Summary
Background
Many patients with acquired thrombotic thrombocytopenic purpura (TTP) harbor autoantibodies that may bind and/or inhibit ADAMTS-13 proteolytic activity and accelerate its clearance in vivo.
Methods
To test this hypothesis, we determined ADAMTS-13 activity and antigen levels in parallel plasma samples from patients clinically diagnosed with TTP. Collagen binding, GST-VWF73 and FRETS-VWF73 assays were used to determine ADAMTS-13 activity and to detect inhibitory autoantibodies. Enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation plus Western blotting (IP/WB) were used to detect total anti-ADAMTS-13 IgG (inhibitory and non-inhibitory).
Results
Among 40 patients with TTP (21 idiopathic and 19 non-idiopathic), inhibitory autoantibodies were detected (by FRETS-VWF73) in 52% of idiopathic and 0% of non-idiopathic TTP patients. In contrast, non-inhibitory IgG autoantibodies were detected in 29% of idiopathic and 50% of non-idiopathic TTP patients. The concentration of inhibitory IgG autoantibody in idiopathic TTP patients was significantly higher than that of non-inhibitory IgG in either idiopathic or non-idiopathic TTP patients. Idiopathic TTP patients demonstrated significantly reduced ADAMTS-13 activity compared with non-idiopathic patients, but only slightly lower ADAMTS-13 antigen levels. Interestingly, patients with inhibitory autoantibodies exhibited significantly lower ADAMTS-13 antigen levels than those with only non-inhibitory IgG autoantibodies or no autoantibody. Serial plasma exchanges increased levels of ADAMTS-13 activity and antigen concurrently in patients with inhibitory autoantibodies.
Conclusion
The identification of severe ADAMTS-13 deficiency and autoantibodies or inhibitors appears to be assay-dependent; the inhibitory IgG autoantibodies, in addition to binding and inhibiting ADAMTS-13 proteolytic activity, may accelerate ADAMTS-13 clearance in vivo.
Keywords: a disintegrin and metalloprotease with thrombospondin type 1 repeats, autoimmune disorder, thrombotic microangiopathy, von Willebrand factor
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disseminated thrombotic microangiopathy (TMA) with platelet clumping in the microvascular circulation [1,2], characterized by thrombocytopenia and microangiopathic hemolytic anemia. The classical definition of TTP also includes neurological symptoms, renal failure, and fever; the latter occurs in one-third of cases [3–6]. Acquired deficiency of ADAMTS-13, the 13th member of the A Disintegrin And Metalloprotease with ThromboSpondin type 1 family [7–11] is considered to be the underlying cause for many cases of idiopathic TTP [12,13].
ADAMTS-13 protease cleaves the Tyr1605–Met1606 bond at the A2 domain of von Willebrand factor (VWF), thereby regulating the sizes of VWF multimers released from the Weibel–Palade bodies of vascular endothelial cells and the α-granules of platelets [14,15]. Inability to cleave the newly released VWF multimers leads to an accumulation of ‘unusually large’ VWF multimers that are very adhesive and may lead to spontaneous platelet adhesion and aggregation at sites of vascular injury. These ‘unusually large’ VWF multimers may be released from the endothelial surface to form micro-thrombi in small arterioles downstream [2,16,17].
Patients with congenital TTP (Upshaw–Schülman syndrome) all have severe deficiency of ADAMTS-13 activity [< 5%–10% of normal human plasma (NHP)], apparently caused by mutations of the ADAMTS-13 gene [11,18]. However, patients with acquired idiopathic TTP may have severe deficiency of ADAMTS-13 activity that is often caused by autoantibodies that neutralize ADAMTS-13 activity [13,19–21]. Patients with non-idiopathic TTP that is associated with pregnancy, autoimmune disease, human immunodeficiency virus infection, hematopoietic progenitor cell transplantation, cancer, chemotherapy, and other drugs (cyclosporine A, FK506, mitomycin, and clopidrogel) usually have normal or moderately reduced ADAMTS-13 activity [20,22]. Severe deficiency of ADAMTS-13 in non-idiopathic TTP is uncommon except for ticlopidine-associated TTP, in which severe ADAMTS-13 deficiency and autoantibodies may be present [23]. The clinical heterogeneity poses a challenge for understanding of the pathogenesis of TTP and selecting appropriate therapies.
The presence of severe ADAMTS-13 deficiency and autoantibody inhibitors increases the likelihood of a diagnosis of TTP and provides a rationale to consider adjunctive immune therapies in a subset of patients [13,20–22,24–26]. However, current functional assays detect autoantibodies in patients with TTP at variable rates. In one report, nearly all patients harbored inhibitors that blocked cleavage of VWF by normal human plasma (NHP) [13]. The likelihood of detecting an anti-ADAMTS-13 autoantibody decreases to 31%–48% in prospective studies in less selective patient populations [20,22]. This low-detection rate may reflect false-negatives in activity-based assays, due to very low autoantibody concentration, presence of denaturing reagents in the assay system or prolonged incubation of the reaction. Alternatively, some patients may harbor autoantibodies that bind ADAMTS-13, but do not inhibit its activity [27]; therefore, they are not detected by the functional assays. Our previous longitudinal study has shown that plasma exchange therapy does not quickly normalize plasma ADAMTS-13 activity as expected in some patients with undetectable autoantibodies. Rather, 2–7 days of plasma exchange were necessary to raise the plasma ADAMTS-13 activity [20], suggesting that the autoantibodies may be present, but undetectable by the functional assays.
To determine the prevalence of the inhibitory and non-inhibitory autoantibodies, we used functional assays (collagen binding, GST-VWF73, and FRETS-VWF73) to identify the inhibitory autoantibodies and immunological assays [enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation plus Western blot] to identify both inhibitory and non-inhibitory autoantibodies in patients with TTP. In addition, we determined ADAMTS-13 antigen levels to assess whether the binding of the inhibitory and non-inhibitory IgG autoantibodies to ADAMTS-13 protease can accelerate its clearance in vivo.
In this study, we showed that the inhibitory autoantibodies were detected in 52% of idiopathic and 0% of non-idiopathic TTP patients; the non-inhibitory IgG autoantibodies were identified in 29% of patients with idiopathic TTP and 50% of patients with non-idiopathic TTP. The patients with inhibitory autoantibodies exhibited significantly reduced levels of plasma ADAMTS-13 antigen, whereas the patients with non-inhibitory autoantibodies did not, suggesting that only inhibitory autoantibodies against ADAMTS-13 bind ADAMTS-13 protease and may accelerate its clearance in vivo.
Materials and methods
Patients
The study protocol was reviewed and approved by the Institutional Review Board at The Children’s Hospital of Philadelphia and The University of Pennsylvania. The informed consent was obtained from patients or patient’s legal guardian. Adult and pediatric patients with a clinical diagnosis of TTP admitted for plasma exchange therapy were enrolled into the study. Patient data and laboratory values are shown in Table 1. Diagnostic criteria of TTP [20,22] and some of patients [20,21] were published previously: (i) microangiopathic hemolytic anemia (hemoglobin < 12.5 g dL−1, three or more schistocytes or helmet cells per high-power field in the peripheral blood smear; (ii) thrombocytopenia (platelet count < 150 × 109 L−1); and (iii) no requirement for neurological symptoms, renal function abnormalities, or fever. Although the syndrome with only thrombocytopenia and microangiopathic hemolytic anemia was traditionally termed thrombotic microangiopathies, some consider it sufficient to enter it as a clinical diagnosis of TTP [20,22,28]. TTP is further classified into idiopathic and non-idiopathic based on etiologies. ‘Idiopathic’ TTP (n = 21 patients) is defined as TTP occurring in patients with no apparent pre-existing or concurrent illness; ‘non-idiopathic’ TTP (n = 19 patients) is defined as TTP occurring in patients after various obvious etiologies including hematopoietic stem cell transplantation, disseminated cancer/chemotherapy, use of certain medications, and pregnancy [20,22,28]. Some may consider this group as ‘thrombotic microangiopathy (TMA) due to other causes’.
Table 1.
Summary of laboratory data in patients with thrombotic thrombocytopenic purpura (TTP)
| Idiopathic TTP (Total n = 21) | Non-idiopathic TTP (Total n = 19) | P-value* | |
|---|---|---|---|
| Age (years) | 46.9 ± 4.9 (17)** | 35.2 ± 4.3 (18) | 0.08 |
| Platelet count (×109 L−1) | 25.2 ± 6.5 (19) | 45.4 ± 11.3 (18) | 0.12 |
| Hematocrit (%) | 26.5 ± 1.5 (19) | 27.1 ± 1.0 (18) | 0.79 |
| Lactate dehydrogenase (U mL−1) | 1170 ± 159 (18) | 2739 ± 1588 (17) | 0.32 |
| Creatinine (mg dL−1) | 1.32 ± 0.13 (15) | 2.66 ± 0.46 (13) | 0.006 |
P-value < 0.05 is considered to be statistically significant;
values are mean ± SE; the numbers in parenthesis are the number of patients for whom data were available. In any case the clinical diagnosis of TTP was made and plasma exchange was initiated.
ADAMTS-13 activity < 10%, 10%–50% and ≥ 50% of normal human plasma was considered to be ‘severe deficiency’, ‘moderate deficiency’, and ‘normal value’. ‘Inhibitory autoantibodies’ were those immunoglobulins (IgG, IgM or other isotypes) that block proteolytic cleavage of ADAMTS-13 substrate (either VWF, GST-VWF73-H or FRETS-VWF73) in the in vitro assays. ‘Inhibitory’ anti-ADAMTS-13 IgG was defined as the immunoglobulin G that binds ADAMTS-13 [detected by immunological assays (see below)] and blocks ADAMTS-13 proteolytic activity (detected by FRET-VWF73 assay). ‘Non-inhibitory’ anti-ADAMTS-13 IgG was defined as the immunoglobulin G that merely binds ADAMTS-13 protease, but does not block ADAMTS-13 activity in the in vitro functional assay (Table 2).
Table 2.
Definition of autoantibodies in patients with thrombotic thrombocytopenic purpura (TTP)
| Inhibitory Ab | Non-inhibitory Ab | No Ab | |
|---|---|---|---|
| ADAMTS-13 activity (by FRETS) | <10% | Any | Any |
| Neutralizing activity (>30%) (by FRETS) | Yes | No | No |
| Anti-ADAMTS-13 IgG (by ELISA) | Positive (>15 U mL−1) | Positive (>15 U mL−1) | Negative (<12 U mL−1) |
ELISA, enzyme-linked immunosorbent assay; Ab, anti-ADAMTS13 IgG autoantibody; FRETS, cleavage of FRETS-VWF73 substrate.
Sample collection
Citrated (3.5%) whole blood (5 mL, adult patients; 1 mL, pediatric patients) was obtained from 40 patients with clinical diagnosis of TTP prior to initiation of plasma exchange therapy. The plasma was prepared after centrifugation at 1500 × g for 10 min, collected and stored at −80 °C. Pooled normal human plasma from 20 healthy donors was used for a reference.
Collagen-binding assay
This assay using purified human plasma VWF as substrate was described previously [20,29]. Briefly, patient plasma was diluted 1:10 with 1.5 M urea in 5 mM Tris–HCl, pH 8.0 and activated with 10 mM BaCl2 for 5 min. It was then mixed with purified VWF (10 μg mL−1) in presence of 0.1% protease inhibitor cocktail (Sigma, St Louis, MO, USA) and incubated at 37 °C overnight. The reaction was stopped with 10 mM of Na2SO4 and centrifuged at 1100 × g for 3 min at room temperature. The supernatant was diluted 1:5 in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA), 0.05% Tween 20, and then added to a MaxiSorb microtiter plate (Nunc, Rochester, NY, USA) that had been precoated with human collagen type III (Southern Biotech, Birmingham, AL, USA). The plate was incubated at 37 °C for 1 h and then washed three times with PBS. Peroxidase-conjugated antihuman VWF antibody (P0226; DakoCytomation, Carpinteria, CA, USA) was diluted 1:3000 in PBS containing 0.5% BSA, 0.05% Tween 20 and incubated at 37 °C for 1 h. After three washes with PBS, the peroxidase substrate o-phenylenediamine, (OPD; Sigma) was added to the plate to identify the bound VWF. The color reaction was stopped with 1.5 N H2SO4 in distilled water and the plate was read at 492/620 nm on Spectra Max 190 ELISA Reader (Molecular Devices, Union City, CA, USA). Normal human plasma was used as a reference.
FRETS-VWF73 assay
The chemically synthesized fluorescence-quenching substrate, FRETS-VWF73 (Peptides International, Louisville, KY, USA), was used for this assay as previously described [30]. Patient plasma was diluted 1:40 in 5 mM Bis–Tris, 25 mM CaCl2, 0.005% Tween 20, pH 6.0 in a black 96-well polypropylene plate (Fisher Scientif, Pittsburgh, PA, USA). FRETS-VWF73 substrate diluted 1:50 in buffer containing 5 mM Bis-Tris, 25 mM CaCl2, 0.005% Tween 20, pH6.0 was added to the plate (final concentration 2.0 μM). The plate was immediately read at λex 340 nm and lambda;em 450 nm at 30 °C on SpectraMax Gemini ELISA Reader (Molecular Devices, Downingtown, PA, USA) every 5 min for 1 h. Data were analyzed with SoftMaxPro 4.8 software (Molecular Devices). Pooled NHP was used as a reference.
GST-VWF73 assay
Recombinant GST-VWF73-His was expressed in Escherichia coli BL21 cells and purified by HiTrap Ni-chelating column and glutathione-agarose (BD Biosciences, San Jose, CA, USA) as previously described [31–33]. It consists of 73 amino acids derived from the central A2 domain of VWF and is flanked by a glutathione S-transferase protein (GST) at its N-terminus and a 6xHis epitope at its C-terminus [31–33]. The molecular weight of uncleaved substrate is ~38 kDa. Specific cleavage of GST-VWF73 at the Tyr–Met bond by plasma ADAMTS-13 protease yielded a N-terminal fragment (~34 kDa) that was detected with anti-GST antibody [31–33] on Western blot. The quantification of the chemiluminescent signal of the cleaved band was performed with densitometry using ImageJ software. Pooled NHP was used as a reference (http://rsb.info.nih.gov/ij/download.html; accessed 16 May 2006).
Inhibitory autoantibody assays
The inhibitory autoantibodies against ADAMTS-13 were determined by the activity assays except for heating patient plasma (NHP as controls) at 56 °C for 30 min to eliminate endogenous ADAMTS-13 protease activity before 50:50 mixing with NHP. The inhibitory autoantibodies were considered to be positive if patient plasma blocked ≥ 30% of proteolytic cleavage of the substrates by NHP and negative if they did not block at least 30% of such cleavage.
Expression of recombinant ADAMTS-13 protease
Human embryonic kidney cells (HEK) 293 (American Type Cell Collection, Manassas, VA, USA) were transfected with pcDNA3.1 carrying a human full-length ADAMTS-13 cDNA tagged with V5-His at its C-terminus using lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) [9,33,34]. After 72 h, the cells were diluted and seeded on 48-well plate in DMEM containing 10% fetal Plex (Genimi Biosciences, Woodland, CA, USA) and 0.5 mg mL−1 G418 (Invitrogen, Carlsbad, CA, USA). The clones expressing high levels of ADAMTS-13-V5-His were screened by proteolytic cleavage of FRETS-VWF73 as described above. The serum-free conditioned medium was collected from stably transfected HEK 293 cells and concentrated with Centriprep-30. The concentrated conditioned medium was kept at −80 °C until use.
Anti-ADAMTS-13 IgG ELISA
The ELISA kit for IgG autoantibodies against ADAMTS-13 was purchased from Technoclone (Vienna, Austria). Patient plasma (diluted 1:100 in assay buffer) was incubated in an ELISA plate that was precoated with recombinant human full-length ADAMTS-13. Calibrated plasma (with known concentration of anti-ADAMTS-13 IgG) was supplied by the manufacturer and used as a standard. The anti-ADAMTS-13 IgG in patient’s plasma were detected by a colorimetric substrate reaction with peroxidase-conjugated antihuman IgG and measured in U mL−1. A patient’s sample was defined as negative for anti-ADAMTS-13 IgG if value < 12 U mL−1, borderline if 12–15 U mL−1, and positive if > 15 U mL−1.
Immunoprecipitation plus Western blotting
The 10-fold concentrated conditioned medium (40 μL) containing full-length recombinant ADAMTS-13-V5-His (~100 ng) was incubated with 20 μL of patient plasma or NHP (control) in presence of 0.1% protease inhibitors (Sigma), at 4 °C overnight. Then protein A-Sepharose 4B (20 μL) (EHealth Care Biosciences, Piscataway, NJ, USA) was added and gently rocked at room temperature for 2 h to allow IgG-ADAMTS-13 complexes to form. After three washes with PBS, pH 7.4, the antigen–antibody complexes were eluted from the protein A-agarose beads by boiling for 5 min with 40 μL of Laemmli buffer containing 5% β-ME. The eluate was separated on 8% sodium dodecyl sulphate-polyacrylamide gel and transferred to the nitrocellular membrane. The recombinant ADAMTS-13-V5-His was detected by monoclonal anti-V5 IgG (1:5000), followed by antimouse IgG (1:5000), peroxidase conjugated (DakoCytomation, Carpinteria, CA, USA) and SuperSignal™ chemiluminescent reagents (Pierce, Rockford, IL, USA). A 190 kDa band indicates full-length ADAMTS-13 bound to IgG–protein A-Sepharose complexes and thus positive anti-ADAMTS-13 IgG in patient plasma.
ADAMTS-13 antigen ELISA
The ADAMTS-13 antigen levels in patient plasma were determined by ELISA kit according to manufacturer’s instructions. ADAMTS-13 antigen in pooled citrated NHP is 540 ± 190 ng mL−1 (n = 22) with intra and inter-assay coefficient of variations for this ELISA are 4.0% and 7.3%, respectively (American Diagnostica, Stamford, CT, USA).
Statistical analysis
The association of categorical variables was evaluated by the chi-squared analysis, continuous variables by Student’s t-test or ANOVA followed by Bonferroni multiple comparison tests. Kruskall–Wallis and Mann–Whitney U-tests were performed for non-parametric tests. A value of P < 0.05 was considered statistically significant. The intra- and inter-assay coefficients of variation for all three functional assays were< 10% and 20%, respectively.
Results
ADAMTS-13 activity in patients with idiopathic and non-idiopathic TTP
There is considerable debate whether ADAMTS-13 deficiency (even severe deficiency) is a specific diagnostic indicator of TTP [26,35]. This disagreement may result from the methodologies being used. Although a previous study demonstrated good overall correlation between many indirect assays (by estimating the loss of VWF or its subunit after incubation with ADAMTS- 13 protease in plasma) [36], the agreement between direct assays (cleavage of a VWF substrate) and indirect assays has not been extensively evaluated. We therefore compared the ability of several commonly used assays including collagen binding [20,29,37–40], GST-VWF73 [31–33] and FRETS-VWF73 [30,33,41,42] to detect ADAMTS-13 deficiency and autoantibody inhibitors.
The ADAMTS-13 activity in parallel plasma samples from 40 patients with TTP was determined by three different functional assays and their correlation coefficients were determined (Fig. 1). The closest correlation occurred between collagen-binding assay and FRETS-VWF73 (r = 0.83), but less so between collagen binding and GST-VWF73 (r = 0.62). At low proteolytic activity, GST-VWF73 showed a systemic bias toward higher values, likely due to the non-linear nature of the chemiluminescent signal. The coefficients of determination indicate that it was unlikely that the correlation observed between any one of the assays resulted from random sampling (collagen binding vs. GST-VWF73, r2 = 0.39, P < 0.0001; collagen binding vs. FRETS-VWF73, r2 = 0.70, P < 0.0001; FRETS-VWF73 vs. GST-VWF73, r2 = 0.53, P < 0.0001) (Fig. 1).
Fig. 1.
Comparison of ADAMTS-13 activity in parallel samples assayed by three different methodologies. Pearson correlation coefficients were determined between collagen binding and GST-VWF73 (A, r = 0.62); between GST-VWF73 and FRETS-VWF73 (B, r = 0.73); between collagen binding and FRETS-VWF73 (C, r = 0.83). The intra- and inter-assay coefficients of variation (CV) for GST-VWF73, collagen binding and FRETS-VWF73 were all< 10% and 20%, respectively.
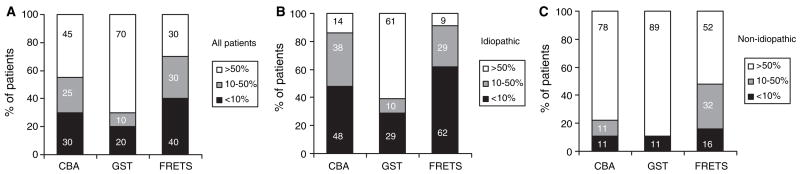
TTP patients with severe ADAMTS-13 deficiency (< 10% activity of NHP) are at highest risk of developing the TTP syndrome [43]. To determine the likelihood that these three assays could identify severe deficiency, we assayed ADAMTS-13 activity in plasma samples from 40 clinically diagnosed TTP being admitted for plasma exchange therapy. Among all TTP patients (21 idiopathic and 19 non-idiopathic), the FRETS-VWF73 assay detected more patients (40%) with severe ADAMTS-13 deficiency than the collagen binding (30%) and the GST-VWF73 (20%) assays (Fig. 2A). This finding was more evident among the subgroup of idiopathic TTP patients, in which the FRETS-VWF73 identified 62% of patients with severe ADAMTS-13 deficiency, compared with 48% by the collagen binding and 29% by the GST-VWF73 (Fig. 2B). Patients with non-idiopathic TTP rarely showed severe deficiency of ADAMTS-13 activity by any method used (Fig. 2C). Random sampling did not likely account for the association between ADAMTS-13 activity (severe deficiency, < 10%; moderate deficiency, 10–50%; and normal activity, > 50%) and the assays used (collagen binding, GST-VWF73, and FRETS-VWF73) in all TTP patients, [χ2(4) = 13.43, P = 0.0094], idiopathic TTP patients only [χ2(4) = 18.39, P = 0.001], or non-idiopathic TTP patients only [χ2(4) = 9.143, P = 0.0576]. These data suggest that the FRETS-VWF73 is more likely to identify TTP patients with severe ADAMTS-13 deficiency.
Fig. 2.
The likelihood of three different assays to detect severe ADAMTS-13 deficiency in patients with thrombotic thrombocytopenic purpura (TTP). The percentage of patients with severe deficiency (< 10%), moderate deficiency (10%–50%) and normal (> 50%) levels of ADAMTS-13 activity among all patients with TTP (A, n = 40), patients with idiopathic TTP (B, n = 21) and non-idiopathic TTP (C, n = 19) by the collagen-binding assay (CBA); GST-VWF73 (GST) and FRETS-VWF73 (FRETS) assays. The number inside bars represents the percentage of patients in each category.
Autoantibodies to ADAMTS-13 in patients with idiopathic and non-idiopathic TTP
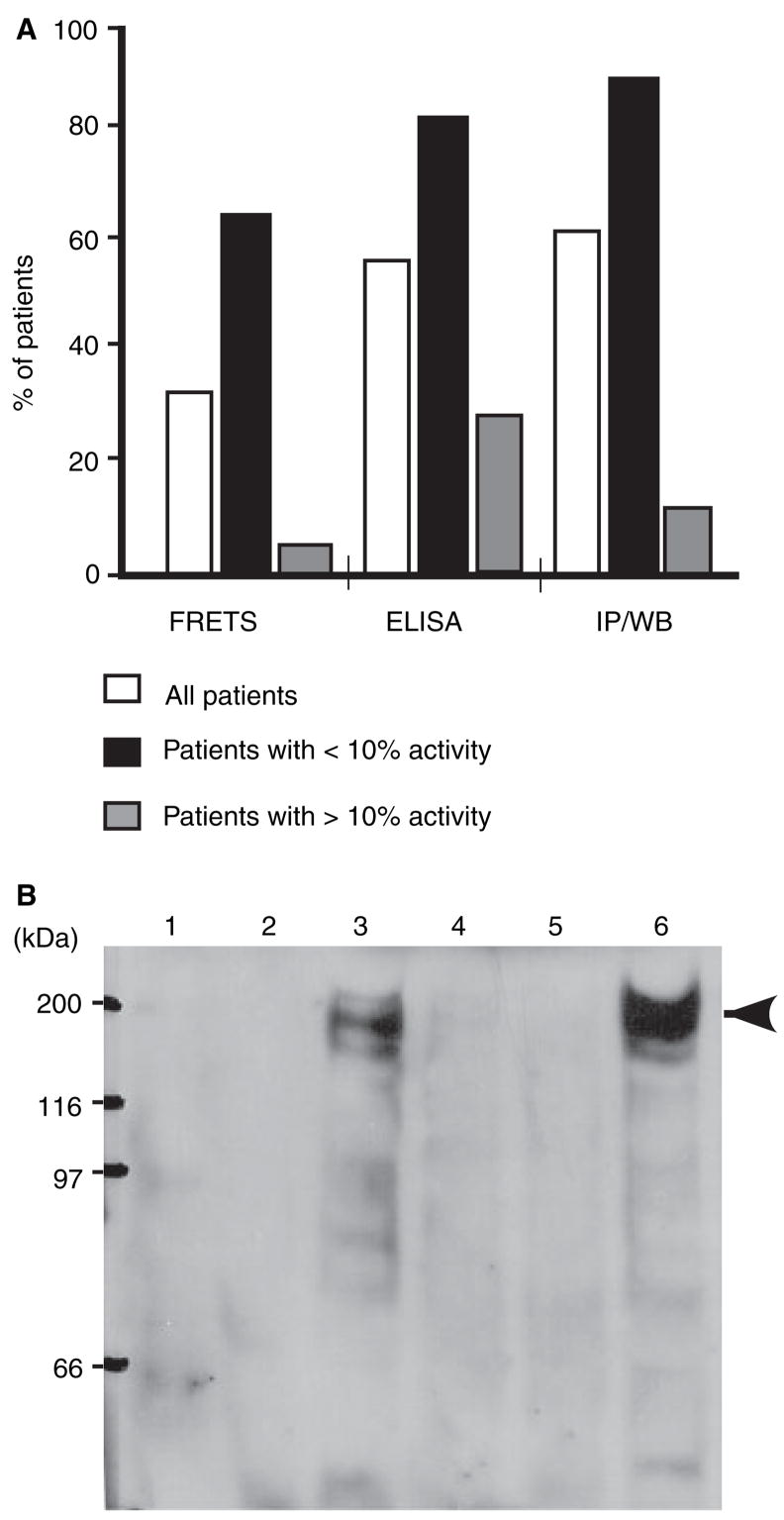
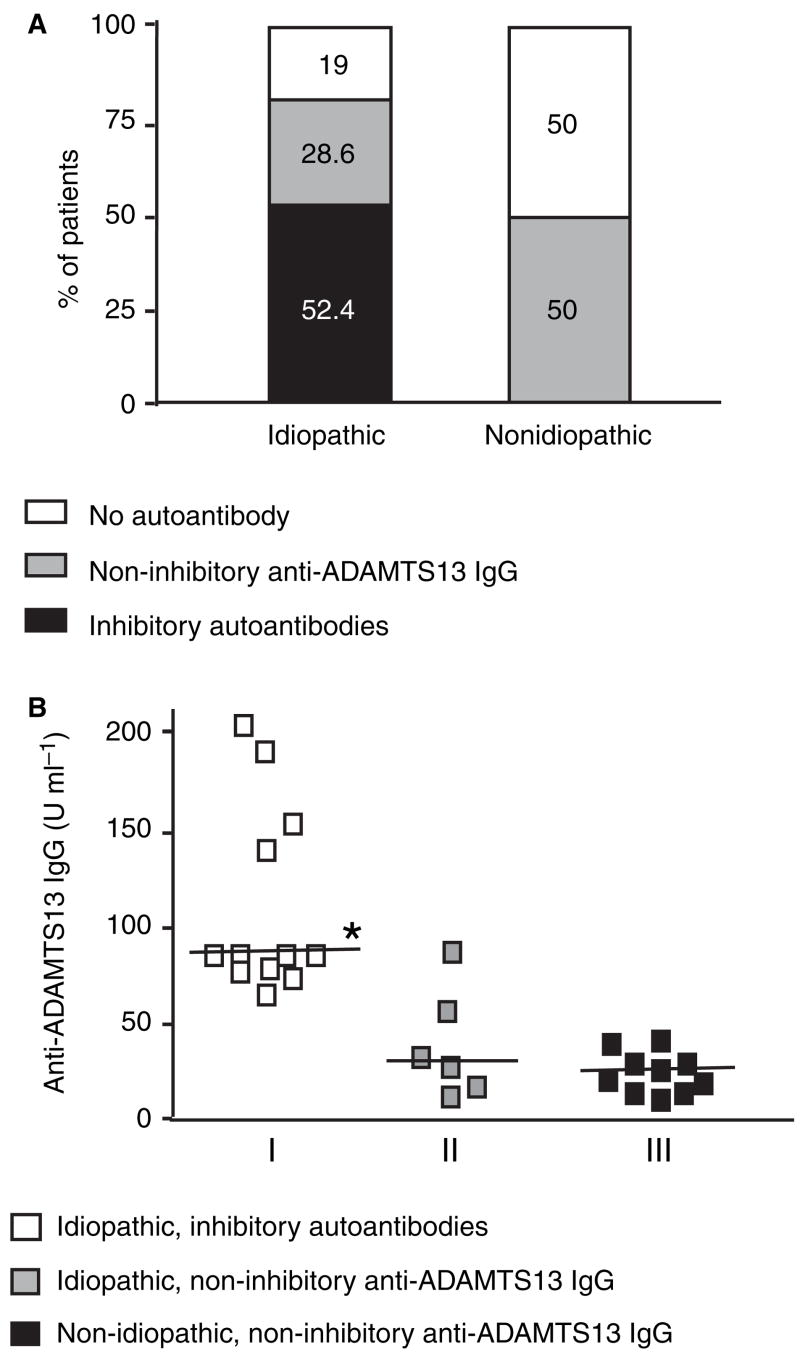
Because of the high likelihood that the FRETS-VWF73 could identify severe ADAMTS-13 deficiency, we used this assay to determine the percentage of patients in our study that harbored inhibitory autoantibodies. In addition, two immunological assays [ELISA; immunoprecipitation followed by Western blot (IP/WB)] were used to determine the percentage of patients with total (inhibitory and non-inhibitory) anti-ADAMTS-13 IgG autoantibodies (Fig. 3). By ELISA, we identified IgG autoantibodies against ADAMTS- 13 protease in 58% of all TTP patients (Fig. 3A). These positive ELISA results were confirmed by immunoprecipitation of recombinant ADAMTS-13-V5-His by patient plasma IgG and detection by Western blot with monoclonal anti-V5 antibody. A 190 kDa band indicates the presence of an anti-ADAMTS-13 IgG in patient plasma (Fig. 3B). The percentage of patients with inhibitory autoantibodies was relatively low among ‘all’ TTP patients, but increased among the subgroup of patients with severe deficiency (< 10% of ADAMTS-13 activity) (Fig. 3A). The ELISA and IP/WB results agreed in 95% of samples, and both of these independently confirmed every positive inhibitory autoantibody identified by FRETS-VWF73 (data not shown), suggesting that FRETS-VWF73 is a sensitive and specific assay to detect inhibitory autoantibodies against ADAMTS-13 protease in patients with TTP. Thus, ELISA and FRETS-VWF73 were used to determine the prevalence of inhibitory and non-inhibitory autoantibodies (defined in Table 2) among patients with idiopathic and non-idiopathic TTP. Among the idiopathic TTP patients, 52% harbored inhibitory autoantibodies, nearly 29% non-inhibitory anti-ADAMTS-13 IgG, and 19% no detectable autoantibody (Fig. 4A). In contrast, among non-idiopathic TTP patients, none demonstrated inhibitory autoantibodies, but 50% had non-inhibitory anti-ADAMTS-13 IgG and the other 50% no anti-ADAMTS-13 IgG (Fig. 4A). Random sampling does not account for the association between the groups (idiopathic vs. non-idiopathic) and the types of autoantibodies detected (inhibitory vs. non-inhibitory or inhibitory vs. no antibody) [χ2(2) = 13.05, P = 0.0015]. The concentration of the anti-ADAMTS-13 IgG in idiopathic TTP samples (73.4 ± 12.9 U mL−1, mean ± SEM) was significantly higher than that in non-idiopathic samples (17.9 ± 2.9 U mL−1) (Mann–Whitney U = 75.5, P = 0.0015). A subgroup analysis showed that the concentration of anti-ADAMTS-13 IgG in idiopathic TTP patients with inhibitory autoantibodies was significantly greater than that of (i) idiopathic patients with non-inhibitory anti-ADAMTS-13 IgG; or (ii) non-idiopathic patients with non-inhibitory anti-ADAMTS-13 IgG (Fig. 4B). In addition, there was no statistically significant difference between the latter two groups.
Fig. 3.
The ability of the different assays to identify autoantibodies in patients with thrombotic thrombocytopenic purpura (TTP). Percentage of patients having anti-ADAMTS-13 IgG or inhibitory autoantibodies in all patients (white bars; n = 40), subgrouped by patients with< 10% (black bars; n = 17) or > 10% (gray bars; n = 23) of ADAMTS-13 activity (black bars; n = 17) (A) was detected by the FRETS-VWF73 (FRETS), enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation plus Western blotting (IP/WB) assays. FRETS-VWF73 detects only inhibitory autoantibodies, whereas the immunoassays (ELISA and IP/WB) identify both inhibitory and non-inhibitory anti-ADAMTS-13 IgG (total). The representative IP/WB signals are shown in (B), in which anti-ADAMTS-13 IgG in patient plasma was designated negative (lanes 1, 2, 4, and 5) or positive (lanes 3 and 6), based on whether a 190 kDa band of recombinant ADAMTS-13-V5-His was detected or not by Western blotting with anti-V5 IgG (arrowheads).
Fig. 4.
The prevalence and concentrations of the inhibitory and the non-inhibitory IgG autoantibodies in patients with thrombotic thrombocytopenic purpura (TTP). Samples were classified as inhibitory autoantibodies or non-inhibitory anti-ADAMTS13 IgG according to the definitions in Table 2. The numbers in the bars represent the percentage of patients in each category of the autoantibodies (A). The concentration of anti-ADAMTS13 IgG (B) in idiopathic TTP with inhibitory autoantibodies (group I) was significantly higher than that in idiopathic TTP (n = 21) with non-inhibitory anti-ADAMTS-13 IgG (group II) or non-idiopathic TTP (n = 19) with non-inhibitory anti-ADAMTS-13 IgG (group III) (Kruskall–Wallis statistic = 18.01, followed by Dunn’s multiple comparison, *P < 0.05). None of the non-idiopathic group’s samples demonstrated inhibitory anti-ADAMTS-13 IgG. There was no statistically significant difference between groups II and III. The horizontal line represents the median of anti-ADAMTS-13 IgG concentration.
ADAMTS-13 antigen levels in patients with idiopathic and non-idiopathic TTP
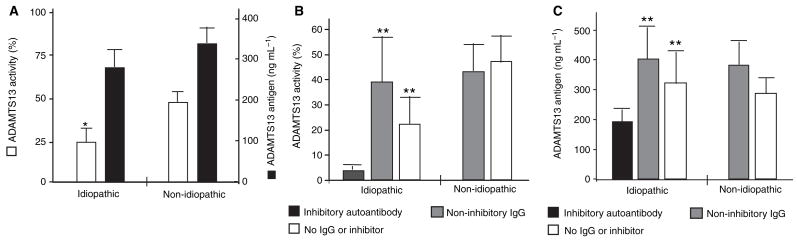
To determine whether inhibitory or non-inhibitory anti-ADAMTS-13 IgG binds the ADAMTS-13 protease and accelerate its clearance in vivo, we compared the ADAMTS-13 antigen levels in TTP patients with inhibitory or non-inhibitory anti-ADAMTS-13 autoantibodies. Overall, there was a positive correlation between ADAMTS-13 activity and ADAMT13 antigen levels (r = 0.665, P < 0.0001). This correlation was not altered even after removing from the analysis samples demonstrating inhibitors and low activity (data not shown). Not surprisingly, the plasma levels of ADAMTS-13 activity (determined by FRETS-VWF73) in patients with idiopathic TTP (16.9 ± 6%, n = 21) were significantly lower than those in patients with non-idiopathic TTP (45.1 ± 5.7%, n = 19; P = 0.002) (Fig. 5A). However, the plasma levels of ADAMTS- 13 antigen in patients with idiopathic TTP (276 ± 46 ng mL−1) were only slightly (not significantly) lower than those of non-idiopathic TTP (328 ± 43 ng mL−1) (Fig. 5A). In contrast, ADAMTS-13 antigen levels were significantly reduced in patients harboring inhibitory autoantibodies, but not in those with non-inhibitory anti-ADMTS13 IgG (Fig. 5C). These data suggest that inhibitory autoantibodies may not only bind and block ADAMTS-13 activity, but also reduce ADAMTS-13 antigen levels, perhaps by accelerating its clearance in vivo.
Fig. 5.
ADAMTS-13 activity and antigen levels in patients with thrombotic thrombocytopenic purpura (TTP). A shows the ADAMTS-13 activity (open bars) and antigen (closed bars) levels in idiopathic and non-idiopathic TTP patients as indicated. B and C show the ADAMTS13 activity and antigen levels in patients with idiopathic TTP harboring inhibitory autoantibodies (black bars), non-inhibitory anti-ADAMTS-13 IgG (gray bars) and no inhibitor or autoantibody (white bars), respectively. ADAMTS-13 antigen in pooled citrated normal human plasma is 540 ± 190 ng mL−1 (see Materials and methods). The statistical analysis was performed using one-factor ANOVA, followed by Bonferroni tests (*P < 0.05 and **P < 0.01) (compared with the lowest value in each panel). A two-way ANOVA to discern the interaction of TTP-type (idiopathic vs. non-idiopathic) and antibody type (inhibitory, non-inhibitory, no antibody or inhibitor) was not performed because the non-idiopathic group did not contain inhibitory autoantibody.
Kinetics of ADAMTS-13 antigen, activity and inhibitors in patients with TTP after therapies
To determine the effect of plasma exchanges on ADAMTS-13 antigen and activity, we analyzed serial plasma samples from two patients that harbored inhibitory autoantibodies. Patient 1 demonstrates a rapid increase in platelet count, followed by a disease flare at days 7 and 8. The platelet count exhibits an uneven progression towards normalization (Fig. 6A), similar to the tracing of ADAMTS-13 activity and antigen (Fig. 6C). Patient 2 shows a more consistent normalization in platelet count (Fig. 6B), ADAMTS-13 activity and antigen (Fig. 6D). Both patients demonstrated a decrease in concentration of anti- ADAMTS-13 IgG, with a more rapid decline in patient 2 (Fig. 6F) than in patient 1 (Fig. 6E).
Fig. 6.
Serial laboratory parameters from two idiopathic thrombotic thrombocytopenic purpura (TTP) patients with inhibitory autoantibodies. Patient platelet counts (A and B), ADAMTS-13 activity and antigen levels (C and D), and anti-ADAMTS-13 IgG levels (E and F) were shown in two patients during the plasma exchange therapy and follow-up. The x-axis indicates the days after initial admission. Both patients received daily plasma exchange therapy, starting from day 1 for 30 days (patient 1) and 12 days (patient 2), after which the plasma exchange was tapered o.. The ADAMTS-13 activity and inhibitor were determined by FRETS-VWF73 assay, whereas anti-ADAMTS-13 IgG was determined by enzyme-linked immunosorbent assay.
Discussion
The values of ADAMTS-13 activity obtained by three different functional assays in this study are closely correlated with the highest correlation coefficient (r = 0.83) seen between the collagen-binding and the FRETS-VWF73 assay (Fig. 1). However, there are differences in the ability of these assays to identify TTP patients with severe ADAMTS-13 deficiency (< 5–10% of NHP) and inhibitors or autoantibodies, which was considered to be critical for diagnosis of idiopathic TTP [13,20,26] and development of TTP symptoms (Fig. 2) [43]. For example, the GST-VWF73 assay tended to overestimate ADAMTS-13 activity in samples with relatively low proteolytic activity, due to the non-linearity of the chemiluminescent signal on the Western blot. Approximately half of the patients with severe deficiency of ADAMTS-13 activity (< 10%) determined by FRETS-VWF73 would be missed by the GST-VWF73 assay. This assay, however, may be useful in determining the specificity of the cleavage at the Tyr–Met bond by patient plasma ADAMTS-13. No aberrant cleavage was detected in any of the 40 patients, suggesting that the truncated form of ADAMTS-13 (after the first TSP-1 repeat or after the metalloprotease domain) [33] was not present in these patient plasmas. Consistent with our published results [20], the collagen-binding assay appeared to be good at identifying samples with severe ADAMTS-13 deficiency in the presence of high titer of inhibitors, but would miss many patients with low titer of inhibitors, perhaps due to a prolonged incubation of substrate and enzyme/inhibitor in presence of 1.5 M urea. The FRETS-VWF73 assay clearly identified more cases with severe ADAMTS-13 deficiency than the collagen-binding and GST-VWF73 assays in ‘all’ TTP patients (Fig. 2A) and among idiopathic TTP patients (Fig. 2B). The FRETS-VWF73 assay is relatively easy, rapid and quantitative [30,41,42] and may become one of the superior assays for measuring plasma ADAMTS-13 activity and inhibitory autoantibodies in patients suspected with TTP, although other rapid and seemingly robust assays are also emerging and deserve evaluation [44–47].
Although the true sensitivity and specificity of each assay for diagnosis of TTP has not been determined due in part, to the lack of the ‘gold standard’ clinical criteria and diagnostic tests for TTP [48], knowing the status of ADAMTS-13 activity and inhibitors is critical for guiding appropriate treatment [20,21,49]. Our data are consistent with the hypothesis that patients with severe deficiency along with detectable autoantibodies against ADAMTS-13 may benefit from adjunctive immune therapies in addition to plasma exchange [13,20,21]. However, interpreting test results may require a careful evaluation of the methodology being used for the test. For example, identifying severe ADAMTS-13 deficiency or inhibitors was less likely to occur using the GST-VWF73 and the collagen-binding assays than using the FRETS-VWF73 assay or immunological assays (Figs 2 and 3). Of notes, the low ADAMTS-13 activity determined by the in vitro assays in this study is not simply due to interference from high endogenous VWF levels released from injured endothelial cells [37,38], although high VWF levels do negatively regulate ADAMTS-13 activity in vivo [38,39]. All of the plasma samples with inhibitory autoantibodies (by FRETS-VWF73) were further verified by the immunological assays (ELISA and IP/WB) (data not shown), suggesting that anti-ADAMTS-13 IgG may be primarily responsible to the inhibitory activity. However, it is ‘theoretically’ possible that a plasma sample harbored a non-inhibitory immunoglobulin (that bound to ADAMTS-13 in the ELISA and IP/WB) to ADAMTS-13, in addition to a non-immunoglobulin inhibitor, the latter of which was responsible for its neutralizing activity.
By the FRETS-VWF73 and immunological assays, we showed that patients with idiopathic TTP had significantly lower ADAMTS-13 activity than those with non-idiopathic TTP, consistent with the previous findings [20,22]. Most patients (~81%) with idiopathic TTP harbored anti-ADAMTS-13 IgG, much higher than previously reported by the collagen-binding [20] and multimeric assays [22,50]. Of these, 65% of patients had inhibitory autoantibodies that blocked the cleavage of FRETS-VWF73 by NHP at 50:50 mixing study. The remaining 35% had non-inhibitory anti-ADAMTS-13 IgG that merely bind ADAMTS- 13 immobilized on the ELISA plate (Fig. 3A) or react with recombinant ADAMTS-13-V5-His in solution (Fig. 3B). We found that 50% of patients with non-idiopathic TTP harbored anti-ADAMTS-13 IgG (Fig. 3).
The concentration of inhibitory anti-ADAMTS-13 IgG in idiopathic TTP patients was significantly higher than that of non-inhibitory anti-ADAMTS-13 IgG in idiopathic and non-idiopathic TTP patients (Fig. 4B). This result is consistent with previous findings that the autoantibodies against ADAMTS-13 at the highest titer exhibited functionally neutralizing activity [51]. Similar findings have been described in heparin-induced thrombocytopenia, another autoantibody-mediated thrombotic disorder, in which high titer IgG correlates with the (functional) ability to activate platelets and cause thrombosis, whereas low-titer IgG do not [52,53]. The ELISA positivity for anti-ADAMTS-13 IgG is rarely (< 4%) found in healthy donors [51] but can be detected in up to 18% of patients with non-TTP thrombocytopenia. In neither of these cases do the anti-ADAMTS-13 IgG show the neutralizing activity toward ADAMTS-13 protease [51].
The inhibitory anti-ADAMTS-13 IgG appears to bind the domains of ADAMTS-13 (particularly the Cys-rich and spacer domains) [54,55] that are essential for recognition and cleavage of VWF [33,34,56,57], thereby blocking ADAMTS-13 proteolytic activity. However, the function of non-inhibitory anti-ADAMTS-13 IgG is not known. It was hypothesized that non-inhibitory anti-ADAMTS-13 IgG might bind the protease and accelerate its clearance in vivo [27,51]. Therefore, we sought to determine the levels of ADAMTS-13 antigen, in idiopathic and non-idiopathic TTP patients, and in patients with inhibitory and non-inhibitory anti-ADAMTS-13 IgG. We found that the levels of ADAMTS-13 antigen in idiopathic TTP patients with non-inhibitory anti-ADAMTS-13 IgG (~400 ng mL−1) were similar to those without detectable autoantibody (~333 ng mL−1) (Fig. 5C), suggesting that the non-inhibitory anti-ADAMTS-13 IgG in patients with TTP may not be able to bind ADAMTS-13 protease and increase its clearance in vivo. In contrast, the levels of ADAMTS-13 antigen in idiopathic TTP patients with inhibitory autoantibodies were significantly lower (~190 ng mL−1) than those without inhibitory autoantibodies or those with non-inhibitory anti-ADAMTS- 13 IgG (Fig. 5). These data suggest that the inhibitory, but not the non-inhibitory autoantibodies, may bind ADAMTS- 13 protease and accelerate its clearance in vivo.
Although certain characteristics of these inhibitory autoantibodies may be associated with lower antigen levels, it is possible that the mere presence of a high titer of anti- ADAMTS-13 IgG among the idiopathic TTP samples with inhibitory autoantibodies (Fig. 4B) may be sufficient to cause the accelerated clearance. Alternative interpretation of the data might be that non-inhibitory anti-ADAMTS-13 IgG bind to ADAMTS-13 protease and decrease its clearance, resulting in a prolongation of half-life of ADAMTS-13 proteases in vivo, akin to the effect of the antibodies against certain drugs such as lepirudin [58].
We conclude that the identification of severe ADAMTS-13 deficiency and autoantibodies is assay-dependent; the FRETS-VWF73 assay appears to be the superior of three functional assays tested for identifying severe ADAMTS-13 deficiency and inhibitory autoantibodies; the ELISA and immunoprecipitation plus Western blot perform equally well for detection of total anti-ADAMTS-13 IgG. Finally, both the FRETS-VWF73 and the ELISA are relatively simple, rapid and reproducible, and may be applied to clinical practice; the inhibitory anti-ADAMTS-13 IgG may not only bind and inhibit ADAMTS-13 activity, but also bind and accelerate its clearance in vivo. The clinical significance of such highly prevalent non-inhibitory anti-ADAMTS-13 IgG detected in patients with idiopathic and non-idiopathic TTP deserves further investigation.
Acknowledgments
The authors thank the medical sta. at the Division of Transfusion Medicine, the Hospital of University of Pennsylvania and the Children’s Hospital of Philadelphia for sample collection. The authors also appreciate Dr Lawrence T. Goodnough at Washington University (now at Stanford University) and Dr J. Evan Sadler at Washington University in St Louis, Missouri and Dr Richard M. Kaufman at Brigham Women’s Hospital, Harvard Medical School, Boston, MA for providing with some of the plasma samples for this study.
This work was supported by grants from National Institute of Health (HL079027 to X.Z. and HL075246 to S.S.); National Blood Foundation and American Heart Association-Delaware and Pennsylvania (0465532U to X.Z.); part of this work was presented in abstract form in the 47th Annual Meeting of the American Society of Hematology, Atlanta, Georgia, December 10–13, 2005.
References
- 1.Moake JL. Thrombotic thrombocytopenic purpura: the systemic clumping ‘plague’. Annu Rev Med. 2002;53:75–88. doi: 10.1146/annurev.med.53.082901.103948. [DOI] [PubMed] [Google Scholar]
- 2.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 3.Amorosi EL, Ultmann JE. Thrombocytopic purpura: report of 16 cases and review of the literature. Medicine (Baltimore) 1966;45:139– 59. [Google Scholar]
- 4.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398–403. doi: 10.1056/NEJM199108083250605. [DOI] [PubMed] [Google Scholar]
- 5.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spaso RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 6.Rock G, Shumak K, Kelton J, Blanchette VS, Buskard N, Nair R, Spaso R. Thrombotic thrombocytopenic purpura: outcome in 24 patients with renal impairment treated with plasma exchange. Canadian Apheresis Study Group. Transfusion. 1992;32:710–4. doi: 10.1046/j.1537-2995.1992.32893032096.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98:1662–6. doi: 10.1182/blood.v98.6.1662. [DOI] [PubMed] [Google Scholar]
- 8.Gerritsen HE, Robles R, Lammle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood. 2001;98:1654–61. doi: 10.1182/blood.v98.6.1654. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–63. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 10.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem (Tokyo) 2001;130:475–80. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 11.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 12.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. NEngl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 13.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–34. [PubMed] [Google Scholar]
- 15.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–44. [PubMed] [Google Scholar]
- 16.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–9. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 17.Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, Lopez JA, Cruz MA. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. J Biol Chem. 2003;278:29633–9. doi: 10.1074/jbc.M301385200. [DOI] [PubMed] [Google Scholar]
- 18.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA. 2002;99:11902–7. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veyradier A, Obert B, Houllier A, Meyer D, Girma JP. von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98:1765–72. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 20.Zheng XL, Richard KM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and non-idiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–9. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng X, Pallera AM, Goodnough LT, Sadler JE, Blinder MA. Remission of chronic thrombotic thrombocytopenic purpura after treatment with cyclophosphamide and rituximab. Ann Intern Med. 2003;138:105–8. doi: 10.7326/0003-4819-138-2-200301210-00011. [DOI] [PubMed] [Google Scholar]
- 22.Vesely SK, George JN, Lammle B, Studt JD, Alberio L, El-Harake MA, Raskob GE. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–8. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 23.Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med. 2000;132:794–9. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furlan M, Robles R, Solenthaler M, Lammle B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood. 1998;91:2839–46. [PubMed] [Google Scholar]
- 25.Remuzzi G, Galbusera M, Noris M, Canciani MT, Daina E, Bresin E, Contaretti S, Caprioli J, Gamba S, Ruggenenti P, Perico N, Mannucci PM. von Willebrand factor cleaving protease (ADAMTS13) is deficient in recurrent and familial thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Blood. 2002;100:778–85. doi: 10.1182/blood-2001-12-0166. [DOI] [PubMed] [Google Scholar]
- 26.Tsai HM. Is severe deficiency of ADAMTS-13 specific for thrombotic thrombocytopenic purpura? Yes. J Thromb Haemost. 2003;1:625–31. doi: 10.1046/j.1538-7836.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 27.Scheiflinger F, Knoebl P, Trattner B, Plaimauer B, Mohr G, Dockal M, Dorner F, Rieger M. Non-neutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura (TTP) Blood. 2003;102:3241–3. doi: 10.1182/blood-2003-05-1616. [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, Sadler JE. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. In: Young NS, Gerson SL, High KA, editors. Clinical Hematology. 1. Philadelphia, PA: Mosby Elsevier; 2006. pp. 802–13. [Google Scholar]
- 29.Gerritsen HE, Turecek PL, Schwarz HP, Lammle B, Furlan M. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP) Thromb Haemost. 1999;82:1386–9. [PubMed] [Google Scholar]
- 30.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Tsai HM. An enzyme immunoassay of ADAMTS13 distinguishes patients with thrombotic thrombocytopenic purpura from normal individuals and carriers of ADAMTS13 mutations. Thromb Haemost. 2004;91:806–11. doi: 10.1160/TH03-11-0675. [DOI] [PubMed] [Google Scholar]
- 32.Kokame K, Matsumoto M, Fujimura Y, Miyata T. VWF73, a region from D1596 to R1668 of von Willebrand factor, provides a minimal substrate for ADAMTS-13. Blood. 2003;103:607–12. doi: 10.1182/blood-2003-08-2861. [DOI] [PubMed] [Google Scholar]
- 33.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280:29428–34. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–41. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remuzzi G. Is ADAMTS-13 deficiency specific for thrombotic thrombocytopenic purpura? No. J Thromb Haemost. 2003;1:632–4. doi: 10.1046/j.1538-7836.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 36.Studt JD, Bohm M, Budde U, Girma JP, Varadi K, Lammle B. Measurement of von Willebrand factor-cleaving protease (ADAMTS-13) activity in plasma: a multicenter comparison of different assay methods. J Thromb Haemost. 2003;1:1882–7. doi: 10.1046/j.1538-7836.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 37.Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98:2730–5. doi: 10.1182/blood.v98.9.2730. [DOI] [PubMed] [Google Scholar]
- 38.Mannucci PM, Capoferri C, Canciani MT. Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol. 2004;126:213–8. doi: 10.1111/j.1365-2141.2004.05009.x. [DOI] [PubMed] [Google Scholar]
- 39.Mannucci PM, Parolari A, Canciani MT, Alemanni F, Camera M. Opposite changes of ADAMTS-13 and von Willebrand factor after cardiac surgery. J Thromb Haemost. 2005;3:397–9. doi: 10.1111/j.1538-7836.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 40.Mannucci PM, Vanoli M, Forza I, Canciani MT, Scorza R. Von Willebrand factor cleaving protease (ADAMTS-13) in 123 patients with connective tissue diseases (systemic lupus erythematosus and systemic sclerosis) Haematologica. 2003;88:914–8. [PubMed] [Google Scholar]
- 41.Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:698–9. doi: 10.1111/j.1538-7836.2005.01767.x. [DOI] [PubMed] [Google Scholar]
- 42.Soejima K, Nakamura H, Hirashima M, Morikawa W, Nozaki C, Nakagaki T. Analysis on the molecular species and concentration of circulating ADAMTS13 in blood. J Biochem (Tokyo) 2006;139:147–54. doi: 10.1093/jb/mvj013. [DOI] [PubMed] [Google Scholar]
- 43.Furlan M, Lammle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol. 2001;14:437–54. doi: 10.1053/beha.2001.0142. [DOI] [PubMed] [Google Scholar]
- 44.Jin M, Cataland S, Bissell M, Wu HM. Arapid test for the diagnosis of thrombotic thrombocytopenic purpura using surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF)-mass spectrometry. J Thromb Haemost. 2006;4:333–8. doi: 10.1111/j.1538-7836.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu JJ, Fujikawa K, Lian EC, McMullen BA, Kulman JD, Chung DW. A rapid enzyme-linked assay for ADAMTS-13. J Thromb Haemost. 2006;4:129–36. doi: 10.1111/j.1538-7836.2005.01677.x. [DOI] [PubMed] [Google Scholar]
- 46.Kostousov V, Fehr J, Bombeli T. Novel, semi-automated, 60-min-assay to determine von Willebrand factor cleaving activity of ADAMTS-13. Thromb Res. doi: 10.1016/j.thromres.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Auton M, Moake J. Attack of the acronyms: TTP, VWF, ADAMTS-13 and SELDI-TOF-MS. J Thromb Haemost. 2006;4:329–32. doi: 10.1111/j.1538-7836.2006.01791.x. [DOI] [PubMed] [Google Scholar]
- 48.George JN, Selby GB. Thrombotic microangiopathy after allogeneic bone marrow transplantation: a pathologic abnormality associated with diverse clinical syndromes. Bone Marrow Transplant. 2004;33:1073–4. doi: 10.1038/sj.bmt.1704513. [DOI] [PubMed] [Google Scholar]
- 49.Shelat SG, Ai J, Zheng XL. Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays. Semin Thromb Hemost. 2005;31:659–72. doi: 10.1055/s-2005-925472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Studt JD, Kremer Hovinga JA, Alberio L, Bianchi V, Lammle B. Von Willebrand factor-cleaving protease (ADAMTS-13) activity in thrombotic microangiopathies: diagnostic experience 2001/2002 of a single research laboratory. Swiss Med Wkly. 2003;133:325–32. doi: 10.4414/smw.2003.10242. [DOI] [PubMed] [Google Scholar]
- 51.Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, Konetschny C, Zimmermann K, Scharrer I, Peyvandi F, Galbusera M, Remuzzi G, Bohm M, Plaimauer B, Lammle B, Scheiflinger F. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–7. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 52.Untch B, Ahmad S, Jeske WP, Messmore HL, Hoppensteadt DA, Walenga JM, Lietz H, Fareed J. Prevalence, isotype, and functionality of antiheparin-platelet factor 4 antibodies in patients treated with heparin and clinically suspected for heparin-induced thrombocytopenia. The pathogenic role of IgG. Thromb Res. 2002;105:117–23. doi: 10.1016/s0049-3848(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 53.Suh JS, Malik MI, Aster RH, Visentin GP. Characterization of the humoral immune response in heparin-induced thrombocytopenia. Am J Hematol. 1997;54:196–201. doi: 10.1002/(sici)1096-8652(199703)54:3<196::aid-ajh4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 54.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, Scheflinger F. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103:4514–9. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 55.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005;93:267–74. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 56.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, Nozaki C, Miyata T, Fujimura Y, Nakagaki T. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–7. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 57.Majerus EM, Anderson PJ, Sadler JE. Binding of ADAMTS13 to von Willebrand factor. J Biol Chem. 2005;280:71773–8. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 58.Liebe V, Bruckmann M, Fischer KG, Haase KK, Borggrefe M, Huhle G. Biological relevance of anti-recombinant hirudin antibodies – results from in vitro and in vivo studies. Semin Thromb Hemost. 2002;28:483–90. doi: 10.1055/s-2002-35289. [DOI] [PubMed] [Google Scholar]