat-specific ADAMTS-13 primers (5′-cgaggacctccaggcta-agat-3′ and 5′-cagcatcccagtggtagaagg-3′) and rat-specific 18S ribosomal RNA primers (5′-cccagtaagtgcgg-gtcataa-3′ and 5′-gatccgagggcctcactaaac-3′) (Integrated DNA technology, Coralville, IA, USA). Serial log-dilutions of the first strand cDNA template were performed in order to reveal the difference in ADAMTS-13 transcript among various treated groups. The amplified fragments were determined by electrophoresis on 1.5% agarose gel with ethidium bromide staining (154 bp). The polymerase chain reaction (PCR) reaction was run for 34 cycles of 95 °C for 30 s, 60 °C for 50 s and 72 °C for 30 s.
For more accurate quantitation, a real-time PCR was performed with same sets of rat ADAMTS-13 primers, rat-specific α-SMA primers (5′-gcttcctcttcttccctggag-3′ and 5′-agatggctggaagagggtctc-3′) and rat-specific 18S ribosomal RNA primers. The PCR reactions were performed in a total volume of 20 μL in the presence of 250 nM of primers, 30 nM diluted reference dye (Stratagene, La Jolla, CA, USA), and brilliant SYBR Green Q PCR master mix (Stratagene). The samples were incubated for 10 min at 95 °C initially, followed by 40 cycles of 95 °C for 30 s, 60 °C for 50 s and 72 °C for 30 s. The fluorescence signal from PCR reactions was monitored in real time on an M × 4000 instrument (Stratagene). The relative amount of ADAMTS-13 was determined by a standard curve generated by a serial dilution of the single-strand cDNA and normalized to the signal of 18S ribosomal RNA.
ADAMTS-13 activity
The ADAMTS-13 activity in cell or tissue lysates or plasma was determined by its ability to cleave fluorescent resonance energy transfer (FRETS)-VWF73 (Peptide International, Lexington, KY, USA), a peptide substrate derived from the amino acid residues between D1595 and R1668 at the central A2 domain of VWF as described previously [30,31]. Briefly, cells or tissues were lyzed with 1% Triton X-100 in the presence of protease inhibitor cocktail (5 mM phenanthroline, 1 mM Pefablock, 10 μg mL−1 pepstatin A, 10 μg mL−1 leupeptin, 1 μg mL−1 aprotinin) (Sigma, St Louis, MO, USA). Ten microliters of the cleared supernatant of the cell lysates were added to FRETS-VWF73 (2 μM) in 5mM Bis-Tris, 25 mM CaCl2, and 0.005% Tween 20, pH 6.0. Fluorescence was determined at 30 °C by a fluorescence spectrophotometer (Molecular Devices Corp., Sunnyvale, CA, USA) (λex = 340 nm and λem = 450 nm) every 5 min for 1 h. The relative proteolytic activity in the samples was derived directly from the slope and compared with that of normal human plasma. The proteolytic activity in normal human plasma was arbitrarily defined as 1 U mL−1.
Protein quantification
Bieinchoninic acid (BCA) reagents (Pierce, Iselin, NJ, USA) were used to quantify total protein concentration in the cell lysates with bovine serum albumin (BSA) as a standard.
Western blotting
Cell lysates or liver tissue lysates and serum-free 24-h conditioned medium were prepared for Western blotting. Briefly, a total of 30 μg protein from rat HSC lysates or a total of 300 μg protein from liver tissue lysates was electrophoretically separated on 8% sodium dodecyl sulfate (SDS)-gels and transferred onto polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). After being blocked by 2.5% non-fat milk (Pathmark, Philadelphia, PA, USA) in 20 mM Tris–HCl, pH 7.5, and 0.05% Tween 20, the membrane was incubated with rabbit antihuman ADAMTS-13 IgG (5 μg mL−1) for 2 h, followed by antirabbit IgG, peroxidase-conjugated (1:5000) (DAKO, Carpinteria, CA, USA) and SuperSignal enhanced chemiluminescence (ECL) reagents (Pierce).
Immunohistochemistry
Frozen tissue sections (6 μm) of rat livers or cultured rat primary HSCs were fixed for 10 min with ethanol: acetic acid (9:1) at −20 °C. After being blocked with 2.5% BSA (Sigma) in phosphate buffered saline (PBS) (Invitrogen), the tissue sections or cells were incubated with rabbit antihuman ADAMTS-13 IgG (40 μg mL−1) and mouse anti-α-SMA IgG (DAKO) in 0.5% BSA in PBS, 0.05% Tween 20 for 2 h, followed by Cy2-conjugated antirabbit IgG (1:100) or Cy3-conjugated antimouse IgG (1:100) (Jackson Immuno-Research Lab, West Grove, PA, USA) as previously described [2]. After being washed three times with PBS, 0.05% Tween 20, the nuclei were stained by 4,6-diamidino-2-phenylindole (DAPI) in Vectorshield™ mounting medium (Vector Lab, Burlingame, CA, USA). The control staining was performed without addition of primary antibody.
Results
ADAMTS-13 antigen and activity, but not the ADAMTS-13 mRNA levels were dramatically increased in rat HSCs upon in vitro activation
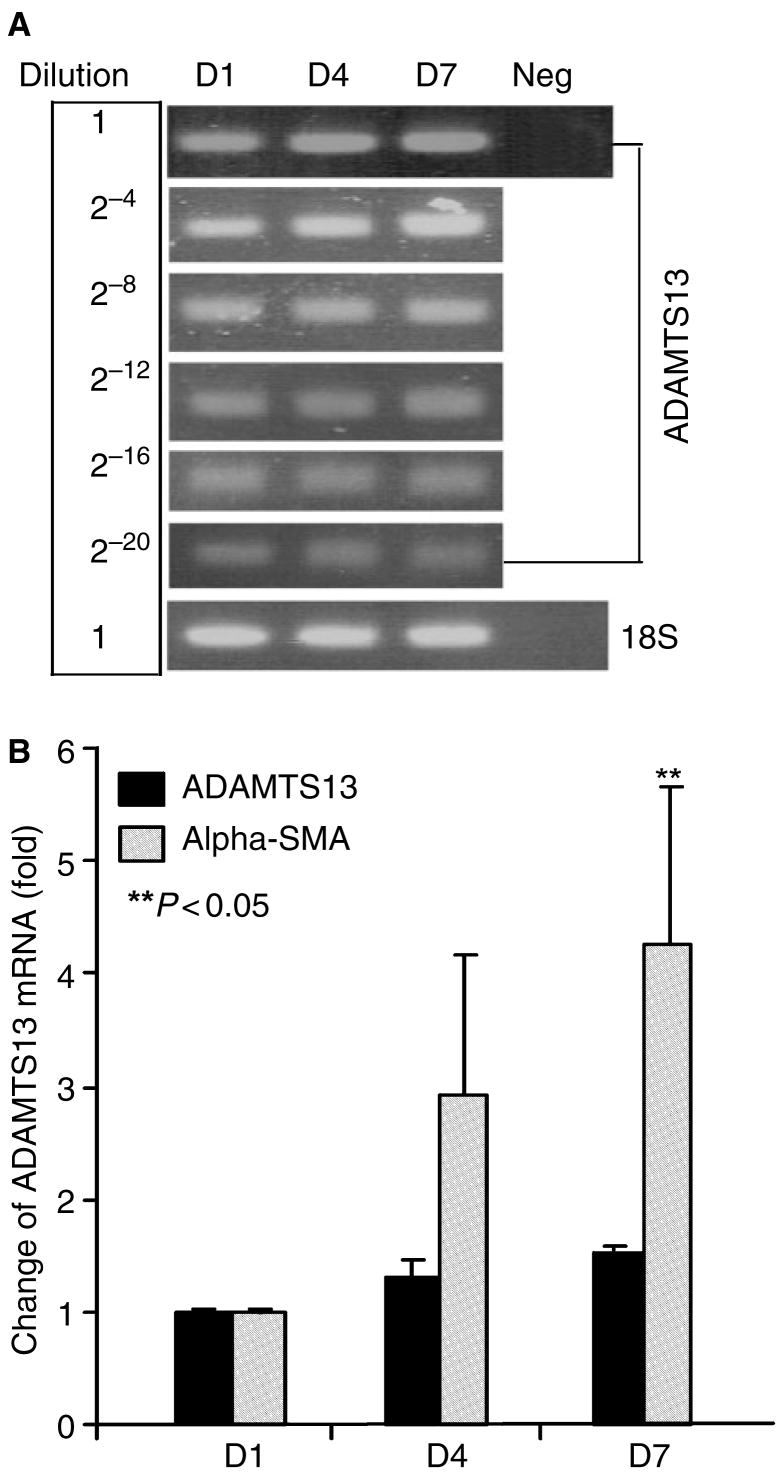
To determine the levels of ADAMTS-13 mRNA in rat HSCs during activation in vitro, we performed both routine and real-time reverse transcriptase polymerase chain reaction (RT-PCR). We first generated a single strand cDNA by random primers. We then amplified ADAMTS-13 cDNA by routine PCR after a serial log dilution of the single strand cDNA templates or by real-time PCR using the standard curve. In the real time PCR, the cDNA fragments corresponding to ADAMTS-13, α-SMA and 18S ribosomal RNA were concurrently amplified in the same reaction. We showed that upon activation of HSCs, the α-SMA mRNA was significantly up-regulated, indicating the activation of HSCs. The 18S ribosomal RNA was, however, not altered during HSCs activation, which was used as an internal control. ADAMTS-13 mRNA (154-bp) was detected after approximately 25 cycles, but the amount of PCR product after 34 cycles of amplification appeared to be equivalent among HSCs at days 1, 4, and 7 after isolation. The serial log dilution of the single-strand cDNA templates did not reveal the difference in ADAMTS-13 mRNA transcript in activated HSCs compared with the quiescent (Fig. 1A). Real time PCR showed that the levels of ADAMTS-13 mRNA in HSCs at days 4 and 7 were only increased by 30% and 50%, respectively, compared with day 1 (Fig. 1B). These data suggest that ADAMTS-13 mRNA was minimally altered in rat HSCs upon activation in vitro.
Fig. 1.
ADAMTS-13 mRNA in rat hepatic stellate cell (HSC) during in vitro activation. Total RNA was isolated from rat primary HSCs at days 1, 4 and 7 after isolation. ADAMTS-13 mRNA was quantified by routine dilution RT-PCR (A) and real-time RT-PCR (B). The amplified ADAMTS-13 fragments (154 bp) are detected by agarose-gel electrophoresis and stained by ethidium bromide after log dilutions of the single strain cDNA template (A). The relative amount of ADAMTS-13 and α-SMA mRNA was determined by standard curve and normalized to the amount of 18S ribosomal RNA in each sample in real-time PCR. While the α-SMA mRNA in rat HSCs during activation in vitro was increased, the ADAMTS-13 mRNA did not alter significantly (the entries are means ± SD, n = 3) (B).
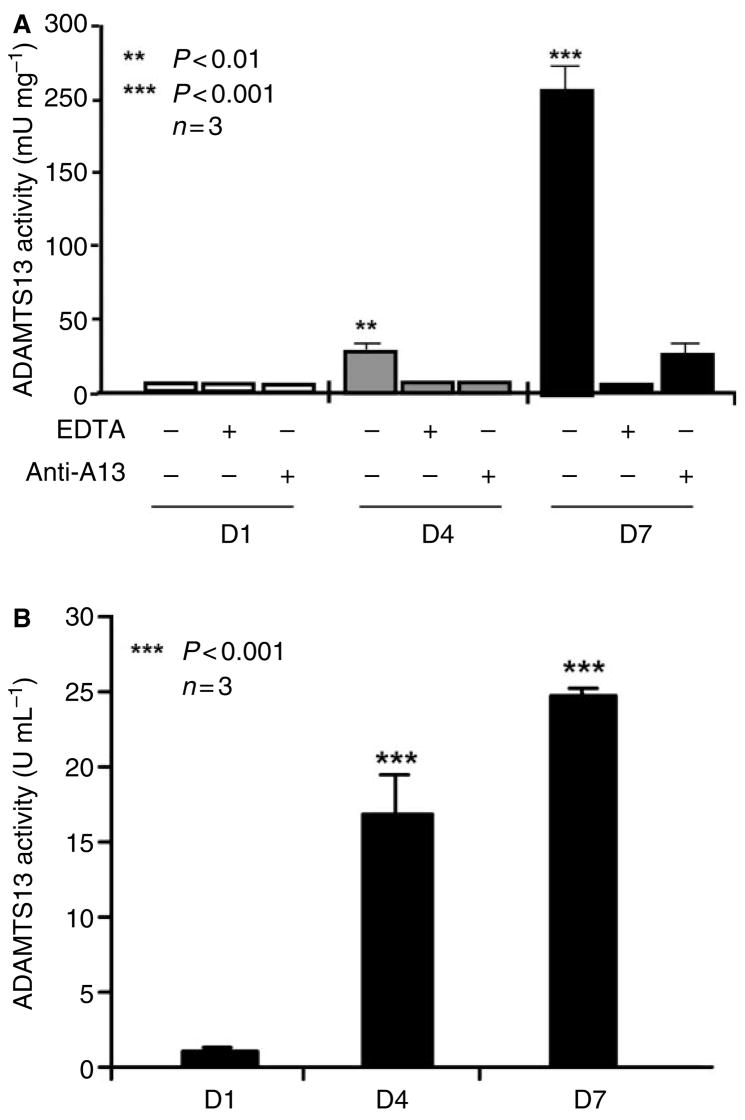
To determine the levels of ADAMTS-13 protein and proteolytic activity in rat HSCs during activation, the total proteins were extracted from cultured rat primary HSCs at days 1, 4, and 7 by 1% Triton X-100, in the presence of 0.2% protease inhibitor cocktail. ADAMTS-13 proteolytic activity was determined by cleavage of FRETS-VWF73 [30,31]. We showed that ADAMTS-13 activity in the rat HSC lysate at day 1 was undetectable, increased to approximately 250 and 2500 mU mg−1 of total protein at days 4 and 7 after isolation, respectively (Fig. 2A). The proteolytic cleavage of FRETS-VWF73 by ADAMTS-13 was not inhibited nor augmented by addition of 1% Triton X-100 and 0.2% protease inhibitor cocktail into the reaction (data not shown). The cleavage of FRETS-VWF73 by rat HSC lysate was, however, completely inhibited by the addition of 10 mM ethyldiamine tetriceticacid (EDTA) or rabbit anti-ADAMTS-13 IgG (40 μg mL−1) (Fig. 2A), suggesting the specific cleavage of the FRETS-VWF73 substrate by ADAMTS-13 protease, not by other non-specific proteases in the cell lysates. In addition, the proteolytic activity in 24 h conditioned medium of rat HSCs was also dramatically increased at days 4 and 7, respectively (Fig. 2B), compared with day 1, suggesting that the ADAMTS-13 is synthesized and secreted from activated rat HSCs.
Fig. 2.
A disintegrin and metalloprotease with thrombospondin-13 activity in cell lysate and conditioned medium of rat hepatic stellate cells (HSCs). Fluorescent resonance energy transfer (FRETS)-VWF73 substrate at 2 μM was incubated with rat HSC lysate (total protein, 10 μg) and 5 μL of 24 h conditioned medium containing 10% fetal bovine serum in the absence (−) or presence (+) of 10 mM EDTA or rabbit anti-ADAMTS-13 IgG (40 μg mL−1) (Anti-A13). A fluorescent ELISA reader detected the cleavage of FRETS-VWF73. The relative activity in the cell lysates and conditioned media was determined by comparison with that of normal human plasma (arbitrarily defined as 1 U or 1000 mU mL−1). The proteolytic activity of ADAMTS-13 in rat HSC lysate (A) and conditioned media (B) at days 4 and 7 was significantly increased compared with that at day 1. The entries are the means ± SEM (n = 3).
The data above were consistent with those obtained from Western blotting (Fig. 3) and immunocytochemical staining of rat HSCs in culture (Fig. 4). We showed that, by Western blot analysis, a 190 kDa band was detected in rat HSC lysates at day 7, but not at days 4 and 1 after isolation (Fig. 3). Immunofluorescent staining with polyclonal antihuman ADAMTS-13 IgG, however, clearly detected very weak positivity of ADAMTS-13 antigen in rat HSCs at day 1 after isolation (Fig. 4A), but strong positivity of ADAMTS-13 antigen in rat HSCs at day 7 after isolation (Fig. 4E). These ADAMTS-13-positive rat HSCs also strongly expressed α-SMA (Fig. 4F), a marker specific for activated HSCs and transdifferentiated myofibroblasts that resemble those in the fibrotic lesions of liver.
Fig. 3.
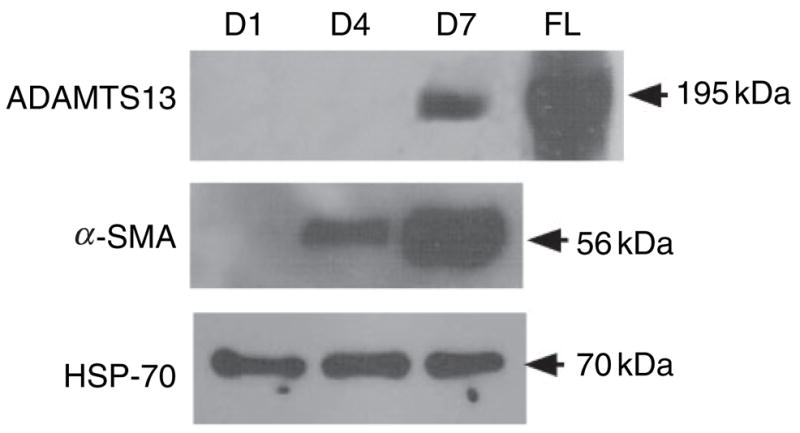
Western blotting analysis of ADAMTS-13 protein in rat hepatic stellate cells (HSCs) during activation in vitro. Total 10 μg of rat HSC cell lysate at days 1, 4 and 7 were used for Western blot. The ADAMTS-13, α-SMA, and heat shock protein-70 (HSP70) were detected by rabbit antihuman ADAMTS-13 IgG, mouse anti-α-SMA IgG, and mouse anti-HSP70 IgG, respectively. The recombinant full-length ADAMTS-13-V5-His was used as a positive control. The molecular weights of ADAMTS-13, α-SMA, and HSP70 are shown in the figure (195, 60, and 70 kDa, respectively).
Fig. 4.
Immunocytochemical staining of ADAMTS-13 antigen in cultured rat hepatic stellate cells (HSCs) during in vitro activation. Isolated primary rat HSCs at days 1 (A–D) and 7 (E–H) after isolation were fixed and stained with rabbit antihuman ADAMTS-13 IgG (1:500) (A, E) and/or mouse anti-α-SMA (1:200) (B, F), followed by a Cy3-conjugated antirabbit (red, A, E) or a Cy2-conjugated antimouse IgG (green, B, F) (1:100) as described in the Materials and methods. The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (blue, C, G, D, H). The merged images are shown in D and H. The original magnification of the digital image was 40×. The levels of ADAMTS-13 antigen in rat primary HSCs were dramatically increased upon in vitro activation.
ADAMTS-13 was significantly up-regulated in liver after CCl4
Several studies have shown the up-regulation of MMPs (MMP-1, -2, -3, and -9) and TIMPs (TIMP-1, -2, and -3) in rat liver after CCl4 [22,24,32] or bile duct ligation-induced liver fibrosis [33]. To determine whether ADAMTS-13 expression was also increased during the evolution of CCl4-induced liver fibrosis, we measured ADAMTS-13 mRNA, protein, and proteolytic activity in the liver tissues prior to injection (normal), 10 (mild fibrosis) and 70 days (severe fibrosis) after injection of CCl4. We found that ADAMTS-13 mRNA in the liver tissue lysates was only increased by 2.5- and 3.0-fold in the livers with mild fibrosis and severe fibrosis after administration of CCl4, respectively, compared with the normal (Fig. 5A,B). However, as in the case of isolated rat HSCs, both ADAMTS-13 antigen (Fig. 6) and proteolytic activity (Fig. 7) were significantly increased in the rat liver after CCl4 injury. The levels of ADAMTS-13 proteolytic activity in the liver were estimated to be approximately 600 and 4800 mU mg−1 of total protein at days 10 and 70, respectively (Fig. 7A). These results were consistent with those obtained by immunohistochemistry, showing that the number of ADAMTS-13-positive and α-SMA-positive cells was significantly higher in the liver tissues at day 10 (Fig. 6E,F) and day 70 (Fig. 6I,J) after CCl4 administration than in the control tissues (Fig. 6A,B). The ADAMTS-13-positive cells were seen in the fibrotic strips that were also expressing α-SMA (Fig. 6I, J, L), but were not seen in the areas of normal liver architecture (Fig. 6A,B,D). All the control slides without incubation with the primary antibody were essentially negative. In no case was the red fluorescence in rat HSCs and liver tissues visible under a green filter or vice versa when ADAMTS-13 or α-SMA was stained separately.
Fig. 5.
A disintegrin and metalloprotease with thrombospondin-13 (ADAMTS-13) mRNA in rat liver after CCl4. ADAMTS-13 and α-SMA mRNAs in rat livers prior to injection (normal), 10 (mild fibrosis) and 70 days (severe fibrosis) after administration of CCl4, were determined by routine RT-PCR after log dilution of the first strand cDNA template (A) and real-time RT-PCR (B) as described in the Materials and methods. The relative amount of ADAMTS-13 and α-SMA mRNAs was determined by a standard curve and normalized to 18S ribosomal RNA in the real-time PCR assay. The entries are the means ± SD (n = 3). While the levels of α-SMA mRNAs in rat HSCs after CCl4 administration were increased dramatically, the ADAMTS-13 mRNAs were only slightly increased.
Fig. 6.
Immunohistochemical staining of ADAMTS-13 antigen in rat liver after CCl4 injection. Cryosections of rat liver obtained prior to (normal, A, B, C, D) 10 (E, F, G, H) and 70 days (I, J, K, L) after administration of CCl4 were fixed and incubated with rabbit antihuman ADAMTS-13 IgG (1:500) (A, E, I) or mouse anti-α-SMA (B, F, J), followed by Cy3-conjugated antirabbit IgG (1:100) (A, E, I) or Cy2-conjugated antimouse IgG (1:100) (B, F, J). The nuclei were stained with DAPI in VECTORSHIELD™-mounting medium (C, G, K). The merged images are shown in D, H and L. The original digital images (magnification 10×) were obtained with a Nikon Inverse fluorescent microscope (Nikon, Melville, NY, USA) and processed by Canvas 7.0.
Fig. 7.
ADAMTS-13 activity in rat liver after CCl4. The proteolytic cleavage of FRETS-VWF73 by rat liver tissue lysates (A) or plasma (B) prior to CCl4 injection (normal), 10 (mild fibrosis) and 70 days (severe fibrosis) after administration of CCl4 was determined as described in the Materials and methods. Although the levels of ADAMTS-13 activity in the liver was dramatically increased 10 and 70 days after CCl4 injection (A), the levels of ADAMTS-13 activity in the plasma were not altered significantly compared with that of normal control (B).
In humans and mice, ADAMTS-13 synthesized and secreted from the activated HSCs were thought to be the major source of plasma ADAMTS-13 protease [14,15], although how such a big protein gets into the blood stream is not known at all. To our surprise, we found that despite the dramatic up-regulation of ADAMTS-13 antigen and proteolytic activity in the liver after CCl4 injection, the plasma ADAMTS-13 activity in rats with mild liver fibrosis and severe liver fibrosis was even lower or slightly higher, although the differences were not statistically significant (P > 0.05) when compared with that in normal rats (Fig. 7B). The reason for slight reduction of ADAMTS-13 activity at day 10 after CCl4 is not known. The CCl4 at concentration of 6.5 mM (similar to initially injected concentration) did not directly enhance or inhibit the cleavage of FRETS-VWF73 by ADAMTS-13 in vitro (data not shown). These data suggest that the activated HSCs may contribute little to regulation of plasma levels of ADAMTS-13 protease.
Discussion
The present study demonstrates for the first time a dramatic up-regulation of ADAMTS-13 antigen and proteolytic activity, but not ADAMTS-13 mRNA in isolated rat primary HSCs upon activation in vitro by culturing them in uncoated plastic plates and in rat liver after CCl4-induced liver fibrosis. We showed by both routine and real-time RT-PCR experiments that the levels of ADAMTS-13 mRNA were only increased by 30% and 50% in rat primary HSC at days 4 and 7 after isolation, respectively, when compared with day 1 (Fig. 1). Similarly, the levels of ADAMTS-13 mRNA in the liver tissues were only increased by 2.5- and 3.0-fold at days 10 (when liver exhibited mild fibrosis) and 70 (when liver exhibited severe fibrosis), respectively, after administration of CCl4 (Fig. 5). These data suggest that regulation of ADAMTS-13 expression in rat HSCs during in vitro and in vivo activation does not occur to a significant degree at the transcriptional level.
Our data are consistent with the results reported by Claus et al. [34] showing that the alteration of ADAMTS-13 mRNA transcript in Hep3B cells is negligible after being stimulated by various pro-inflammatory stimuli such as endotoxin, tissue necrosis factor-α, interleukin-2 and -1β, and immunosuppressive agents such as cyclosporine and cortical steroids. However, the levels of ADAMTS-13 antigen and proteolytic activity in Hep3B cells and conditioned medium prior to or after various stimuli were not investigated in their study [34]. We showed, by FRETS-VWF73 and Western blotting, that the levels of ADAMTS-13 activity and antigen were dramatically increased in rat HSCs upon activation both in vitro (Figs 2 and 3) and in vivo (Fig. 7) after CCl4-induced liver fibrosis. These results were corroborated by immunohistochemical staining demonstrating that ADAMTS-13 immunereactivity was also dramatically enhanced in activated rat HSCs (Fig. 4) and in liver tissues after CCl4-induced liver injury and fibrosis (Fig. 6). Surprisingly, the plasma ADAMTS-13 proteolytic activity did not change significantly in rats with mild-and-severe fibrosis after CCl4 injection despite of a dramatic up-regulation of ADAMTS-13 antigen and activity in activated rat HSCs (Fig. 7), suggesting that the activated HSCs may not be the primary source of plasma ADAMTS-13. This hypothesis is consistent with the phenomenon observed in humans in which plasma ADAMTS-13 activity is quite variable or frequently reduced in patients with liver cirrhosis [35]. Indeed, recent studies have suggested that ADAMTS-13 is also synthesized and secreted from arterial and venous vascular endothelial cells [36], megakaryocytes, and platelets [37,38]. The ADAMTS-13 mRNA is detected in brain, heart, lung, liver, kidney, adrenal glands, uterus, placenta, and prostate in humans by RT-PCR [11,39], suggesting a small amount of ADAMTS-13 protease may be produced in various organ tissues that contribute to the regulation of plasma levels of ADAMTS-13.
The mechanisms of the apparent discrepancy in the increment of ADAMTS-13 protein or activity and ADAMTS-13 mRNA remain to be determined. One could speculate that the increased ADAMTS-13 protein upon rat HSC activation or CCl4-induced liver injury or fibrosis may be resulted from an increase in protein translation efficiency and/or a reduction in protein degradation upon activation of HSCs in vitro and in vivo. It may also be possible that the increased ADAMTS-13 proteolytic activity in the activated rat HSCs is because of up-regulation of certain cofactors [40,41] such as heparin sulfate or other unknown factors for the ADAMTS-13 protease in the cells. However, the ADAMTS-13 activity in the conditioned medium of cultured rat HSCs correlated well with the increased expression of ADAMTS-13 in the cells (Fig. 2B), suggesting that the ADAMTS-13 is readily secreted from activated rat HSCs in culture.
Several other MMPs [24,42] including MMP-3, -13, pro-MMP-2, and MT1-MMP and TIMPs, particularly TIMP-1 and TIMP-2 are up-regulated in rat livers upon CCl4-injury [23,24,43], suggesting that the presence of active MMPs in acute and chronic liver injury may cleave extracellular matrix proteins, limit an excessive deposition of such matrix proteins and prevent liver fibrosis. This sequence of events may have a physiological function in liver regeneration. Considering that the only known substrate for ADAMTS-13, VWF [5,44,45], which is up-regulated in the microvascular endothelial cells in the area of necrosis and fiber-like structure during liver injury [46], one may speculate that the presence of the ADAMTS-13 protease at the time of tissue injury may also limit formation of microvascular thrombosis in situ or prevent excessive matrix protein deposition [46] and development of fibrosis in the liver [47,48]. However, there is no evidence thus far to demonstrate that ADAMTS-13 cleaves other matrix proteins except for VWF. The interaction between VWF and ADAMTS-13 protease do, however, occur intracellularly, particularly in the endoplasmic reticulum when both of them are expressed in the same cells [3]. The exact contribution of the enhanced ADAMTS-13 expression and proteolysis to liver fibrosis is, however, yet to be confirmed in future studies in animal models.
In summary, our data suggest that the dramatic up-regulation of ADAMTS-13 antigen and proteolytic activity during rat HSC activation in vitro and in vivo by CCl4 injection may be crucial for the biological functions of the activated HSCs. ADMTS13 proteolysis may play a role in modulating liver regeneration or formation of fibrotic scar in injured liver after various acute and chronic insults. The data also suggest the sources of plasma ADAMTS-13 may be multiple. Finally, we believe that rat HSCs may be an excellent rat model for studying the regulation of ADAMTS-13 expression at posttranscriptional level.
Acknowledgments
The authors thank Dr Suresh Shelat for his comments on the manuscript. This study was supported in part by grants from National Institute of Health (HL079027 and DK58123 to XLZ and RGW, respectively), the Doris Duke Charitable Foundation (to ESP) and the American Heart Association (to XLZ).
References
- 1.Gerritsen HE, Robles R, Lammle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood. 2001;98:1654–61. doi: 10.1182/blood.v98.6.1654. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–41. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majerus EM, Zheng X, Tuley EA, Sadler JE. Cleavage of the ADAMTS13 propeptide is not required for protease activity. J Biol Chem. 2003;278:46643–8. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono T, Mimuro J, Madoiwa S, Soejima K, Kashiwakura Y, Ishiwata A, Takano K, Ohmori T, Sakata Y. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107:528–34. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 5.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–34. [PubMed] [Google Scholar]
- 6.Hulstein JJ, de Groot PG, Silence K, Veyradier A, Fijnheer R, Lenting PJ. A novel nanobody that detects the gain-of-function phenotype of Von Willebrand factor in adamts13 deficiency and Von Willebrand disease type 2B. Blood. 2005;106:3035–42. doi: 10.1182/blood-2005-03-1153. [DOI] [PubMed] [Google Scholar]
- 7.Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, Azocar J, Seder RH, Hong SL, Deykin D. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–5. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 8.Moake JL, Chow TW. Willebrand factor (vWf) binding to platelets associated with impaired vWf breakdown in thrombotic thrombocytopenic purpura. J Clin Apheresis. 1998;13:126–32. doi: 10.1002/(sici)1098-1101(1998)13:3<126::aid-jca6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–63. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 11.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 12.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem (Tokyo) 2001;130:475–80. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 13.Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143–7. doi: 10.1136/bmj.327.7407.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–4. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Inada M, Lee TP, Benten D, Lyubsky S, Bouhassira EE, Gupta S, Tsai HM. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–8. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Gaca MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem. 2003;278:11721–8. doi: 10.1074/jbc.M207728200. [DOI] [PubMed] [Google Scholar]
- 17.Milani S, Herbst H, Schuppan D, Grappone C, Heinrichs OE. Cellular sources of extracellular matrix proteins in normal and fibrotic liver. Studies of gene expression by in situ hybridization. JHepatol. 1995;22:71–6. [PubMed] [Google Scholar]
- 18.Ramadori G, Knittel T, Odenthal M, Schwogler S, Neubauer K, Meyer zum Buschenfelde KH. Synthesis of cellular fibronectin by rat liver fat-storing (Ito) cells: regulation by cytokines. Gastroenterology. 1992;103:1313–21. doi: 10.1016/0016-5085(92)91522-6. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SL, Yamasaki G, Wong L. Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem. 1994;269:10551–8. [PubMed] [Google Scholar]
- 20.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 21.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–9. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 22.Takahara T, Furui K, Funaki J, Nakayama Y, Itoh H, Miyabayashi C, Sato H, Seiki M, Ooshima A, Watanabe A. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology. 1995;21:787–95. [PubMed] [Google Scholar]
- 23.Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13:S33–8. doi: 10.1111/jgh.1998.13.s1.33. [DOI] [PubMed] [Google Scholar]
- 24.Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443–53. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850–60. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann A, Zhao D, Reichen J. Myofibroblasts in the cirrhotic rat liver reflect hepatic remodeling and correlate with fibrosis and sinusoidal capillarization. J Hepatol. 1999;30:646–52. doi: 10.1016/s0168-8278(99)80195-0. [DOI] [PubMed] [Google Scholar]
- 27.Nagaoka MR, Kouyoumdjian M, Borges DR. Hepatic clearance of tissue-type plasminogen activator and plasma kallikrein in experimental liver fibrosis. Liver Int. 2003;23:476–83. doi: 10.1111/j.1478-3231.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- 28.Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B, Feirt N, Mei B, Cho MS, Ramamoorthi R, Roldan G, Ng P, Lum P, Hirth-Dietrich C, Tomkinson A, Brenner DA. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106–15. doi: 10.1002/hep.20425. [DOI] [PubMed] [Google Scholar]
- 29.Drano JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–24. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 31.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280:29428–34. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okazaki I, Watanabe T, Hozawa S, Arai M, Maruyama K. Molecular mechanism of the reversibility of hepatic fibrosis: with special reference to the role of matrix metalloproteinases. J Gastroenterol Hepatol. 2000;15:D26–32. doi: 10.1046/j.1440-1746.2000.02185.x. [DOI] [PubMed] [Google Scholar]
- 33.Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895–902. doi: 10.1016/S0002-9440(10)65703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claus RA, Bockmeyer CL, Kentouche K, Sieber MW, Oberle V, Kaufmann R, Deigner HP, Losche W. Transcriptional regulation of ADAMTS13. Thromb Haemost. 2005;94:41–5. doi: 10.1160/TH04-08-0498. [DOI] [PubMed] [Google Scholar]
- 35.Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98:2730–5. doi: 10.1182/blood.v98.9.2730. [DOI] [PubMed] [Google Scholar]
- 36.Shang D, Zheng X, Ai J, Zheng X. Biosynthesis and apical secretion of ADAMTS13 metalloprotease in vascular endothelial cells and Madin-Darby canine kidney cells. Blood. 2005;106:744a. doi: 10.1182/blood-2006-02-002139. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived VWF-cleaving metalloprotease ADAMTS-13. J Thromb Haemost. 2005;3:2536–44. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, Murata M, Matsubara Y, Uchida T, Ishihara H, Shibano T, Ashida S, Soejima K, Okada Y, Ikeda Y. Detection of von Willebrand factor-cleaving protease (ADAMTS-13) in human platelets. Biochem Biophys Res Commun. 2004;313:212–6. doi: 10.1016/j.bbrc.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 39.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, Furlan M, Gerritsen H, Lammle B, Schwarz HP, Scheiflinger F. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100:3626–32. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 40.Nishio K, Anderson PJ, Zheng XL, Sadler JE. Binding of platelet glycoprotein Ibalpha to von Willebrand factor domain A1 stimulates the cleavage of the adjacent domain A2 by ADAMTS13. Proc Natl Acad Sci USA. 2004;101:10578–83. doi: 10.1073/pnas.0402041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Cleavage of ultralarge multimers of von Willebrand factor by C-terminal-truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–3. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373–84. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- 43.Murphy G, Ward R, Hembry RM, Reynolds JJ, Kuhn K, Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989;258:463–72. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–44. [PubMed] [Google Scholar]
- 45.Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389–94. doi: 10.1097/00062752-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Knittel T, Neubauer K, Armbrust T, Ramadori G. Expression of von Willebrand factor in normal and diseased rat livers and in cultivated liver cells. Hepatology. 1995;21:470–6. [PubMed] [Google Scholar]
- 47.Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK. Extra cellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- 48.Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]