Abstract
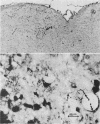
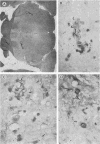
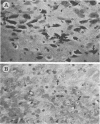
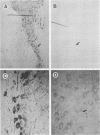
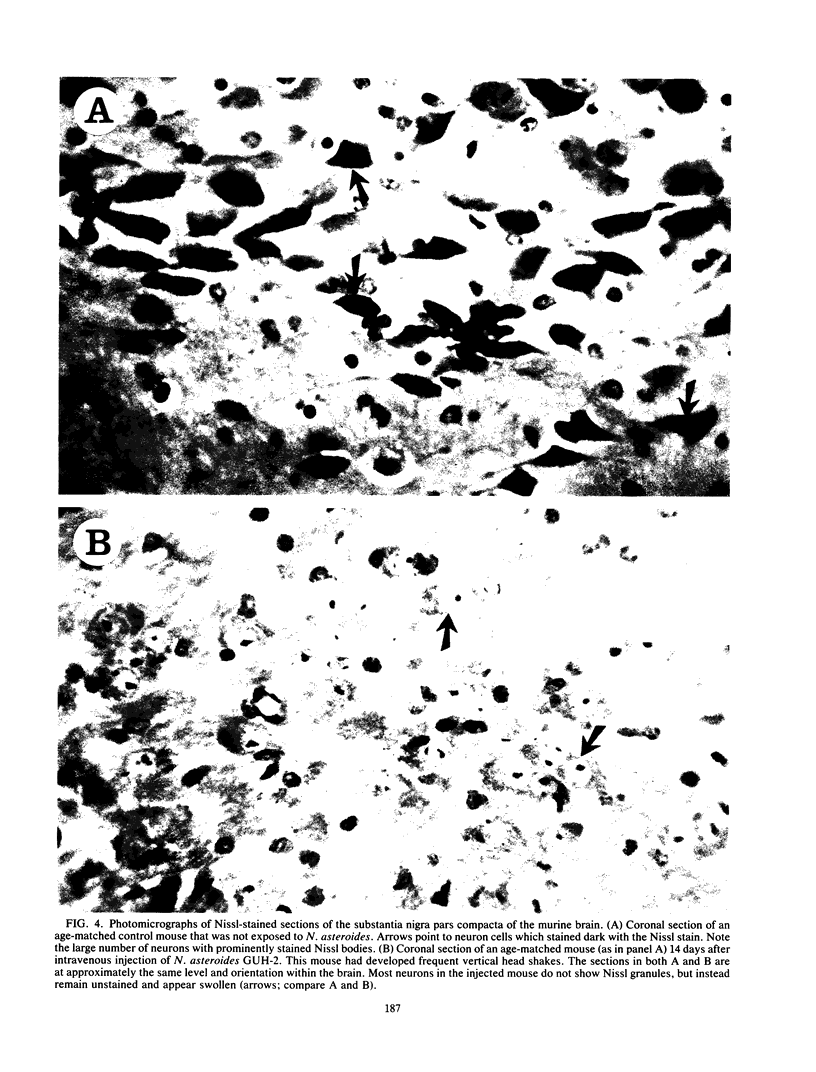
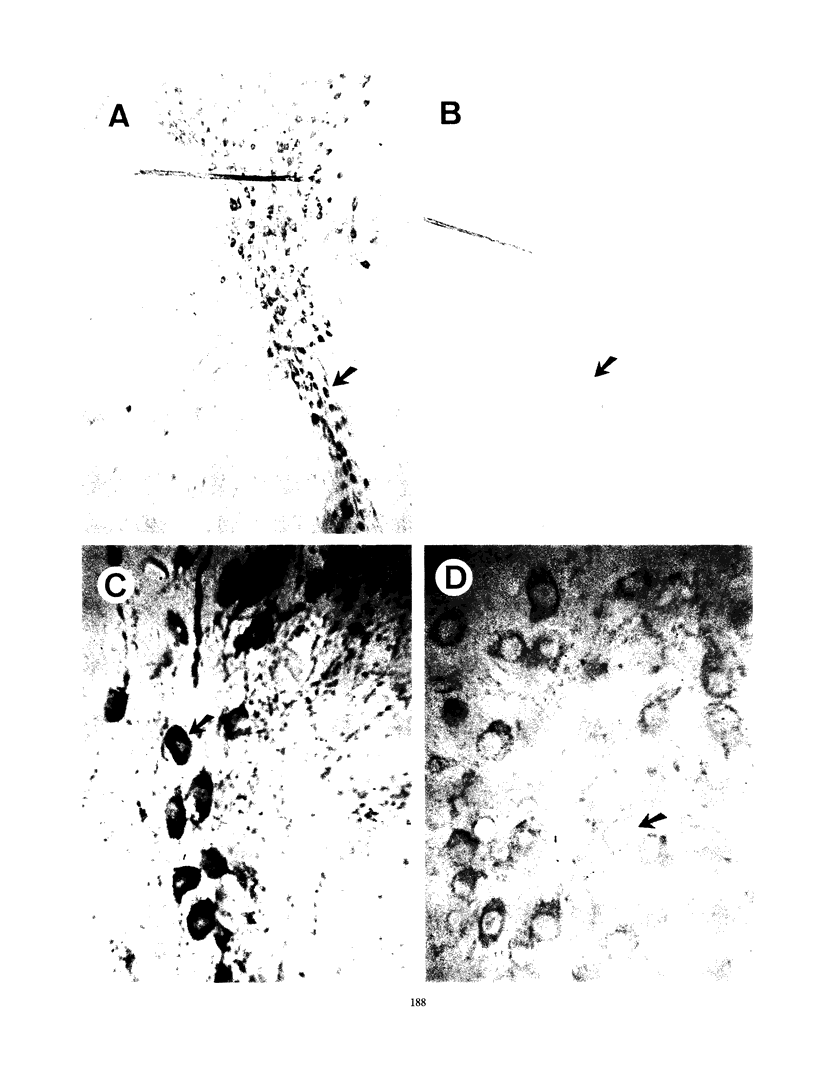
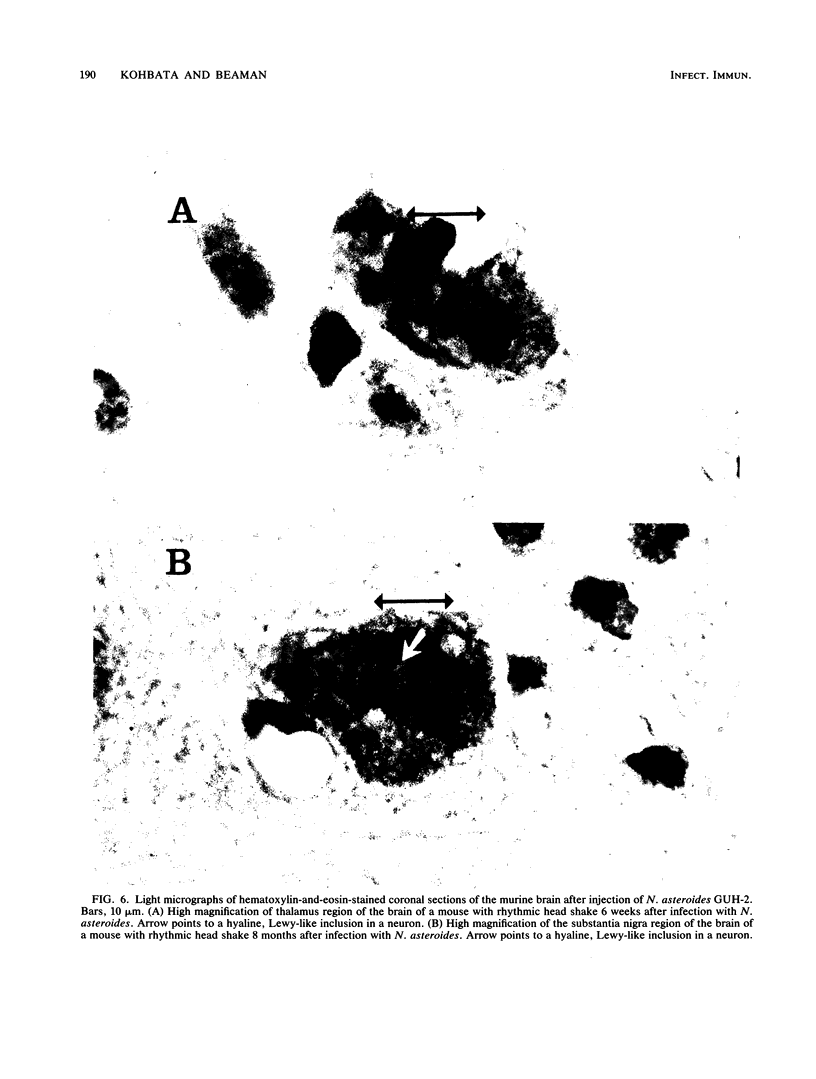
Nocardia asteroides can cause infections in the brain of humans and a variety of animals. In mice, invasion of the central nervous system results in specific neurologic signs. Following intravenous injection of various doses of log-phase N. asteroides GUH-2 into female BALB/c mice, localization and growth of nocardial cells within the brains were determined, histopathological sections were prepared, and Nissl substance and tyrosine hydroxylase immunoreactivity were observed. Mice were monitored for the development of neurologic signs, and their responsiveness to L-dopa was determined. It was shown that nocardial cells became localized within specific regions of the brain and then underwent rapid growth followed by a delayed clearance, and there was no inflammatory response at the site of invasion for 24 h. Mice that received a subclinical dose of nocardiae developed specific neurologic signs that emerged following the elimination of nocardial cells from the brain. On the basis of the specific signs, mice could be divided into distinct groups. One group consisted of animals that had a form of hemiparesis that did not respond to L-dopa. They expressed a deviation of the head and a tendency to roll, and when suspended by the tail they would spin rapidly. The second group of mice developed a rhythmic, uncontrolled vertical shake of the head (four to five times per s) tremulous movement, stooped posture, restlessness, and no signs of hemiparesis. The head shakes were temporarily stopped by treatment with L-dopa. Mice that expressed head shakes had a loss of Nissl substance and tyrosine hydroxylase immunoreactivity in the neurons of the substantia nigra and ventral tegmental areas of the brain. Hyaline inclusion bodies that resembled Lewy bodies were found in the neurons of mice with head shake 1 month after infection. Therefore, mice infected with N. asteroides may serve as a model for studying parkinsonian signs and other degenerative diseases involving extrapyramidal and pyramidal systems.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRIOLE V. T., BALLAS M., WILSON G. L. THE ASSOCIATION OF NOCARDIOSIS AND PULMONARY ALVEOLAR PROTEINOSIS. A CASE STUDY. Ann Intern Med. 1964 Feb;60:266–275. doi: 10.7326/0003-4819-60-2-266. [DOI] [PubMed] [Google Scholar]
- BIRKMAYER W., HORNYKIEWICZ O. [The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. Wien Klin Wochenschr. 1961 Nov 10;73:787–788. [PubMed] [Google Scholar]
- Baily G. G., Neill P., Robertson V. J. Nocardiosis: a neglected chronic lung disease in Africa? Thorax. 1988 Nov;43(11):905–910. doi: 10.1136/thx.43.11.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnicoat M. J., Wierzbicki A. S., Norman P. M. Cerebral nocardiosis in immunosuppressed patients: five cases. Q J Med. 1989 Aug;72(268):689–698. [PubMed] [Google Scholar]
- Bauman J. M., Osenbach R., Hartshorne M. F., Youngblood L., Crooks L., Landry A. J., Cawthon M. A. Positive indium-111 leukocyte scan in Nocardia brain abscess. J Nucl Med. 1986 Jan;27(1):60–62. [PubMed] [Google Scholar]
- Beaman B. L., Burnside J., Edwards B., Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976 Sep;134(3):286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Moring S. E. Relationship among cell wall composition, stage of growth, and virulence of Nocardia asteroides GUH-2. Infect Immun. 1988 Mar;56(3):557–563. doi: 10.1128/iai.56.3.557-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojinov S. Encephalitis with acute Parkinsonian syndrome and bilateral inflammatory necrosis of the substantia nigra. J Neurol Sci. 1971 Apr;12(4):383–415. doi: 10.1016/0022-510x(71)90109-2. [DOI] [PubMed] [Google Scholar]
- Burns R. S., LeWitt P. A., Ebert M. H., Pakkenberg H., Kopin I. J. The clinical syndrome of striatal dopamine deficiency. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Engl J Med. 1985 May 30;312(22):1418–1421. doi: 10.1056/NEJM198505303122203. [DOI] [PubMed] [Google Scholar]
- Calne D. B., Duvoisin R. C., McGeer E. Speculations on the etiology of Parkinson's disease. Adv Neurol. 1984;40:353–360. [PubMed] [Google Scholar]
- Calne D. B., Eisen A., McGeer E., Spencer P. Alzheimer's disease, Parkinson's disease, and motoneurone disease: abiotrophic interaction between ageing and environment? Lancet. 1986 Nov 8;2(8515):1067–1070. doi: 10.1016/s0140-6736(86)90469-1. [DOI] [PubMed] [Google Scholar]
- Calne D. B., Langston J. W. Aetiology of Parkinson's disease. Lancet. 1983 Dec 24;2(8365-66):1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 20-1980. N Engl J Med. 1980 May 22;302(21):1194–1199. doi: 10.1056/NEJM198005223022109. [DOI] [PubMed] [Google Scholar]
- Cotzias G. C., Van Woert M. H., Schiffer L. M. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967 Feb 16;276(7):374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- DUVOISIN R. C., YAHR M. D. ENCEPHALITIS AND PARKINSONISM. Arch Neurol. 1965 Mar;12:227–239. doi: 10.1001/archneur.1965.00460270003001. [DOI] [PubMed] [Google Scholar]
- ERCHUL J. W., KOCH M. L. Cerebral nocardiosis with coexistent pulmonary tuberculosis; report of a fatal case. Am J Clin Pathol. 1955 Jul;25(7):775–781. doi: 10.1093/ajcp/25.7.775. [DOI] [PubMed] [Google Scholar]
- Fraser H. Eosinophilic bodies in some neurones in the thalamus of ageing mice. J Pathol. 1969 Jul;98(3):201–204. doi: 10.1002/path.1710980307. [DOI] [PubMed] [Google Scholar]
- Frazier A. R., Rosenow E. C., 3rd, Roberts G. D. Nocardiosis. A review of 25 cases occurring during 24 months. Mayo Clin Proc. 1975 Nov;50(11):657–663. [PubMed] [Google Scholar]
- GREENFIELD J. G., BOSANQUET F. D. The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiatry. 1953 Nov;16(4):213–226. doi: 10.1136/jnnp.16.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. The significance of the Lewy body in the diagnosis of idiopathic Parkinson's disease. Neuropathol Appl Neurobiol. 1989 Jan-Feb;15(1):27–44. doi: 10.1111/j.1365-2990.1989.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Hadley M. N., Spetzler R. F., Martin N. A., Johnson P. C. Middle cerebral artery aneurysm due to Nocardia asteroides: case report of aneurysm excision and extracranial-intracranial bypass. Neurosurgery. 1988 May;22(5):923–928. [PubMed] [Google Scholar]
- Hoehn M. M., Yahr M. D. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Howard R. S., Lees A. J. Encephalitis lethargica. A report of four recent cases. Brain. 1987 Feb;110(Pt 1):19–33. doi: 10.1093/brain/110.1.19. [DOI] [PubMed] [Google Scholar]
- KAUFMAN N., PRIETO L. C., Jr Cerebral nocardiosis; report of a case with necropsy. AMA Arch Pathol. 1952 Apr;53(4):379–384. [PubMed] [Google Scholar]
- Kohbata S., Yokoyama H., Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30(12):1225–1237. doi: 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Haltia M. Cytochemical and ultrastructural studies of chromatolysis in neurones infected with Herpes simplex virus. Virchows Arch B Cell Pathol. 1970;4(4):335–344. doi: 10.1007/BF02906088. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lieberman A. N. Parkinson's disease: a clinical review. Am J Med Sci. 1974 Feb;267(2):66–80. [PubMed] [Google Scholar]
- MURRAY J. F., FINEGOLD S. M., FROMAN S., WILL D. W. The changing spectrum of nocardiosis. A review and presentation of nine cases. Am Rev Respir Dis. 1961 Mar;83:315–330. doi: 10.1164/arrd.1961.83.3.315. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Cordell J. L., Abdulaziz Z., Naiem M., Bordenave G. Preparation of peroxidase: antiperoxidase (PAP) complexes for immunohistological labeling of monoclonal antibodies. J Histochem Cytochem. 1982 Nov;30(11):1114–1122. doi: 10.1177/30.11.6183312. [DOI] [PubMed] [Google Scholar]
- Richter R. W., Silva M., Neu H. C., Silverstein P. M. The neurological aspects of Nocardia asteroides infection. Res Publ Assoc Res Nerv Ment Dis. 1968;44:424–444. [PubMed] [Google Scholar]
- Roberts G. D., Brewer N. S., Hermans P. E. Diagnosis of nocardiosis by blood culture. Mayo Clin Proc. 1974 May;49(5):293–296. [PubMed] [Google Scholar]
- Rosett W., Hodges G. R. Recent experiences with nocardial infections. Am J Med Sci. 1978 Nov-Dec;276(3):279–285. doi: 10.1097/00000441-197811000-00004. [DOI] [PubMed] [Google Scholar]
- SALTZMAN H. A., CHICK E. W., CONANT N. F. Nocardiosis as a complication of other diseases. Lab Invest. 1962 Nov;11:1110–1117. [PubMed] [Google Scholar]
- STEVENS H. Actinomycosis of the nervous system. Neurology. 1953 Oct;3(10):761–772. doi: 10.1212/wnl.3.10.761. [DOI] [PubMed] [Google Scholar]
- Smith P. W., Steinkraus G. E., Henricks B. W., Madson E. C. CNS nocardiosis: response to sulfamethoxazole-trimethoprim. Arch Neurol. 1980 Nov;37(11):729–730. doi: 10.1001/archneur.1980.00500600077017. [DOI] [PubMed] [Google Scholar]
- Torvik A., Heding A. Histological studies on the effect of actinomycin D on retrograde nerve cell reaction in the facial nucleus of mice. Acta Neuropathol. 1967 Oct 20;9(2):146–157. doi: 10.1007/BF00691440. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D., Blevins A., Lieberman P. Nocardia asteroides infection complicating neoplastic disease. Am J Med. 1971 Mar;50(3):356–367. doi: 10.1016/0002-9343(71)90224-5. [DOI] [PubMed] [Google Scholar]