Abstract
Many plant species exhibit seasonal variation of flowering time in response to daylength. Arabidopsis (Arabidopsis thaliana) flowers earlier under long days (LDs) than under short days (SDs). This quantitative response to photoperiod is characterized by two parameters, the critical photoperiod (Pc), below which there is a delay in flowering, and the ceiling photoperiod (Pce), below which there is no further delay. Thus Pc and Pce define the thresholds beyond which maximum LD and SD responses are observed, respectively. We studied the quantitative response to photoperiod in 49 mutants selected for early flowering in SDs. Nine of these mutants exhibited normal Pce and Pc, showing that their precocious phenotype was not linked to abnormal measurement of daylength. However, we observed broad diversification in the patterns of quantitative responses in the other mutants. To identify factors involved in abnormal measurement of daylength, we analyzed the association of these various patterns with morphogenetic and rhythmic defects. A high proportion of mutants with altered Pce exhibited abnormal hypocotyl elongation in the dark and altered circadian periods of leaf movements. This suggested that the circadian clock and negative regulators of photomorphogenesis may contribute to the specification of SD responses. In contrast, altered Pc correlated with abnormal hypocotyl elongation in the light and reduced photosynthetic light-input requirements for bolting. This indicated that LD responses may be specified by positive elements of light signal transduction pathways and by regulators of resource allocation. Furthermore, the frequency of circadian defects in mutants with normal photoperiodic responses suggested that the circadian clock may regulate the number of leaves independently of its effect on daylength perception.
The understanding of normal biological processes has been consistently enhanced by the study of abnormal development, or teratology. Among teratology tools, mutants are particularly useful because alterations in their development are, to a large extent, reproducible and inheritable. Thus, mutant analyses have allowed the dissection of complex molecular pathways. However, the number of individual mutants that can be characterized by molecular genetics methods is limited by the sophistication of the methods employed. Furthermore, studies of small numbers of mutants do not necessarily reveal the broad significance of the findings. In contrast, analyses of large and genetically varied populations as part of agricultural botany, ecology, and evolutionary studies do not allow detailed characterization of regulatory pathways at a molecular level, but can yield more general information. Such studies may identify recurrent patterns associated with a given phenotype, such as alterations within specific signaling, metabolic, or developmental pathways (Lu et al., 2008). Studies of wide populations of mutants thus offer a means to either validate predictions drawn from molecular analyses or raise new predictions to be tested.
One example is the regulation of flowering time in plants. A large number of mutants with altered flowering times have been described in Arabidopsis (Arabidopsis thaliana). Analysis of these mutants has uncovered an intricate network of at least 100 genes regulating the floral transition (Koornneef et al., 2004; Putterill et al., 2004; Bernier and Périlleux, 2005). The growing complexity of the model derived from these analyses has prompted novel interest in natural variation of flowering time as a complementary source of investigation. Arabidopsis is generally described as a quantitative long day (LD) species because it flowers much later under short days (SDs) than it does under LDs. However, the photoperiodic responsiveness of natural accessions ranges, in fact, from day neutrality to a strong quantitative LD requirement. Physiological and molecular genetic analyses have shown that daylength signals are perceived by photoreceptors and integrated by a circadian clock (Yanovsky and Kay, 2003). The integration relies on the coincidence of light with the clock-regulated expression of CONSTANS (CO), a key regulator of flowering time (Suárez-López et al., 2001). In theory, both the phase of the CO rhythm and the light sensitivity of the coincidence perception process should contribute to the daylength-dependent pattern of floral induction.
Our previous work (Pouteau et al., 2004) described the isolation and phenotypic profiling of a collection of 61 mutants that flowered earlier than their wild-type progenitor, Wassilewskija (Ws), under SDs. About one-third of these mutants only showed an early flowering phenotype under SDs, whereas the remaining two-thirds were also early under LDs. Within this second group, six mutants were more or less insensitive to photoperiod. We also characterized the pattern of quantitative variation in flowering time in wild-type plants and showed that leaf number and bolting time indicators are differentially regulated by photoperiod (Pouteau et al., 2006). The objective of this article is first to examine to what extent the quantitative, daylength-dependent pattern of floral induction and, in particular, the specification of SD and LD responses are affected in the mutants. We then evaluate the contribution of various factors to the observed changes in photoperiodic responses. In particular, we test whether specific types of alterations of the daylength-dependent pattern of floral induction are associated with light and dark perception defects or abnormal circadian rhythms.
RESULTS
Genetic Characterization of the Mutant Collection
For this analysis, we randomly selected 49 mutants out of a collection of 61 early-flowering T-DNA lines named eav1 to eav61 (for early flowering from Versailles). Details of their genetic characterization are provided in Supplemental Table S1 and a summary of their phenotypes in Supplemental Table S2. The majority of the mutations were recessive, but 10 of them were semidominant. Complementation analyses revealed a low level of genetic redundancy and only five allelic groups could be identified, each comprising no more than two or three alleles. This included two lhp1 alleles, two elf4 alleles, and one allele of elf3 (Hicks et al., 1996, 2001; Gaudin et al., 2001; Doyle et al., 2002).
Additional information on the mutated loci was sought by sequencing flanking sequence tags (FSTs) for mutants showing a genetic linkage with a T-DNA insertion. For two tagged alleles, the identified insertions localized near or within the AGL18 and AGL27 loci that have been reported to contribute to the regulation of flowering time (Oh et al., 2004; Werner et al., 2005; Adamczyk et al., 2007). The encoded factors belong to the MADS-box protein family and act as floral repressors. Other insertions occurred within or near candidate loci that have not been previously associated with flowering-time phenotypes. The encoded factors included the circadian-regulated transcription factor GATA6 (Manfield et al., 2007), a regulator of pathogen responses and senescence known as CPR5 or HYS1 (Yoshida et al., 2002), the cell wall enzyme XTH3 (Yokoyama and Nishitani, 2001), and a gene encoding a KOW-domain protein similar to the transcription factor GTA02. One insertion was observed in a transposable element and one within a pseudogene. The causal link between these insertions and the observed flowering-time phenotypes awaits further analysis.
The majority of eav mutants appear to have no known counterparts among the flowering-time mutants described in the literature. The collection exhibits wide genetic diversity and broad phenotypic variation (Pouteau et al., 2004; Supplemental Table S2). It thus constitutes a suitable tool to analyze the range of phenotypic alterations that may be associated with precocious flowering-time phenotypes.
Characterization of Quantitative Responses across Photoperiods
Our previous work (Pouteau et al., 2004) showed that 43 of 49 eav mutants retained sensitivity to photoperiod. To test whether the precocious flowering phenotypes of these mutants under SD was linked to altered quantitative responses to photoperiods, flowering time was assayed under photoperiods ranging from 6 to 24 h. Multiple indicators were monitored, including the total number of nodes bearing leaves and the number of days to bolting.
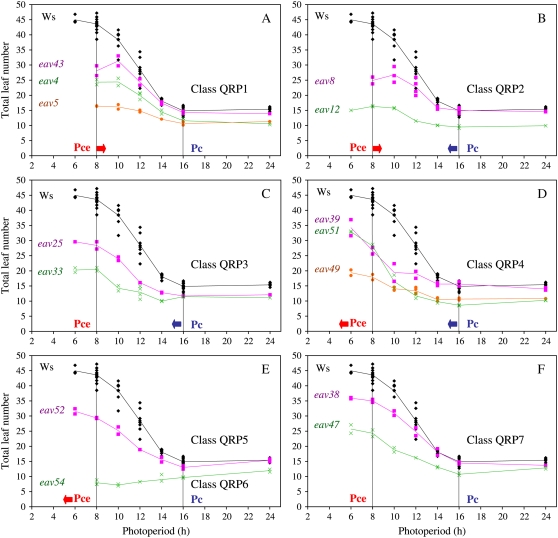
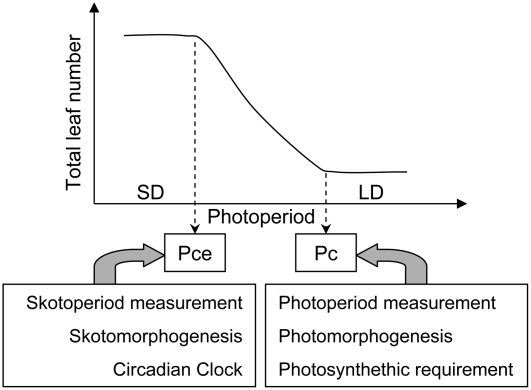
In the wild type, the quantitative response to photoperiod exhibited a sigmoidal shape (Fig. 1; Pouteau et al., 2006). The variation in leaf numbers was bound by two stationary plateaus defining, respectively, SD and LD responses: a ceiling plateau under photoperiods shorter than 8 h where leaf numbers were highest, and a base plateau under photoperiods longer than 16 h where leaf numbers were lowest. Intermediate numbers of leaves were observed between the two plateaus, corresponding to nonoptimal SD and/or LD responses. Here, we describe this response using two parameters previously defined by Roberts and Summerfield (1987): (1) the critical photoperiod (Pc), below which there is a delay in flowering; and (2) the ceiling photoperiod (Pce), below which there is no further delay. It should be noted that this definition for the Pc is distinct from the one adopted in some other works that used this single parameter to describe the daylength response (Thomas and Vince-Prue, 1997). Here, the Pc corresponds to the minimum photoperiod at which an optimal LD response is observed, whereas the Pce corresponds to the maximum photoperiod at which an optimal SD response is observed. Thus, Pc and Pce are indicators of a given plant's definition of LDs and SDs, respectively.
Figure 1.
Altered patterns of photoperiodic responses in early-flowering mutants. Quantitative responses to photoperiod (QRP) were assessed by monitoring total leaf numbers at flowering. A to F, Examples of the seven different QRP classes of mutations defined in Table I. Mutant data are shown in color and wild-type (Ws) controls in black. Vertical lines indicate the position of Pc and Pce for Ws. Red and blue arrows show the direction of the shifts in Pce and Pc relative to wild-type values. Each of the points represents averaged data from 10 individuals in one independent experiment.
Using leaf numbers as an indicator of flowering time, we found that either one or both of these parameters were altered in 34 of 43 mutants studied. Fourteen mutants exhibited changes in Pce only, eight in Pc only, and 12 in both Pce and Pc. Changes in Pce or Pc ranged from 1 to 4 h. Three main patterns of responses were identified based on increases or decreases in Pce or Pc or both (Fig. 1; Table I). The most obvious was the day-neutral pattern already reported for other mutants and accessions (Koornneef et al., 2004; Pouteau et al., 2004) and corresponding to an absence of response to photoperiod. The second pattern was described as qualitative-like because the maximum number of leaves continued to rise under photoperiods shorter than wild-type Pce as in species with an obligate LD flowering response (Roberts and Summerfield, 1987). Third, we identified a pattern defined as tropical-like because it was reminiscent of common photoperiodic responses in tropical species (although these are usually SD species rather than LD species, e.g. rice [Oryza sativa]; Summerfield et al., 1997). This pattern was characterized by a narrow photoperiod interval of sensitivity with longer Pce and shorter Pc. Intermediate patterns with either longer Pce or shorter Pc were also identified, resulting in a total of seven different classes.
Table I.
Classification of the patterns of quantitative response to photoperiod in the eav mutant collection
The Pc and Pce are compared with those of wild-type plants. Nb, Number of mutants in the different QRP classes; QRP, quantitative response to photoperiod. In this classification and other analyses presented in this article, the six mutants insensitive to photoperiod were considered to have alterations in both Pce and Pc. These include the elf3 allele, the two elf4 alleles, and the two lhp1 alleles (Hicks et al., 1996, 2001; Gaudin et al., 2001; Doyle et al., 2002).
| Class | Description | Pce | Pc | Nb |
|---|---|---|---|---|
| QRP 1 | Narrow sensitivity window, tropical-like response | Longer | Normal | 13 |
| QRP 2 | Narrow sensitivity window, tropical-like response | Longer | Shorter | 6 |
| QRP 3 | Narrow sensitivity window, tropical-like response | Normal | Shorter | 8 |
| QRP 4 | No ceiling under common SD, qualitative-like response | Shorter | Shorter | 6 |
| QRP 5 | No ceiling under common SD, qualitative-like response | Shorter | Normal | 1 |
| QRP 6 | Day neutral | – | – | 6 |
| QRP 7 | Normal response | Normal | Normal | 9 |
These results demonstrated that plant definitions of Pce and Pc can be uncoupled genetically. This suggested that regulation of SD or LD responses may, at least in part, be mediated by distinct processes.
Is Altered Measurement of SDs and LDs Related to Reduction in Leaf Numbers?
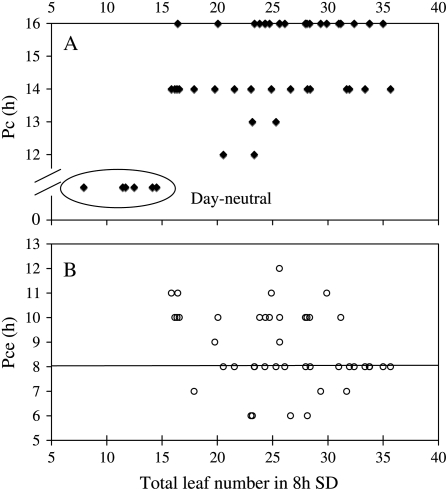
Because we used leaf numbers to monitor flowering time, there was a possibility that our results might be skewed by abnormal leaf development in some mutants. We therefore examined whether there was any correlation between the Pc or the Pce and leaf production under SDs and LDs. All of the mutants in the collection flowered with lower numbers of leaves than the wild-type under SDs, because this had formed the basis of the original mutant screen. However, Figure 2 shows that there was no relationship between their Pce or Pc and their leaf production under SDs. This demonstrates that alterations in plant quantitative response to photoperiod are generally not linked to changes in the plant's ability to keep producing leaves under short photoperiods.
Figure 2.
Independent variation of Pc or Pce from leaf production. Here, the mutants' ability to produce leaves was evaluated by their total leaf numbers under 8-h (SD) photoperiods. Values for 49 eav mutants were indicated. No correlation was observed with either Pc (A, black diamonds) or Pce (B, white circles). Mutants insensitive to photoperiod (day neutral) were shown in A as having Pc = 0 h. In the wild type, Pce was 8 h and Pc was 16 h.
A subset of 32 mutants flowered earlier than wild type under LD conditions and thus had a lower minimum number of leaves. Figure 3 shows a remarkable overlap between this category of mutants and mutants with altered Pc. This suggests that the plant's definition of LD may be important to specify leaf numbers under long photoperiods. Nevertheless, Pc was unaltered in 11 mutants that were early in LD and therefore other factors may also contribute to this precociousness. No correlation could be observed between changes in Pce and early flowering in LD.
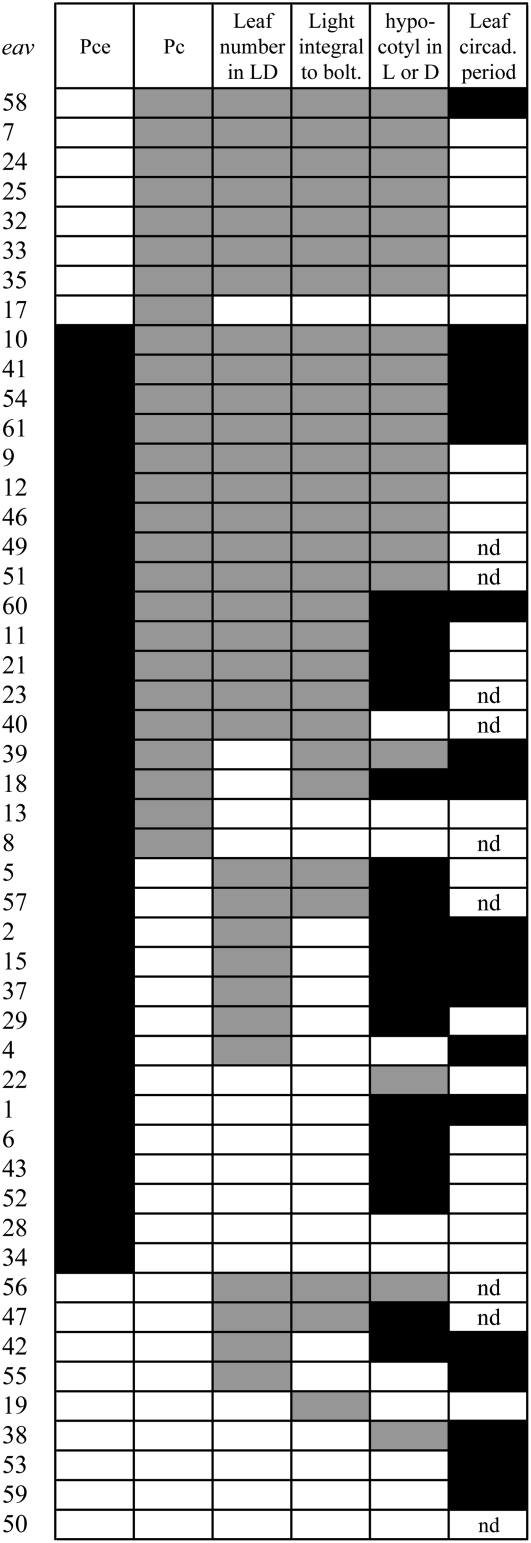
Figure 3.
Associations between photoperiodic time measurement, phototrophic requirement, photomorphogenic, and circadian phenotypes in the eav mutant population. Sorting by phenotype was applied from left to right. Solid boxes indicate significant changes. Gray filling was used to stress correlations between changes in Pc, decreases in leaf numbers in LDs, reduced photosynthetic requirements (light integral) for bolting, and abnormal hypocotyl elongation in the light (L). Black filling was used to highlight the overlap between changes in Pce and abnormal hypocotyl elongation in the dark (D). Note that some of these mutants also showed abnormal hypocotyl elongation in the light. Changes in the circadian period of leaf movements were also shown in black to emphasize their frequent association with changes in Pce. Nd, Not determined. See Supplemental Table S2 for a more detailed description of mutant phenotypes.
Do Phototrophic Effects Play a Role in Daylength Measurements?
Our previous work (Pouteau et al., 2006) indicated that the time to bolting does not necessarily correlate with leaf numbers at flowering and is strongly influenced by phototrophic input. Although total leaf numbers changed with photoperiod, the total number of days of photosynthetically active light or light integral required for bolting remained constant for the wild type, perhaps reflecting the need to achieve a certain biomass to ensure optimal seed production. Here, we tested whether this phototrophic input requirement was modified in early-flowering mutants. As previously shown for wild-type plants, the mutants exhibited little or no variation in the light integral required for bolting under photoperiods ranging from 6 to 16 h (data not shown). However, the average light integral was significantly reduced compared to wild type in 28 of 49 mutants (see Supplemental Table S2), suggesting a lower phototropic input requirement. Figure 3 shows that most of these mutants also produced fewer leaves in LDs and showed abnormal Pc. These findings suggest a possible link between perception of the phototrophic input, leaf numbers in LDs, and correct measurement of long photoperiods.
May Light and Dark Signaling Contribute Differentially to the Measurement of SDs and LDs?
A majority of early-flowering mutants within the Versailles collection exhibited hypocotyl elongation phenotypes in either light or darkness, suggesting abnormal perception of these environmental signals (Pouteau et al., 2004; Fig. 3; see Supplemental Table S2). Here, we tested whether the quantitative response to photoperiod was altered differentially in mutants that showed abnormal phenotypes in the light or in the dark.
Figure 3 shows that 17 of 20 mutants with abnormal phenotypes in the light had a shorter Pc. Furthermore, 17 flowered early in LDs and 18 bolted after shorter times under photosynthetically active light. These results indicate a link between these three phenotypes and suggest that light signaling plays a role in the measurement of Pc and the specification of LD responses. In contrast, Pce was modified in 15 of 17 mutants with abnormal phenotypes in the dark, suggesting that proper function of light signal transduction pathways during what would normally be a long night may be needed for a measurement of Pce and the specification of SD responses. The maximal delay in flowering under SDs may thus correspond to a long night response and depend on the measurement of a critical skotoperiod.
What Is the Contribution of the Circadian Clock?
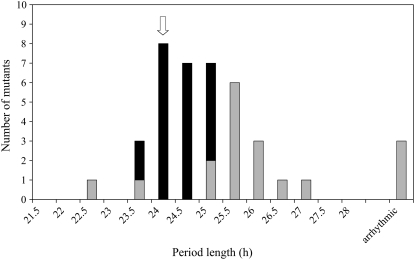
To assess the role of the circadian clock in defining the quantitative floral response to photoperiod, we tested whether some of the mutants exhibited abnormal circadian rhythms of leaf movements and whether these rhythmic defects correlated with changes in Pce, Pc, or both. Eighteen of 40 mutants tested for circadian phenotypes exhibited altered rhythmic behavior (see Supplemental Table S2). Figure 4 shows that the majority of these mutations (13 in total) lengthened the circadian period by 1 to 3 h. Only two of the mutations shortened the circadian period. Three of the mutants were arrhythmic and photoperiodically insensitive and were shown to correspond to alleles of the previously described elf3 and elf4 mutations (Hicks et al., 1996; Doyle et al., 2002; Supplemental Table S1).
Figure 4.
Distribution of leaf movement period phenotypes in the mutant population. Mutants with period values that were statistically different from Ws are indicated by the gray bars and mutants with wild-type period values by the black bars. The vertical arrow indicates the average period value for Ws (24.1 h).
Figure 3 shows that abnormal circadian rhythms were often observed in mutants with an altered Pce, revealing that the measurement of Pce and the specification of SD responses may rely to a large degree on the circadian clock. Rhythmic defects were most frequent in mutants with alterations in both Pce and Pc. However, seven of eight mutants with altered Pc only exhibited normal leaf movement periods. These findings indicate no obvious link between circadian regulation and the measurement of Pc (i.e. the specification of LD responses). Furthermore, rhythmic defects were observed in mutants with no change in Pce and Pc, suggesting that normal measurement of daylength is not necessarily linked to correct circadian function.
Circadian clocks regulate many aspects of plant physiology and defects in this regulation have been associated with a number of phenotypes, including abnormal hypocotyl elongation (Dowson-Day and Millar, 1999), abnormal light-regulated gene expression (Covington et al., 2001), and abnormal carbon assimilation (Dodd et al., 2005). We therefore tested for associations between aberrant rhythmic phenotypes and other defects frequently observed in early-flowering mutants. Figure 3 shows that almost all the mutants with circadian phenotypes displayed abnormal hypocotyl elongation. Seven circadian mutants showed hypocotyl elongation defects in the light only. Seven further circadian mutants showed hypocotyl phenotypes in constant darkness. Only four of the circadian mutants showed normal hypocotyl phenotypes. These results indicate that circadian clock defects are associated with altered photomorphogenesis and skotomorphogenesis within this group of flowering-time mutants.
DISCUSSION
Diversification of the Photoperiodic Response in Early-Flowering Mutants
Previous characterization of flowering-time mutants has been relatively simplistic and limited to flowering-time phenotypes under commonly used LDs and SDs. This article constitutes a description of quantitative responses across a wide range of photoperiods and in a large collection of flowering-time mutants. Because of the high level of phenotypic and genetic diversity in this collection, we believe that our results can be of broad significance. Phenotypic diversity was sought in the initial mutant screen that only eliminated sterile plants. Thus, the level of pleiotropy in the recovered mutants was generally high. Only a few, previously characterized, early-flowering mutants could be identified, possibly because previous studies focused on mutants with minimal pleiotropic phenotypes. Furthermore, our preliminary molecular characterization of tagged alleles identified a number of new candidate loci that, to our knowledge, have not yet been described as flowering-time regulators. Thus, the number of genetic alterations that allow the expression of an early-flowering phenotype may be broader than anticipated.
Based on the measurement of Pce and Pc, we identified changes in the response of 40 of 49 early-flowering mutants analyzed. Starting with a typical LD quantitative response in the wild-type accession Ws, three main patterns were identified: reduced photoperiod interval of sensitivity, enlarged photoperiod interval of sensitivity under SDs, and day neutrality. The occurrence of these different patterns demonstrated that early-flowering phenotypes can be associated with diversification in photoperiodic responses. Changes in Pce and Pc were not simply a biophysical consequence of general changes in the plant architecture because no correlation was identified between the maximum number of leaves under SDs and either of these parameters.
The Circadian Clock Is Not the Sole Factor Defining Pce and Pc
The circadian clock is generally recognized to regulate photoperiod sensing in living organisms. In plants, the timing of circadian rhythms relative to dawn and dusk (and more specifically of the rhythmic CO mRNA accumulation) is a key determinant of the floral response (Suárez-López et al., 2001; Roden et al., 2002; Yanovsky and Kay, 2002; Valverde et al., 2004): (1) mutations that alter the phase of the CO expression rhythm to increase its level of coincidence with light result in accelerated flowering; and (2) experimental conditions that restore normal timing of expression to the CO rhythm also restore normal photoperiodic responses. We thus anticipated that the circadian clock would be the main determinant of Pce and Pc. In agreement with this hypothesis, our detailed characterization of photoperiodic timing showed that changes in Pce were frequently associated with rhythmic defects. Altered circadian rhythms were also often linked to reduced number of leaves under LD. In contrast, no correlation was found between changes in Pc and altered circadian clock function. These results suggested a differential contribution of circadian regulation to the measurement of SD and LD.
Different factors may explain why incorrect definitions of Pce or Pc were not strictly correlated to rhythmic defects and reciprocally why a correct definition of Pce and Pc may not be affected by circadian period changes. First, relevant circadian defects may have been overlooked in our survey. We tested effects of the mutations on circadian period, rather than on phase, but some mutations may affect phase without altering period. However, the phase of circadian rhythms is generally related to period (shorter period rhythms correlating with advanced phases) and cases of specific phase defects have been scarcely reported (Salome et al., 2002). Alternatively, some circadian defects may not be detected by measuring leaf movement because these defects do not affect petiole tissues. Cells important for photoperiodic regulation of flowering are expected to include those in which CO is expressed. CO expression was shown to be restricted to vascular tissue in cotyledons and leaves and in the hypocotyl (An et al., 2004). Altered clock function in these cells only would explain changes in photoperiodic timing. Such tissue-specific regulation of circadian rhythms has been reported for the PRR3 gene, which is specifically expressed in the vasculature (Para et al., 2007).
Second, additional components of the quantitative response across photoperiods may have been overlooked in our study. We measured the Pce and Pc components, but did not estimate the slope and changes in amplitude of the response. Effects on these parameters may be associated with some of the circadian defects detected in this work. Such effects may, for example, be mediated by changes in the amplitude of the CO expression rhythm without altering its phase. Thus, the early-flowering phenotype of the fiona1 mutant under SD conditions was associated with increased amplitude, but no phase alteration of the CO rhythm (Kim et al., 2008).
Third, factors other than the circadian clock probably contribute to the definition of Pce and Pc. We show below that these factors include light signal transduction pathways. Finally, it is not currently possible to rule out the possibility that normal circadian regulation may not be an absolute prerequisite for a correct definition of Pce and Pc.
Skotomorphogenic and Photomorphogenic Pathways May Contribute to the Definition of Pce and Pc, Respectively
In general, changes in Pce and Pc correlated with different environmental perception defects. Changes in Pce (i.e. plant perception of a SD) correlated with altered elongation phenotypes in darkness, suggesting that skotomorphogenesis is involved in the appropriate perception of SDs. In contrast, changes in Pc (i.e. plant perception of a LD) correlated with altered elongation phenotypes in the light, decreased number of leaves under LDs, and a reduced photosynthetic requirement for bolting. These phenotypes are reminiscent of the phenotypes of wild-type plants grown under low red/far-red ratios (Franklin and Whitelam, 2005) and may therefore be related to the shade avoidance response. Therefore, Pce and Pc appear to be defined through different regulatory processes. Pce may be defined by negative regulators of light signaling, such as the SPA and COP1 proteins that interact with CO to promote its degradation in darkness and thus ensure suppression of flowering under SDs (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). Pc may be specified by photoreceptors and positive regulators of light signal transduction pathways that act to either promote (PhyB) or inhibit (PhyA and CRY2) the degradation of the CO protein in the light (Valverde et al., 2004). To test these possibilities, expression of the CO protein will have to be analyzed in some representative eav mutants. Furthermore, the quantitative response to photoperiod of previously characterized photomorphogenic mutants will need to be examined.
The importance of skotomorphogenesis for the specification of Pce may indicate that quantitative LD species are able to measure the duration of the night, or skotoperiod, in a manner similar to SD species in which much of the early studies of the photoperiodic regulation of flowering have been conducted (e.g. Pharbitis nil; O'Neill, 1992). These studies pointed to the importance of the skotoperiod for correct measurement of inductive SD. More recently, the homologs of CO in SD plants (rice and P. nil) and LD plants (wheat [Triticum aestivum] and Arabidopsis, respectively) were shown to be functionally equivalent (Putterill et al., 2004). In spite of this equivalence and the presence of similar regulatory factors in rice and Arabidopsis, CO activity generates opposite flowering responses to photoperiod in these two species. Hd1, the CO homolog in rice, acts as a repressor in LDs, but is a promoter of flowering in SDs. Furthermore, long-night induction of flowering is apparently not dependent on Pn CO, the CO homolog in P. nil, although the downstream floral activator (FLOWERING LOCUS T) identified in Arabidopsis and rice is present and plays a similar role in this species (Hayama et al., 2007). The dark-dependent regulation of flowering in P. nil may share some common basis with the definition of Pce in Arabidopsis for which a molecular basis has yet to be identified.
Maintenance of Normal Patterns of Daylength Measurement
Altered flowering time under SD and/or LD is usually interpreted as an indication of photoperiodic perturbation. We show here that this may be an overinterpretation since 17 early-flowering mutants under SDs have no defect in the specification of Pce and nine of these mutants have a completely normal response to photoperiod. Thus, in the absence of a complete characterization of flowering responses across photoperiods, the hypothesis of photoperiodic alteration can only be provisional. A summary of the factors involved in the specification of Pce and Pc and the maintenance of normal photoperiodic response patterns is presented in Figure 5. Our data also show that abnormal flowering time in mutants with normal Pce and Pc is often associated with circadian period defects. This may indicate that the circadian clock can regulate flowering time and leaf numbers independently of its function in the measurement of day and night, possibly by modulation of resource allocation or developmental rate (Wiltshire et al., 1994; Diggle, 1999).
Figure 5.
Summary of regulatory pathways that may contribute to the pattern of quantitative response to photoperiod in Arabidopsis.
CONCLUSION
This study shows that large-scale profiling approaches and complete characterization of photoperiodic phenotypes can help clarify the models drawn from the analyses of individual mutants. Because only few mutations recovered in our collection have been characterized at a molecular level, it will be important to further our findings on the differential measurement of SDs and LDs by characterizing the photoperiodic response patterns and rhythmic responses in mutants with known molecular defects. It would be particularly relevant to analyze early- and late-flowering mutants, including circadian clock and light signal transduction mutants. Complete characterization of photoperiodic phenotypes should help to determine whether uncoupling between altered flowering time and abnormal daylength measurement is also observed in some of these mutants.
MATERIALS AND METHODS
Plant Material
The natural accession Ws was used. A subset of 49 of 61 T-DNA insertion lines, eav1 to eav61, obtained from the Versailles collection (INRA, France) in the Ws background (Bechtold et al., 1993; Pouteau et al., 2001, 2004) were analyzed.
Analyses of T-DNA FSTs
DNA from mutants was extracted according to Doyle and Doyle (1990). FSTs were produced using a protocol based on gene walking (Devic et al., 1997) and optimized for large-scale amplification and systematic sequencing (Balzergue et al., 2001). FSTs with a reference number can be found in the FLAGdb++ database (http://urgv.evry.inra.fr/projects/FLAGdb++/HTML/index.shtml; Samson et al., 2002).
Growth Conditions for Flowering-Time Assays
Mutant and wild-type seeds were sown on soil (Stender A240; Bluemendenwerk Stender GmbH) and grown in Sanyo Gallenkamp SGC660 growth cabinets at 20°C ± 0.2°C and 70% ± 2% relative humidity. The soil was kept moist by application of nutrient solution three times a week. The light during the whole-day period was provided with mixed fluorescent and incandescent tubes and the photon flux density measured at soil level was 230 ± 20 μmol m−2 s−1 and 2 ± 0.2 μmol m−2 s−1, respectively. Developmental uniformity was obtained by selecting the 10 most uniform plants on average about 12 d after sowing, bringing the plant density to one plant per pot, and rotating the trays three times a week.
Measurement of Flowering-Time Indicators
The total number of leaves produced by the apical meristem was recorded on bolted plants. Bolting time was measured as the number of days from sowing to the first elongation of the floral stem at 0.1-cm height. No major variation was observed in two to four independent repeats for the mutants. The total amount of photosynthetically active light (light integral) received before bolting was calculated as follows: number of days to bolting x hours under photosynthetically active light/24.
Estimation of the Pce and Pc
The Pce and Pc in the mutants were determined by measuring the ratios dTLN8-x = (TLN8 − TLNx)/TLN8 and dTLN16-x = (TLN16 − TLNx)/TLN16 (where TLNx corresponds to the TLN at a photoperiod of x h), respectively, and comparing them with the values obtained for Ws (Pce = 8 h and Pc = 16 h, as previously defined by Pouteau et al., 2006). Pce was longer when dTLN8-10 was markedly lower than in Ws (0.12) and was defined as the photoperiod x where 0.05 < [mutant dTLN8-(x + 1)] < [Ws dTLN8-(x-1)] or [mutant dTLN8-x] < 0.05. Pce was shorter when dTLN6-8 was markedly higher than in Ws (0.03) and was defined as 6 h where [mutant dTLN6-8] > 0.12 and 7 h where 0.12 > [mutant dTLN6-8] > 0.07. Pc was shorter when the ratio dTLN16-14 was markedly lower than in Ws (0.22) and was defined as the photoperiod x where 0.1 < [mutant dTLN16-(x-1)] < [Ws dTLN16-(x + 1)] or [mutant dTLN16-x] < 0.1.
Measurement of Rhythmic Leaf Movements
Plants were grown under a 12-h photoperiod (12 L–12 D cycles) until emergence of primary leaves (approximately 10 d), then transferred to constant light (70–100 μmol m−2 s−1). The vertical position of growing leaves was tracked using the Kujata imaging system and the circadian period was determined by fast Fourier transform-nonlinear least squares analysis as previously described (Dowson-Day and Millar, 1999).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Molecular genetic description of the early-flowering mutant collection.
Supplemental Table S2. Phenotypic characterization of the early-flowering mutant collection.
Supplementary Material
Acknowledgments
We thank Hervé Ferry for technical assistance with plant culture, Sandrine Balzergue (URGV, INRA Evry) for FST production, and Matthieu Simon and the staff of the Arabidopsis Resource Centre for Genomics (SGAP, INRA Versailles) for their help with resource databases. We are grateful to Yves Chupeau and Herman Höfte for their support.
This work was supported by the European Union (grant no. BI04–CT97–2340 to V.F.); by INRA (grant to D.L.); and by the University of Warwick (Research and Teaching Development grant to M.W.). The leaf movement imaging system at Warwick was funded by the Royal Society. Collaboration between the two laboratories was supported by the Biotechnology and Biological Sciences Research Council (ISIS grant to I.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sylvie Pouteau (sylvie.pouteau@versailles.inra.fr).
The online version of this article contains Web-only data.
References
- Adamczyk B, Lehti-Shiu M, Fernandez D (2007) The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J 50 1007–1019 [DOI] [PubMed] [Google Scholar]
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131 3615–3626 [DOI] [PubMed] [Google Scholar]
- Balzergue S, Dubreucq B, Chauvin S, Le-Clainche I, Le Boulaire F, de Rose R, Samson F, Biaudet V, Lecharny A, Cruaud C, et al (2001) Improved PCR-walking for large-scale isolation of plant T-DNA borders. Biotechniques 30 496–498, 502, 504 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Ser III Sci Vie 316 1194–1199 [Google Scholar]
- Bernier G, Périlleux C (2005) A physiological overview of the genetics of flowering time control. Plant Biotechnol J 3 3–16 [DOI] [PubMed] [Google Scholar]
- Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA (2001) ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13 1305–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic M, Albert S, Delseny M, Roscoe T (1997) Efficient PCR walking on plant genomic DNA. Plant Physiol Biochem 35 331–339 [Google Scholar]
- Diggle CS (1999) Heteroblasty and the evolution of flowering phenologies. Int J Plant Sci 160 S123–S134 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Tóth EK, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309 630–633 [DOI] [PubMed] [Google Scholar]
- Dowson-Day MJ, Millar AJ (1999) Circadian dysfunctions causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J 17 63–71 [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle DJ (1990) Isolation of plant DNA from fresh tissues. Focus 12 13–15 [Google Scholar]
- Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma-Bognar L, Nagy F, Millar AJ, Amasino RM (2002) The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature 419 74–77 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC (2005) Phytochromes and shade avoidance responses in plants. Ann Bot (Lond) 96 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V, Libault M, Pouteau S, Juul T, Zhao G, Lefebvre D, Grandjean O (2001) Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 128 4847–4848 [DOI] [PubMed] [Google Scholar]
- Hayama R, Agashe B, Luley E, King R, Coupland G (2007) A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis. Plant Cell 19 2988–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Albertson TM, Wagner DR (2001) EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Millar AJ, Carré IA, Somers DE, Straume M, Meeks-Wagner DR, Kay SA (1996) Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science 274 790–792 [DOI] [PubMed] [Google Scholar]
- Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27 1277–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim Y, Yeom M, Nam HG (2008) FIONA1 is essential for regulating period length in the Arabidopsis circadian clock. Plant Cell 20 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55 141–172 [DOI] [PubMed] [Google Scholar]
- Laubinger S, Marchal V, Gentilhomme J, Wenkel S, Adian J, Jang S, Kulajta G, Braun H, Coupland G, Hoecher U (2006) Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133 3213–3222 [DOI] [PubMed] [Google Scholar]
- Liu L-J, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20 292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Savage LJ, Ajjawi I, Imre KM, Yoder DW, Benning C, DellaPenna D, Ohlrogge JB, Osteryoung KW, Weber AP, et al (2008) New connections across pathways and cellular processes: industrialized mutant screening reveals novel associations between diverse phenotypes in Arabidopsis. Plant Physiol 146 1482–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfield I, Devlin P, Jen C, Westhead D, Gilmartin P (2007) Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiol 143 941–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Zhang H, Ludwig P, van Nocker S (2004) A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell 16 2940–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SD (1992) The photoperiodic control of flowering: progress toward understanding the mechanism of induction. Photochem Photobiol 56 789–801 [Google Scholar]
- Para A, Farre EM, Imaizumi T, Pruneda-Paz JL, Harmon FG, Kay SA (2007) PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell 19 3462–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S, Ferret V, Gaudin V, Lefebvre D, Sabar M, Zhao G, Prunus F (2004) Extensive phenotypic variation in early flowering mutants of Arabidopsis. Plant Physiol 135 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S, Ferret V, Lefebvre D (2006) Comparison of environmental and mutational variation in flowering time in Arabidopsis. J Exp Bot 57 4099–4109 [DOI] [PubMed] [Google Scholar]
- Pouteau S, Gaudin V, Ferret V, Lefebvre D, Libault M, Prunus F, Sabar M, Zhao G (2001) Analysis of the floral repression process in Arabidopsis. Flowering Newsl 32 3–9 [Google Scholar]
- Putterill J, Laurie R, MacKnight R (2004) It's time to flower: the genetic control of flowering time. Bioessays 26 363–373 [DOI] [PubMed] [Google Scholar]
- Roberts EH, Summerfield RJ (1987) Measurement and prediction of flowering in annual crops. In JG Atherton, ed, Manipulation of Flowering. Butterworths, London, pp 17–50
- Roden LC, Song HR, Jackson S, Morris K, Carré IA (2002) Floral responses to photoperiod are correlated with the timing of rhythmic expression relative to dawn and dusk in Arabidopsis. Proc Natl Acad Sci USA 99 13313–13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome PA, Michael TP, Kearns EV, Fett-Neto AG, Sharrock RA, McClung CR (2002) The out of phase1 mutant defines a role for PHYB in circadian phase control in Arabidopsis. Plant Physiol 129 1674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Brunaud V, Balzergue S, Dubreucq B, Lepiniec L, Pelletier G, Caboche M, Lecharny A (2002) FLAGdb/FST: a database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Res 30 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410 1116–1120 [DOI] [PubMed] [Google Scholar]
- Summerfield RJ, Ellis RH, Craufurd PQ, Aiming Q, Roberts EH, Wheeler TR (1997) Environmental and genetic regulation of flowering of tropical annual crops. Euphytica 96 83–91 [Google Scholar]
- Thomas B, Vince-Prue D (1997) Photoperiodism in Plants. Academic Press, San Diego
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoperiod regulation of CONSTANS protein in photoperiodic flowering. Science 303 1003–1006 [DOI] [PubMed] [Google Scholar]
- Werner J, Borevitz J, Warthmann N, Trainer G, Ecker J, Chory J, Weigel D (2005) Quantitative trait locus mapping and DNA array hybridization identify an FLM deletion as a cause for natural flowering-time variation. Proc Natl Acad Sci USA 102 2460–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire RJE, Murfet IC, Reid JB (1994) The genetic control of heterochrony: evidence from developmental mutants of Pisum sativum L. J Evol Biol 7 447–465 [Google Scholar]
- Yanovsky MJ, Kay SA (2002) Molecular basis of seasonal time measurement in Arabidopsis. Nature 419 308–312 [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA (2003) Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Biol 4 265–275 [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Nishitani K (2001) A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol 42 1025–1033 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ito M, Nishida I, Watanabe A (2002) Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defence responses in Arabidopsis thaliana. Plant J 29 427–437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.