Abstract
The cytosolic pools of glucose-1-phosphate (Glc-1-P) and glucose-6-phosphate are essential intermediates in several biosynthetic paths, including the formation of sucrose and cell wall constituents, and they are also linked to the cytosolic starch-related heteroglycans. In this work, structural features and biochemical properties of starch-related heteroglycans were analyzed as affected by the cytosolic glucose monophosphate metabolism using both source and sink organs from wild-type and various transgenic potato (Solanum tuberosum) plants. In leaves, increased levels of the cytosolic phosphoglucomutase (cPGM) did affect the cytosolic heteroglycans, as both the glucosyl content and the size distribution were diminished. By contrast, underexpression of cPGM resulted in an unchanged size distribution and an unaltered or even increased glucosyl content of the heteroglycans. Heteroglycans prepared from potato tubers were found to be similar to those from leaves but were not significantly affected by the level of cPGM activity. However, external glucose or Glc-1-P exerted entirely different effects on the cytosolic heteroglycans when added to tuber discs. Glucose was directed mainly toward starch and cell wall material, but incorporation into the constituents of the cytosolic heteroglycans was very low and roughly reflected the relative monomeric abundance. By contrast, Glc-1-P was selectively taken up by the tuber discs and resulted in a fast increase in the glucosyl content of the heteroglycans that quantitatively reflected the level of the cytosolic phosphorylase activity. Based on 14C labeling experiments, we propose that in the cytosol, glucose and Glc-1-P are metabolized by largely separated paths.
During photosynthesis, most plant species utilize a major proportion of the carbon fixed to accumulate transitory starch, which is deposited in the stromal space of the chloroplasts. Chloroplastic starch is degraded in the subsequent dark period and, thereby, plants are able to sustain growth and developmental processes in the absence of photosynthetic carbon assimilation (Smith et al., 2005; Fettke et al., 2007; Smith and Stitt, 2007; Zeeman et al., 2007a, 2007b). Starch mobilization appears to be initiated by a combined action of starch phosphorylating dikinases (Ritte et al., 2002; Hejazi et al., 2008) and various hydrolases, such as plastidial β-amylase isozymes (Scheidig et al., 2002; Fulton et al., 2008) and a debranching isoenzyme (ISA3; Edner et al., 2007). This process leads to the formation of maltose and a series of maltodextrins, which are further metabolized by the plastidial disproportionating isozyme 1 (DPE1) to yield Glc (Chritchley et al., 2001). However, most of the starch-derived neutral sugars are exported by the chloroplasts as maltose (Weise et al., 2004). Following the transport into the cytosol via selective transporters (designated MEX and pGT; Weber et al., 2000; Niittylä et al., 2004), both Glc and maltose are converted to phosphorylated sugars, which fuel a variety of biosynthetic processes, including the formation of Suc and cell wall constituents (Reiter, 2008). The initial reactions of this path are unexpectedly complex and contain, as an indispensable step, a reversible glucosyl transfer from maltose to a glycan-type acceptor and the release of the other maltose-derived glucosyl residue (the one containing the reducing end of the former disaccharide) as Glc. This reaction is catalyzed by a cytosolic transglucosidase (EC 2.4.1.25; also designated amylomaltase, glucosyl transferase, or DPE2). DPE2 is a modular protein containing two putative N-terminal carbohydrate-binding modules and a C-terminal domain similar to that of glycoside hydrolases of family 77, which, however, is split by a large insertion (Steichen et al., 2008). Arabidopsis (Arabidopsis thaliana) mutants deficient in DPE2 differ from wild-type plants by elevated levels of both maltose and starch and by a strongly reduced growth (Chia et al., 2004; Lu and Sharkey, 2004). Based on sequence analyses, both DPE2 and MEX are widely distributed in higher and lower plants, including Ostreococcus tauri and Chlamydomonas reinhardtii (Deschamps et al., 2008; Steichen et al., 2008). This implies that both proteins exert an important function in starch metabolism throughout the plant kingdom.
Under in vitro conditions, DPE2 catalyzes the repetitive transfer of glucosyl residues from maltose molecules to nonreducing ends of glycogen (Chia et al., 2004) and also transfers glucosyl residues from glycogen to monosaccharides, thereby forming disaccharides (Fettke et al., 2006). Glycogen is likely to act as a substitute of an endogenous highly branched polysaccharide residing in the same compartment as DPE2. In an attempt to identify the physiological polyglycan substrate of DPE2, we recently analyzed water-soluble heteroglycans (SHG) from leaves of several plant species, including pea (Pisum sativum), Arabidopsis, and potato (Solanum tuberosum). These glycans are heterogeneous both in subcellular distribution and biochemical features. For this reason, we established a procedure allowing their physical separation. The total soluble heteroglycans were separated into a low molecular mass fraction (SHGS; apparent size ranging from 1 to 10 kD) and a high molecular mass fraction (SHGL; above 10 kD; Fettke et al., 2004). By field flow fractionation (FFF) or, alternatively, by incubation with the β-glucosyl Yariv reagent (Yariv et al., 1962; Nothnagel, 1997), the latter was resolved into two subfractions designated subfraction I (which is Yariv nonreactive) and subfraction II (which is, conversely, Yariv reactive). Both aqueous and nonaqueous fractionation techniques revealed that subfraction I is located in the cytosol of mesophyll cells, whereas subfraction II resides in the apoplast and appears to represent cell wall-related glycans (Fettke et al., 2004, 2005a, 2005b). Subfraction I contains, as major constituents, Gal, Ara, and Glc and possesses in excess of 20 glycosidic linkages (Fettke et al., 2004, 2005a, 2005b).
On the basis of several independent lines of evidence, subfraction I is likely to be the in vivo substrate of DPE2 in leaves and, therefore, may be intimately involved in the cytosolic metabolism of starch-derived monosaccharides and disaccharides. Recombinant DPE2 transfers glucosyl residues from maltose to subfraction I and from subfraction I to Glc, thereby yielding Glc and maltose, respectively. By contrast, DPE2 does not utilize subfraction II as glucosyl acceptor or as glucosyl donor (Fettke et al., 2006). Moreover, in DPE2-deficient Arabidopsis mutants, subfraction I possesses a higher glucosyl content and undergoes structural changes that significantly affect the interaction with cytosolic glucosyl transferases. As an example, recombinant DPE2 transfers glucosyl residues from maltose to the mutant-derived subfraction I at a rate that is approximately 1 order of magnitude higher than that of the glycan isolated from wild-type leaves (Fettke et al., 2006). Furthermore, DPE2-deficient mutants of Arabidopsis possess a 3- to 4-fold higher activity of cytosolic phosphorylase (Pho2 or, in Arabidopsis, AtPHS2; Chia et al., 2004). Interestingly, in vitro studies have revealed that recombinant cytosolic phosphorylase, like DPE2, utilizes subfraction I as substrate and, moreover, the two glucosyl transferases have been shown to act on the same glucosyl donor/acceptor sites (Fettke et al., 2006). Finally, in transgenic potato plants that overexpress or underexpress the cytosolic phosphorylase, both the monomer patterns and the size distribution of subfraction I differ from that of the wild type (Fettke et al., 2005b). On the basis of all these data, a pathway (Fig. 1) has been proposed that defines the conversion of the two starch-derived neutral sugars, maltose and Glc, to Suc and intermediates of the cellular metabolism. It should be noted that the path relies on an existing pool of cytosolic heteroglycans, as the biosynthesis of these polysaccharides is not included (see below). While the data mentioned above strongly suggest that the reaction sequence outlined in Figure 1 is functional in mesophyll cells, very little is known about the occurrence and functions of cytosolic heteroglycans in heterotrophic tissues.
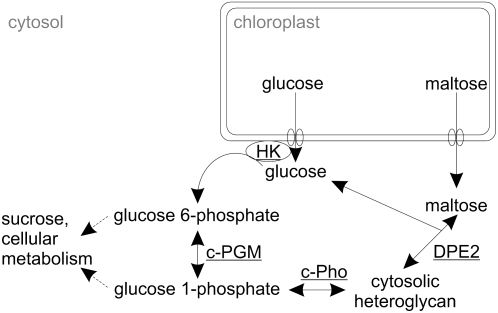
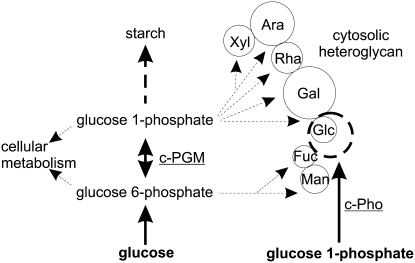
Figure 1.
Proposed cytosolic metabolism of starch-derived Glc and maltose following their export from the chloroplast. The carbon flux from the chloroplast to the cytosol is restricted to the export of two neutral sugars, maltose and Glc, via the Glc and maltose transporter. Both the cytosolic transglucosidase (DPE2) and the cytosolic phosphorylase isozyme (c-Pho) transfer glucosyl residues to the cytosolic heteroglycans. Both maltose and Glc-1-P act as glucosyl donors. Due to the reversibility of the two glucosyl transfer reactions, glucosyl residues can also be transferred from the nonreducing ends of the heteroglycans to Glc or to orthophosphate, yielding maltose and Glc-1-P, respectively. The cytosolic hexokinase (HK) is thought to be associated with the outer envelope membrane of the chloroplast.
According to this path (Fig. 1), the cytosolic pools of both maltose and Glc are immediately or via a short sequence of reactions linked to the heteroglycans. DPE2 transfers a glucosyl residue from maltose to the heteroglycans and also from the glycans to Glc, yielding maltose. When using maltose as glucosyl donor, the glucosyl moiety containing the former reducing end of the disaccharide is released as Glc, which enters the cytosolic pool as does Glc generated from starch inside the chloroplast, and is subsequently exported to the cytosol. By the action of hexokinase, the cytosolic Glc pool is phosphorylated to yield Glc-6-P, which, subsequently, is converted to Glc-1-P by the cytosolic isozyme(s) of phosphoglucomutase (PGM; EC 2.7.5.1). The two Glc-Ps enter many pathways, among which, in quantitative terms, the biosynthesis of Suc and the formation of cell wall constituents are most prominent (Scheible and Pauly, 2004). Alternatively, Glc-1-P can act as glucosyl donor for the Pho2-mediated transfer of glucosyl residues to the heteroglycans. Due to the reversibility of the Pho2-catalyzed reaction, the enzyme can also utilize the heteroglycan as a glucosyl donor and orthophosphate as an acceptor, catalyzing the formation of Glc-1-P.
The cyclic process outlined in Figure 1, which includes the interconversion of Glc-6-P and Glc-1-P, enables the cell to balance varying influxes of monosaccharides and disaccharides from the numerous chloroplasts into the cytosol. Recently, several transgenic lines of potato were phenotypically characterized that possess altered cytosolic phosphoglucomutase (cPGM) activity. Some of these lines express a PGM from Escherichia coli in addition to the endogenous PGM isoforms. Expression of the transgenes was controlled either by the tuber-specific B33 patatin promoter or the tissue-constitutive 35S promoter. In leaves possessing an elevated cPGM activity, photosynthesis rates were essentially unchanged but the Suc content was increased. Interestingly, the level of maltose (and that of isomaltose as well) was significantly decreased in all transgenic lines. In addition, the content of minor monosaccharides, such as Gal and Ara, was lowered (Lytovchenko et al., 2005a). Intriguingly, both monomers are prominent constituents of the heteroglycans (Fettke et al., 2005a, 2005b). These data thus suggest that overexpression of the cPGM results in a complex change in the intracellular carbon fluxes that also affect features of the cytosolic heteroglycans. For this reason, we have studied the glycans in leaves from cPGM-overexpressing lines from potato. Properties of the heteroglycans were compared with those isolated from wild-type plants and also from transgenic lines that, due to the expression of the transgene in the antisense orientation, possess decreased levels of the cPGM (Fernie et al., 2002b; Lytovchenko et al., 2002a).
The reason for analyzing heteroglycans from potato tuber was 2-fold. First, almost nothing is known about the structure and functions of these glycans in heterotrophic tissues. In this system, major carbon fluxes are directed from the cytosol toward the plastid, whereas in autotrophic cells, fluxes from the chloroplast into the cytosol are dominant. Second, exogeneous sugars are efficiently taken up by tuber discs and are utilized for a massive reserve starch biosynthesis inside the amyloplast (Geiger et al., 1998; Lytovchenko et al., 2005a, 2005b). Therefore, feeding experiments can contribute to the understanding of the biochemical functions and structural features of the heteroglycans under prevailing metabolic conditions.
RESULTS
PGM Activity in the Transgenic Potato Plants
Four previously generated transgenic potato lines that either overexpress or underexpress the cPGM were grown under controlled conditions together with wild-type plants. Source leaves or growing tubers freshly removed from the mother plants were harvested, and the total extractable PGM activities were monitored (Table I). In leaves, the protein-based total PGM activity of the most efficiently overexpressing line (denoted line B here) and the most strongly reduced line (denoted line C here) differ by approximately 5-fold (the original designations of these lines are provided in “Materials and Methods”). For the activity of the cPGM isozyme(s), this range is likely to be even larger, as it represents approximately 55% of the total extractable enzyme activity (Tauberger et al., 2000; Fernie et al., 2002b). Remarkably, leaves and tubers differ in the efficiency of the PGM overexpression: while leaves from line B possess a specific PGM activity 2-fold higher than that of wild-type leaves, the PGM activity in tubers from the same transgenic line is only slightly increased in comparison with that found in wild-type tubers. Similar effects have been frequently observed, as the constitutive expression of a transgene, as mediated by the 35S promoter, requires both specific cis elements in the regulatory regions of the promoter and the corresponding trans-acting proteins (Benfey and Chua, 1990). The latter are likely to vary depending on the plant organ.
Table I.
Total PGM activities in extracts from potato leaves and tubers
Proteins were extracted from leaves or from growing tubers of two cPGM-overexpressing (lines A and B) or two cPGM-underexpressing (lines C and D) potato lines. Total PGM activities and protein concentrations were monitored. Specific activities are given as percentage of the respective wild-type control, which was designated 100%. Mean values and sd are given (three independently performed experiments, each with three replicas).
| Line | PGM Activity
|
|
|---|---|---|
| Leaves | Tubers | |
| A | 133 ± 13 | 123 ± 7 |
| B | 201 ± 36 | 108 ± 5 |
| C | 38 ± 2 | 52 ± 5 |
| D | 73 ± 3 | 68 ± 8 |
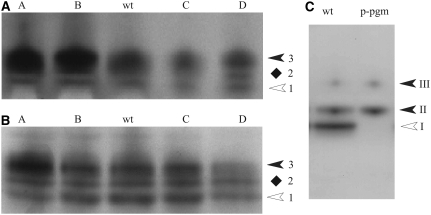
Next, we determined the PGM isoform patterns of leaves (Fig. 2A) and tubers (Fig. 2B) by native PAGE followed by an enzyme activity staining that is based on the PGM-dependent conversion of Glc-1-P to Glc-6-P and two subsequent redox reactions that finally result in the formation of insoluble formazan (Kofler et al., 2000). In all samples, three bands of PGM activity were resolved, all of which strictly depend upon the addition of Glc-1-P (data not shown). In the transgenic lines that express the PGM from E. coli in the cytosol (lines A and B; Lytovchenko et al., 2005b), the transgenic PGM comigrated with the most slowly moving endogenous PGM form (band 3; Fig. 2, A and B). A strong overexpression of the bacterial PGM tends to diminish the resolution of the three bands of activity. In antisense lines (C and D), the staining of the slowest moving band is diminished (Fig. 2, A and B). These results suggest that band 3 represents the cPGM isozyme. However, due to the instability of the PGM activity from potato leaves or tubers, verification of this assumption by nonaqueous fractionation was unsuccessful.
Figure 2.
PGM isoform patterns in potato and Arabidopsis as revealed by native PAGE followed by enzyme activity staining. Extracts from leaves (A) or growing tubers (B) of two overexpressing (lines A and B) and two underexpressing (lines C and D) transgenic lines from potato and from wild-type plants (wt) were separated by native PAGE. In A, 20 μg of protein was loaded in each lane; in B, 30 μg of tuber protein was applied per lane; in C, 20 μg of protein from leaves of Arabidopsis wild type (ecotype Columbia) or from the mutant lacking the plastidial PGM (p-pgm) was loaded in each lane. Following electrophoresis, the slab gel was stained for PGM activity. In A and B, the three PGM forms were designated 1 (having the highest mobility) to 3 (lowest mobility). Plastidial (white arrowheads) and cytosolic (black arrowheads) isozymes of PGM are marked. The location of form 2 (diamonds) remains to be clarified. In C, the white arrowhead marks the plastidial PGM isozyme (band I) and the two closed arrowheads (bands II and III) mark the two cPGM isozymes.
Therefore, we compared the PGM isozyme pattern from potato with that of leaves from Arabidopsis plants, and we included a mutant that is deficient in the plastidial isoform (Caspar et al., 1985). For leaves from Arabidopsis wild-type plants, three bands of PGM activity were observed that, according to the order of decreasing mobilities, were designated bands I to III. In the mutant, the two slower moving bands (bands II and III; i.e. the cytosolic forms) were present but the fast moving band (band I; the plastidial isozyme) was undetectable (Fig. 2C). Interestingly, essentially the same PGM isozyme pattern was observed when leaf extracts were separated by a continuous electrophoresis in starch gels (Caspar et al., 1985).
In summary, it is highly likely that in extracts from both leaves and tubers from potato, band 3 is located in the cytosol whereas band 1 is plastidial. The subcellular location of band 2 remains to be defined. This isoform could be attributed to the cytosol (like band II from Arabidopsis) or it could represent a modified plastidial isozyme.
Some features of the photometrically assayed PGM activity, as shown in Table I and the zymograms (Fig. 2), should be noted. In the photometric assay, the total PGM activity is monitored under steady-state conditions using a saturating substrate (i.e. Glc-1-P) concentration. All PGM isoforms contribute to the measured total enzyme activity, but it is unknown whether the assay conditions used are optimal for all isoforms. Therefore, it is not known whether the total PGM activity measured equals the sum of the Vmax values of all PGM isoforms. For the zymograms, equal amounts of buffer-soluble proteins were applied to each lane. PGM activity detection was optimized to obtain the most sensitive enzyme activity staining, the highest possible resolution of the PGM isoforms, and a low background staining as well. Based on these criteria, the native PAGE system originally described by Davis (1964) was found to be most suitable. For two reasons, the zymograms obtained do not reflect a truly quantitative measure of the activity of the PGM isoforms resolved. First, the substrate (i.e. Glc-1-P) concentration in the vicinity of the PGM isoforms is determined by both the diffusion into the separation gel and the rate of consumption by the PGM activity; therefore, substrate saturation is difficult to ensure. Second, the activity staining is based on the local formation of insoluble formazan, which may affect the catalytic process of the various PGM isoforms. If so, steady-state conditions are unlikely to be maintained throughout the entire staining period.
We also tested both potato leaf and tuber extracts from the four transgenic lines and from wild-type plants for possible variation in transglucosidase and phosphorylase isozyme patterns; however, neither qualitative nor quantitative differences were observed among all the lines. Therefore, we conclude that the altered expression of the cPGM does not noticeably affect the activities of the two subfraction I-related glucosyl transferases, Pho2 and DPE2 (Supplemental Fig. S1).
Heteroglycans from Leaves of the PGM Transgenic Potato Lines
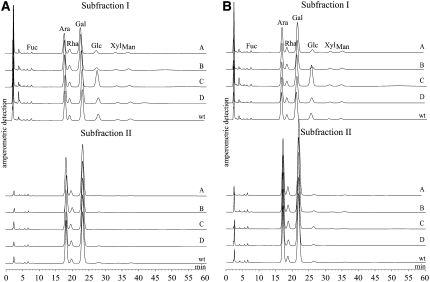
Heteroglycans were isolated from the leaves of two overexpressing lines (lines A and B), the two underexpressing lines (lines C and D), and the wild type. Leaves were harvested both in the middle of the light period (8 h after the onset of the illumination) and in the middle of the dark period (4 h after the beginning of the dark period). Subfractions I and II were separated by FFF and subjected to acid hydrolysis. The monomeric patterns of the hydrolysates were determined by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Fig. 3). In subfraction I, Gal and Ara were the two most prominent compounds, whereas Fuc, Rha, Glc, Xyl, and Man were minor constituents (compare with Fettke et al., 2005b). Transgenic lines overexpressing the cPGM (lines A and B) differed in the monomer patterns of subfraction I from the wild-type control, since they possessed a lower glucosyl content (Fig. 3). By contrast, underexpressing lines had the same (line D) or even an increased (line C) relative Glc content compared with the wild type. Given that in line C the antisense inhibition was more efficient than in line D, as the specific PGM activity is approximately 50% of that of line D (Table I), this effect appears to correlate with the activity of PGM. The effects of both the overexpressing and the underexpressing lines were consistently observed during both the light and dark periods (Fig. 3). By contrast, the monomer patterns of subfraction II did not significantly differ from those of the wild-type control (Fig. 3).
Figure 3.
Monosaccharide patterns from subfractions I and II from leaves of cPGM-overexpressing (lines A and B) or cPGM-underexpressing (lines C and D) potato lines. Leaves were harvested in the middle of the light (A) or the dark (B) period. As a control, wild-type leaves (wt) were harvested at the same time. Following acid hydrolysis, equal amounts of carbohydrates (4 μg of Glc equivalents) were analyzed by HPAEC-PAD. The monosaccharide patterns of subfractions I and II (representing the cytosolic and apoplastic heteroglycans, respectively, whose apparent sizes exceed 10 kD) are shown. For both panels, a typical chromatogram from three independently performed preparations (two independent batches of plants) is presented. All chromatograms were normalized to Ara.
As shown previously, both subfractions I and II possess a relatively wide and overlapping size distribution (Fettke et al., 2005a, 2005b). In wild-type potato leaves, the size distribution of subfraction I increases during the dark period, whereas that of subfraction II remains constant during the light-dark period (Fettke et al., 2005b).
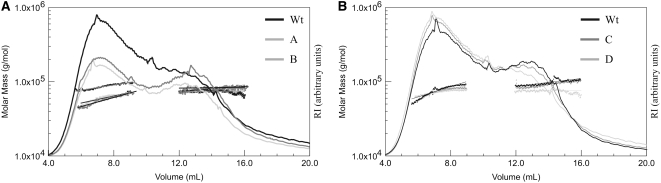
For a more detailed analysis, SHGL was prepared from leaves of the four transgenic lines harvested at the middle of the light period and equal amounts (80 μg of Glc equivalents each) were subjected to FFF, and both the multi-laser light scattering and the delta refractive index (MALLS-DRI) were monitored. As controls, wild-type leaves were harvested and processed under identical conditions (Fig. 4). Two observations were made. First, in SHGL fractions derived from the two overexpressing lines (lines A and B), the size distribution of subfraction I was shifted toward the lower molecular mass region (Fig. 4A). Second, in these lines, the ratio between subfractions I and II was decreased. By contrast, both subfractions I and II from underexpressing lines (lines C and D) were quantitatively and qualitatively essentially undiscernible from those of the wild type. The same differences between SHGL preparations derived from line A or B and from the wild-type control were observed when the glycans were prepared from leaves that had been harvested during darkness. Similarly, the two underexpressing lines (lines C and D) did not differ from the wild type (data not shown).
Figure 4.
Size distribution of subfractions I and II from leaves of wild-type plants and from cPGM-overexpressing (A) and cPGM-underexpressing (B) lines was analyzed by FFF coupled to MALLS-DRI. From each line, three independently prepared SHGL samples were subjected to FFF. A typical elution profile for each line and for the wild type (Wt) is shown. The refractive index (RI) signals (solid lines) and the molar mass distributions obtained by MALLS-DRI (dots) are plotted against the eluate volume. A and B designate the two overexpressing lines, and C and D designate the two underexpressing lines (Table I).
Taken together, the data presented in Figures 3 and 4 are fully consistent with the cyclic path outlined in Figure 1: elevated levels of cPGM activity favor the phosphorylase-mediated conversion of glucosyl residues from subfraction I into the cytosolic hexose-P pools. However, this increased flux from the cytosolic heteroglycans is not restricted to the glucosyl residues; rather, it is accompanied by a more general decrease in the total amount and size of the cytosolic heteroglycans. By contrast, more glucosyl residues are retained in subfraction I when the levels of cPGM activity are decreased. Under these conditions, neither the amount nor the size distribution of subfraction I is altered (Fig. 4B).
The monomer patterns of the SHGs were also analyzed (Supplemental Fig. S2, A and B). In these glycans, Glc is the dominant monomer, but it is more prominent during the light period than during darkness. This effect was also observed in the SHGS preparations from all four transgenic lines. In comparison with the wild type, the relative Glc levels of the mutant-derived SHGs preparations were either unchanged or lower. As SHGS comprises several glycan pools that reside in different compartments, however, these differences are difficult to explain at the molecular level (Fettke et al., 2005a).
Heteroglycans from Tubers of Transgenic Lines
Growing potato tubers are characterized by a massive starch biosynthetic flux and act as major sinks of the entire plant (Geigenberger et al., 2004). Following long-distance transport, reduced carbon compounds are imported into the parenchyma cells of the tubers and are then imported into the amyloplast, most likely in the form of Glc-6-P (Tauberger et al., 2000; Zhang et al., 2008). Thus, in growing tubers, major intracellular carbon fluxes are directed from the cytosol into the plastid and thereby differ considerably from those of leaf mesophyll cells (Geigenberger et al., 2004). Little is currently known about the occurrence, structure, and metabolic functions of cytosolic heteroglycans in heterotrophic organs. Therefore, we performed a detailed study of SHGL that had been isolated from growing potato tubers.
When using essentially the same procedure that has been established for leaves from Arabidopsis or potato, the SHGL preparation obtained still contained minor protein bands. As revealed by tryptic digestion and matrix-assisted laser-desorption ionization mass spectrometry analyses, these proteins were unrelated to any glycoproteins but rather represented major storage proteins of the tuber that had been incompletely removed during deproteinization of the glycans (Supplemental Fig. S3). By contrast, noticeable protein contaminations were never observed in heteroglycans isolated from any leaf material.
As contaminating proteins of the tuber-derived SHGL preparations could interfere both with FFF-MALLS analyses and with the quantification of the total monosaccharide content, several procedures were tested in an attempt to achieve complete removal of contaminating proteins. Treatment of SHGL with proteinase K was found to be most efficient (Supplemental Fig. S3). Presumably, the autodegradation catalyzed by proteinase K is advantageous for the enzymatic deproteinization of the tuber-derived SHGL (Bajorath et al., 1988), as peptides with an apparent size of less than 10 kD are not retained in the heteroglycan preparations.
Following proteinase K treatment, SHGL preparations from growing tubers of the various transgenic lines (and from wild-type tubers as well) were separated into subfractions I and II either by FFF or by reaction with the β-glucosyl Yariv reagent. As revealed by HPAEC-PAD analyses, subfractions I and II isolated from wild-type tubers possess similar monomer patterns as the respective subfractions from wild-type leaves (see below). Likewise, the ratio and size distribution of subfractions I and II, as revealed by FFF-MALLS-DRI, were essentially as described previously for leaves (data not shown; Fettke et al., 2005b). However, unlike in leaves, subfraction I isolated from tubers of the four transgenic lines was undiscernible from that of wild-type tubers. Irrespective of the level of cPGM activity, neither the size distribution nor the monomer patterns of subfractions I and II differed significantly from those of the wild-type tubers (data not shown).
Utilization of External Glc by Tuber Discs
Given that it is likely that only major structural alterations of the heteroglycans are reflected by changes in the size distribution or the monomer pattern, we next attempted a more subtle analysis based on evaluating the metabolic fate of the supplied radiolabel. Labeling experiments were performed with tuber discs from wild-type plants and both a cPGM-overexpressing line (line A) and a cPGM-underexpressing transgenic line (line C). All discs were prepared from growing potato tubers that were removed from the mother plant immediately before the experiment. Discs were incubated with uniformally 14C-labeled Glc in sealed flasks, and the CO2 released was trapped in an alkaline solution. At intervals, the alkaline solution was substituted and discs were removed. Subsequently, they were analyzed to determine the distribution of label. The data obtained are compiled in Table II. Total uptake of 14C-labeled Glc continued over the entire incubation period, the rate of uptake being approximately the same in line A as in the wild type but approximately 20% lower in the antisense line C. Interestingly, in keeping with the changes in steady-state metabolite levels previously recorded in this antisense lines (Fernie et al., 2002b), label incorporation into organic acids, Suc, and proteins and even carbon dioxide evolution were also strongly decreased in this line. By contrast, in keeping with a previous feeding experiment conducted on other overexpression lines than the one used here, the incorporation into organic acids, amino acids, and proteins did not differ significantly between line A and the wild-type control (Lytovchenko et al., 2005a). That said, labeling of Suc was significantly diminished in line A (as recorded previously in a line exhibiting corresponding levels of overexpression); furthermore, the incorporation into both starch and the cell wall fraction was, at least after 4 h, significantly increased in comparison with that observed in the wild type.
Table II.
Uptake and metabolic conversion of exogeneous [U-14C]Glc by potato tuber discs
Discs from wild-type, cPGM-overexpressing (line A), and cPGM-underexpressing (line C) transgenic plants were incubated with [U-14C]Glc. Values are given as Bq g−1 fresh weight. They represent the normalized mean ± se of measurements from two tuber discs and six replicas. Numbers in boldface indicate those values that, according to the t test, are estimated to be significantly different from the respective wild-type value (P < 0.05).
| Variable | Time | Line A | Wild Type | Line C |
|---|---|---|---|---|
| min | ||||
| Total uptake | 30 | 1,980.6 ± 61.2 | 2,175.7 ± 104.7 | 1,793.9 ± 78.8 |
| 120 | 4,613.0 ± 288.8 | 5,085.2 ± 140.4 | 4,191.1 ± 243.9 | |
| 240 | 8,410.4 ± 646.0 | 8,578.0 ± 425.3 | 6,661.9 ± 265.0 | |
| Total metabolized | 30 | 1,170.9 ± 34.4 | 1,279.7 ± 48.2 | 1,088.2 ± 44.3 |
| 120 | 3,609.6 ± 256.1 | 4,002.9 ± 116.2 | 3,041.8 ± 162.4 | |
| 240 | 7,410.7 ± 531.0 | 7,412.1 ± 363.9 | 5,257.2 ± 185.2 | |
| Organic acids | 30 | 164.8 ± 13.8 | 177.1 ± 14.8 | 90.8 ± 8.0 |
| 120 | 683.8 ± 45.1 | 735.0 ± 25.1 | 462.3 ± 60.9 | |
| 240 | 1,321.1 ± 88.5 | 1,208.6 ± 94.8 | 781.1 ± 79.1 | |
| Amino acids | 30 | 166.2 ± 7.5 | 199.3 ± 14.3 | 170.6 ± 8.5 |
| 120 | 332.5 ± 10.3 | 355.6 ± 11.2 | 317.0 ± 18.8 | |
| 240 | 420.6 ± 3.9 | 435.9 ± 26.5 | 337.6 ± 8.6 | |
| Total hexose-P | 30 | 148.8 ± 14.7 | 130.9 ± 12.6 | 111.8 ± 8.1 |
| 120 | 148.8 ± 14.9 | 148.8 ± 16.1 | 138.0 ± 15.9 | |
| 240 | 97.3 ± 31.6 | 31.2 ± 6.9 | 38.8 ± 22.6 | |
| Suc | 30 | 336.0 ± 19.7 | 417.9 ± 30.4 | 291.4 ± 17.4 |
| 120 | 1,202.1 ± 78.8 | 1,450.1 ± 74.0 | 1,220.3 ± 104.2 | |
| 240 | 3,092.9 ± 381.4 | 4,301.9 ± 244.4 | 2,744.9 ± 224.6 | |
| Starch | 30 | 213.8 ± 23.3 | 229.7 ± 33.8 | 222.8 ± 30.2 |
| 120 | 993.9 ± 167.0 | 1,085.5 ± 108.4 | 680.6 ± 67.9 | |
| 240 | 1,887.1 ± 160.2 | 948.6 ± 56.8 | 954.7 ± 106.3 | |
| Cationic (proteins) | 30 | 5.5 ± 1.3 | 7.2 ± 0.8 | 5.2 ± 0.8 |
| 120 | 38.5 ± 5.0 | 45.9 ± 1.7 | 25.8 ± 2.0 | |
| 240 | 142.0 ± 7.0 | 136.7 ± 9.2 | 57.7 ± 6.2 | |
| Anionic (cell wall) | 30 | 20.2 ± 1.4 | 20.6 ± 1.8 | 18.9 ± 2.2 |
| 120 | 94.1 ± 14.4 | 116.2 ± 13.5 | 67.6 ± 6.4 | |
| 240 | 282.9 ± 8.0 | 215.8 ± 23.0 | 148.0 ± 18.4 | |
| CO2 released | 30 | 2.2 ± 0.2 | 3.8 ± 1.0 | 4.1 ± 1.2 |
| 120 | 46.7 ± 4.4 | 52.0 ± 1.5 | 38.1 ± 3.2 | |
| 240 | 165.7 ± 13.7 | 154.4 ± 5.7 | 101.1 ± 3.7 |
Next, we evaluated the 14C incorporation into heteroglycans within the exact same tuber material used for the experiments described above. SHGL was separated into subfractions I and II by treatment with the β-glucosyl Yariv reagent (Yariv et al., 1962). As demonstrated previously, the apoplastic subfraction II strongly interacts with this reagent, whereas the cytosolic subfraction I is nonreactive (Fettke et al., 2004). As incorporation of radiolabel was low, analyses were restricted to the longer incubation periods of the experiment described above. Incorporation into subfraction I was greater than that into subfraction II. However, no significant differences were observed between wild-type controls and the two transgenic lines. The antisense line (line C) exhibited a slightly decreased labeling of subfraction I in comparison with the wild-type control (Fig. 5A).
Figure 5.
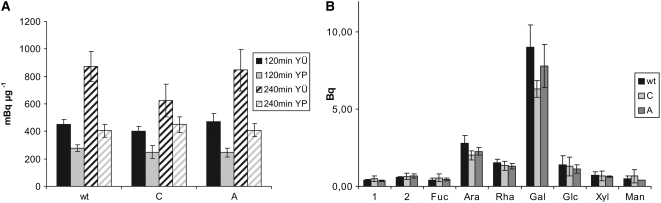
[U-14C]Glc-dependent labeling of heteroglycans in potato tuber discs. A, Following 2 or 4 h of incubation with uniformally labeled [U-14C]Glc (Table II), SHGL was isolated and separated into subfraction I (which is Yariv nonreactive and, therefore, remains in the supernatant; YÜ) and subfraction II (which is pelleted by the Yariv reagent; YP). For each subfraction, the total carbohydrate content was determined (as Glc equivalents) and the radioactivity was quantified. For each line, the average of three experiments and sd are given. B, An aliquot of SHGL that had been isolated after 4 h of incubation was subjected to acid hydrolysis, and the monosaccharides released were resolved by HPAEC-PAD. In the eluate, each monosaccharide was collected separately, and the total content of radioactivity of each eluate fraction was determined. For each line, the average of three independently performed experiments and the sd are given. Abbreviations are as in Figure 3.
We then determined the labeling pattern of the individual constituents of SHGL (Fig. 5B). Given the low rate of 14C incorporation into the heteroglycan, analyses were restricted to the 240-min incubation period.
For this purpose, we performed an acid hydrolysis, and equal amounts of the monosaccharides released were applied to an HPAEC column. Eluate fractions were collected that each represented a distinct monosaccharide, and the radiolabel content of each fraction was monitored. Galactose, which is the most prominent constituent of SHGL, contained the majority of the 14C label, whereas incorporation into the other monosaccharides was minor. In the antisense line (line C) and in the overexpressing line (line A), labeling of the various monosaccharides did not differ noticeably from that of the wild type. In summary, the 14C incorporation into the various monosaccharides roughly reflects their relative abundance within the heteroglycans. This strongly suggests that even during the prolonged incubation, only a small proportion of the labeled Glc is partitioned to the various constituents of the glycans, without any clear preference being noticeable.
When comparing the labeling of the heteroglycans with the 14C incorporation into major cellular constituents of the tuber, it becomes apparent that, at least under the conditions used here, the Glc-driven heteroglycan biosynthesis represents only a very minor intracellular flux within the potato tuber (their total 14C content per gram fresh weight is less than 1% of that found in starch). This low labeling of the heteroglycans also has an important methodological implication: it clearly indicates that the heteroglycans isolated from potato tubers lack, to any noticeable extent, starch-derived contaminations; otherwise, a much higher labeling, especially of the glucosyl residues within the heteroglycans, would be expected.
Utilization of External Glc-1-P by Tuber Discs
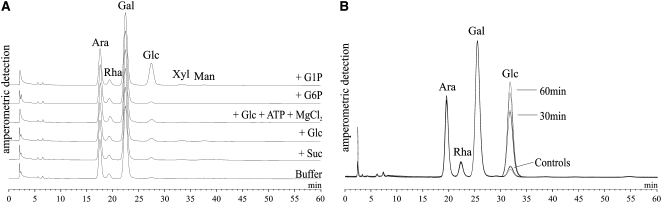
Potato tuber discs were incubated with Glc-1-P (or, alternatively, with equimolar concentrations of other sugars) and, subsequently, the monomer pattern of SHGL was analyzed by HPAEC-PAD (Fig. 6A). Following a 60-min incubation with Glc-1-P, the glucosyl content of SHGL was increased significantly. This effect was not detectable when Glc-1-P was replaced by Glc-6-P, Glc, or Suc. In a further control experiment, discs were incubated with a mixture containing Glc, ATP, and MgCl2 in order to facilitate the in situ formation of Glc-6-P by any endogenous extracellular hexokinase. However, the effect of external Glc-1-P could not be mimicked (Fig. 6A). In summary, the data shown in Figure 6A suggest that external Glc-1-P and free Glc exert different effects on the cytosolic carbon fluxes.
Figure 6.
Monosaccharide patterns of SHGL in potato tuber discs as affected by various sugars. A, Discs from potato tubers were incubated as indicated for 60 min. Subsequently, SHGL was isolated and hydrolyzed and the resulting hydrolysates were analyzed by HPAEC-PAD. A typical chromatogram from six independently performed experiments (three independent batches of plants) is shown. B, Monosaccharide pattern of SHGL following 0, 30, and 60 min of incubation of tuber discs with Glc-1-P. As a control, Glc-1-P was omitted and SHGL was prepared after 0, 30, and 60 min of incubation. Typical chromatograms from six independently performed experiments (three independent batches of plants) are shown.
The above conclusion was supported by further experiments. Discs from wild-type tubers were incubated for varying periods of time with unlabeled Glc-1-P. As a control, Glc-1-P was omitted. For each incubation time, SHGL preparations were isolated and their monosaccharide patterns were analyzed by HPAEC-PAD. The Glc-1-P-dependent increase in the glucosyl content of SHGL is remarkably fast, as most of the change in the monosaccharide pattern occurs during 30 min following the onset of the incubation (Fig. 6B). Subsequently, the increase in the glucosyl content slows, and following prolonged incubation with Glc-1-P (for 2 or 3 h) the glucosyl content even decreases (data not shown). As revealed by native PAGE, the phosphorylase and the DPE2 patterns were not significantly altered during the incubation with any of the compounds supplied (Supplemental Fig. S4).
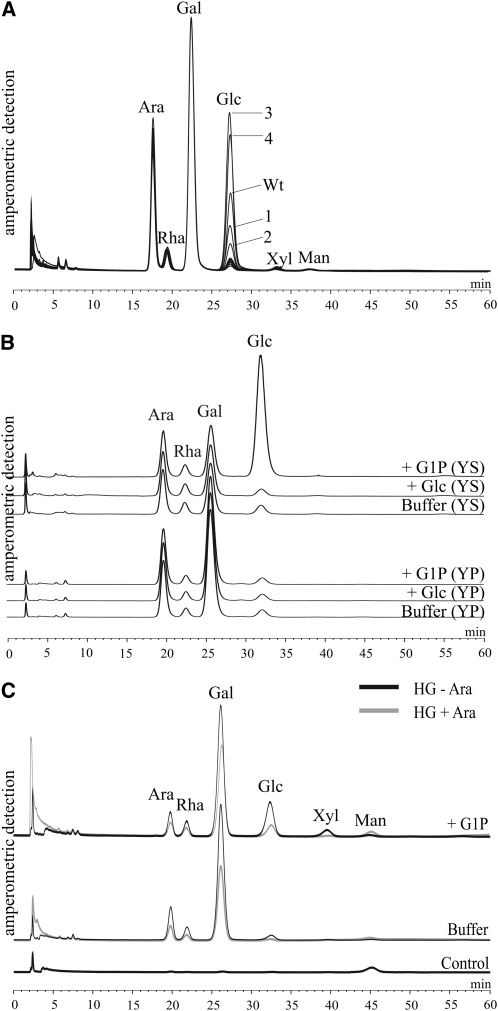
According to the path proposed for mesophyll cells (Fig. 1), the cytosolic phosphorylase isozyme (Pho2) catalyzes the reversible glucosyl transfer from Glc-1-P to the heteroglycans. If this path is also functional in tuber cells, the Glc-1-P-dependent increase in the glucosyl content of SHGL should be correlated with the level of Pho2 activity. In order to test this assumption, tuber discs from four transgenic potato lines that possess elevated (lines 3 and 4) or reduced (lines 1 and 2) levels of the cytosolic phosphorylase were incubated with Glc-1-P. In a previous publication, these lines were characterized in detail (Fettke et al., 2005b). In discs derived from the overexpressing lines, the glucosyl content of the SHGL preparation was higher compared with that of the wild-type control. By contrast, in discs from the underexpressing lines, the increase in the glucosyl content was less than in the wild type (Fig. 7A). When the tuber discs were incubated in a medium lacking Glc-1-P, none of the transgenic lines deviated significantly from the wild-type controls (Fig. 7A). That this increase reflects the in situ activity of Pho2 is considered to be a strong argument for a direct interaction between Pho2 and the cytosolic heteroglycans occurring in the intact cells.
Figure 7.
Glc-1-P-related changes in SHGL. A, Discs were prepared from tubers of transgenic potato plants that possess elevated (lines 3 and 4) or decreased (lines 1 and 2) levels of the cytosolic phosphorylase isozyme. As a control, tubers from wild-type plants (Wt) were used. Following the incubation with Glc-1-P for 30 min, SHGL was isolated and hydrolyzed and the resulting monosaccharide pattern was analyzed by HPAEC-PAD. From each preparation, equal amounts of monosaccharides (4 μg of Glc equivalents each) were applied to the column. For each line, a typical chromatogram from three independently performed experiments is shown. B, Monosaccharide patterns of the Yariv-reactive (subfraction II; YP) or the Yariv-nonreactive (subfraction I; YS) subfractions of SHGL that had been isolated from tuber discs (wild type) following 60 min of incubation. As controls, tubers were incubated in a medium in which Glc-1-P was either omitted (buffer) or replaced by equimolar Glc. Equal amounts of carbohydrates (4 μg of Glc equivalents each) were applied to the HPAEC column. A typical chromatogram from two independently performed experiments is shown. C, SHGL (20 μg) prepared from tuber discs (wild type) following 20 min of incubation with Glc-1-P was treated for 3 h with endo-α-1,5-arabinanase from A. niger. Subsequently, compounds having an apparent size of less than 10 kD were removed by membrane filtration and the residual >10-kD material was subjected to acid hydrolysis and applied to the column. As a control, arabinanase was incubated in the absence of the SHGL preparation. A typical chromatogram from three independently performed experiments is shown.
In order to further substantiate this conclusion, two additional experiments were performed. In the first experiment, wild-type tuber discs were incubated in three mixtures containing equimolar concentrations of Glc-1-P or Glc or lacking any carbohydrate. SHGL was isolated from each set of discs and was then resolved into subfractions I and II by treatment with β-glucosyl Yariv reagent. The increase in the glucosyl content was restricted to the Yariv nonreactive subfraction (i.e. the cytosolic subfraction I), and this increase was only observed following incubation with Glc-1-P (Fig. 7B). In the second experiment, SHGL was treated with an endo-α-1,5-arabinanase from Aspergillus niger and, subsequently, the residual (>10 kD) heteroglycan was freed of low molecular mass glycans (<10 kD) and was then subjected to acid hydrolysis. The treatment with endo-arabinanase that selectively cleaves inter-Ara linkages has previously been applied successfully to characterize cytosolic heteroglycans from leaves of both Arabidopsis and potato (Fettke et al., 2005b, 2006). The monosaccharide pattern of the residual SHGL is shown in Figure 7C. Two control experiments were performed. In the first control, SHGL was treated in exactly the same way except that the endo-arabinanase was omitted (Fig. 7C). In the second control, SHGL was replaced by an equal amount of glycogen. The incubation with the arabinanase did not result in any noticeable hydrolytic activity (data not shown). Due to the action of the arabinanase, the >10-kD heteroglycan contains less Ara, Rha, Gal, and Glc. However, the loss of Glc content exceeds that of the other monosaccharides. Similar results have been obtained for SHGL that had been isolated from freshly harvested potato leaves (Fettke et al., 2005b). Since phosphorylases catalyze the reversible transfer of glucosyl residues from Glc-1-P to the nonreducing ends of acceptor molecules, it is reasonable to assume that most of the glucosyl residues observed in subfraction I are placed at the periphery of the polyglycan molecules. The susceptibility of these glucosyl residues (and of a few other monosaccharide residues as well) to arabinanase treatment clearly indicates that they have been transferred to a heteroglycan rather than to an as yet unidentified homoglucan.
Selective Uptake of Glc-1-P by Tuber Discs
The data shown in Figures 6 and 7 clearly demonstrate that external Glc and Glc-1-P differently affect the monomer pattern of cytosolic heteroglycans. The increase in the glucosyl content of subfraction I is strictly dependent on Glc-1-P and is mediated by the cytosolic Pho2 (Fig. 7). Therefore, it is likely that following uptake, the external Glc-1-P directly enters the cytosolic pool of this sugar phosphate.
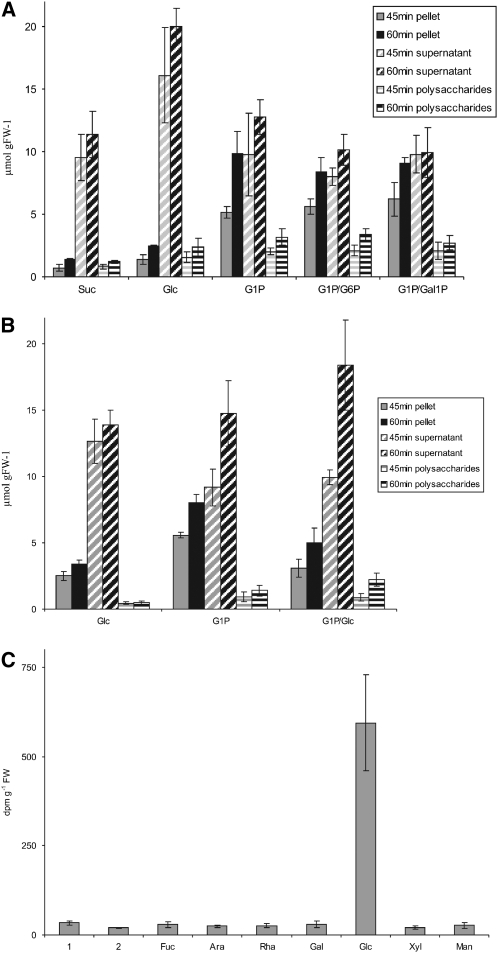
The import of Glc-1-P into intact amyloplasts isolated from tubers or suspension-cultured cells from potato has been reported (Kosegarten and Mengel, 1994; Naeem et al., 1997), but the uptake of this sugar phosphate by intact cells has not been demonstrated so far. To gain further insight into this uptake process, 14C-labeling experiments were performed with tuber discs that were incubated with several 14C-labeled sugars or sugar derivatives, such as Suc, Glc, or Glc-1-P, both in the presence and absence of unlabeled sugar compounds (Fig. 8, A and B). Using this approach, we intended to determine whether the uptake processes and intracellular carbon fluxes are separated from each other. Following an incubation for 45 or 60 min, discs were withdrawn, homogenized, and processed to yield three fractions: first, insoluble material that essentially consists of starch particles; second, heat-stable polysaccharides that are water soluble but insoluble in 70% (v/v) ethanol (this fraction essentially represents SHGL); and, finally, the heat-stable supernatant fraction that is soluble in 70% (v/v) ethanol and contains low molecular mass compounds, including water-soluble glycans with an apparent size of less than 10 kD (SHGS; Fettke et al., 2005a). Labeling of these three fractions was different when 14C-labeled Suc, Glc, or Glc-1-P was supplied in the absence of any other unlabeled compound. The supply of [U-14C]Glc-1-P resulted in an enhanced 14C incorporation into starch (i.e. pellet) and the water-soluble polysaccharides (i.e. SHGL). Interestingly, the simultaneous supply of unlabeled Glc-6-P, Gal-1-P, or Glc did not significantly affect the Glc-1-P-dependent 14C incorporation into SHGL (Fig. 8, A and B). However, the presence of unlabeled Glc did, to some extent, decrease the flux from 14C-labeled Glc-1-P to the starch fraction (Fig. 8B). This indicates that the entire paths of the Glc-starch conversion and the Glc-1-P-starch conversion are not completely separated from each other. By contrast, the incorporation into the heteroglycan fraction, as initiated by external Glc-1-P, is almost unaffected by the presence of Glc. This conclusion is confirmed by the analysis of the monosaccharide residues within the heteroglycans (i.e. SHGL). When tuber discs were incubated for 30 min with [U-14C]Glc-1-P, almost all label was recovered in the glucosyl residues of the heteroglycans (Fig. 8C). Given that the incubation was restricted to 30 min, this incorporation must be rapid. By contrast, incubation with [U-14C]Glc results in a much slower incorporation into the constituents of SHGL, and under these conditions, most of the label is retained in the most prominent constituent, the galactosyl residue (Fig. 5B).
Figure 8.
Incorporation of 14C-labeled carbohydrates into constituents of potato tuber discs from wild-type plants. A, Potato tuber discs were incubated with [U-14C]Suc (Suc), [U-14C]Glc (Glc), [U-14C]Glc-1-P (G1P), [U-14C]Glc plus unlabeled Glc-6-P (G1P/G6P), or [U-14C]Glc-1-P plus unlabeled Gal-1-P (G1P/Gal1P). After 45 or 60 min of incubation (see inset), discs were removed and processed. Subsequently, the 14C contents of the pellet, the supernatant, and the polysaccharides were determined. B, In a similar experiment, tuber discs were incubated with [U-14C]Glc (Glc), [U-14C]Glc-1-P (G1P), or [U-14C]Glc-1-P plus unlabeled Glc (G1P/Glc). At intervals (see inset), discs were removed and processed (see A). In A and B, the vast majority of the pellet consists of starch, and minor constituents are compounds derived from the cell wall. The polysaccharides are essentially SHGL. The supernatant contains low molecular mass compounds, such as SHGS monosaccharides and disaccharides. C, Tuber discs were incubated for 30 min in [U-14C]Glc-1-P. Subsequently, SHGL was isolated and subjected to acid hydrolysis. The monosaccharides released were separated by HPAEC-PAD. In the eluate, each monosaccharide was collected separately and monitored for 14C content. Bars 1 and 2 represent unidentified compounds that elute early during HPAEC. All data shown are averages of three independently performed experiments and sd.
DISCUSSION
In this article, we have analyzed cytosolic heteroglycan-related processes in two distinct systems of potato. One system is the fully developed leaf, which acts as the major source organ of the entire plant. Within this system, carbon fixed during photosynthesis is exported from the chloroplast to the cytosol utilizing either intermediates of the Calvin cycle (during the light period) or products formed by the degradation of transitory starch (during the dark period). Presumably, at least two paths exist by which plastidial metabolites are linked to the cytosolic heteroglycans: one predominantly acts during net starch degradation and includes both maltose and Glc transporters, and the other one is mediated by the triose-P transporter and links Calvin cycle intermediates with the cytosolic heteroglycans. The latter process is consistent with the fact that cytosolic heteroglycans have been isolated from starch-deficient Arabidopsis mutants (Fettke et al., 2005a) and, therefore, that the in vivo biosynthesis of the glycans proceeds even if starch-derived monosaccharides or disaccharides are not available. Furthermore, in vivo labeling of Arabidopsis wild-type plants using 14CO2 clearly indicated that the labeled carbon is incorporated into the glycans during the light period (Fettke et al., 2005a). The evidence for the DPE2-mediated involvement of the heteroglycans in the cytosolic maltose metabolism is based on both in vitro and in vivo studies and includes the massive structural alterations observed in heteroglycans from DPE2-deficient Arabidopsis mutants (Fettke et al., 2006).
In the leaf system, heteroglycans were isolated from transgenic plants that possess altered levels of the cPGM activity. The total extractable PGM activity was resolved into three isoforms, and the same pattern was obtained for tubers (Fig. 2, A and B). The fastest moving isoform appears to be plastidial, whereas the slowest moving form is attributed to the cytosol. The subcellular location of the third band (the one having an intermediate mobility) remains uncertain. A similar PGM pattern was obtained for Arabidopsis wild-type leaves. In the mutant lacking the plastidial PGM isozyme, the fastest moving form was undetectable (Fig. 2C). For Arabidopsis, two genes encoding cPGM isozymes (At1g23190 and At1g70730) have been identified. Single knockout mutants in which either At1g23190 or At1g70730 is nonfunctional possess an approximately 50% effect on starch and Suc content (J. Fettke, unpublished data). Therefore, the two gene products appear to exert redundant rather than selective metabolic functions. In transgenic potato lines that express the E. coli-derived PGM in the cytosol (overexpressing lines A and B), the transgenic product comigrates with the slowest moving endogenous PGM isoform (Fig. 2, A and B). In the two antisense lines, the slowest moving cPGM isoform is most efficiently repressed (Fig. 2). It is important to note that, in contrast to Arabidopsis, the potato genome appears to encode only two PGMs. In keeping with this partial purification, studies revealed two major distinct peaks (Takamiya and Fukui, 1978; Tauberger et al., 2000) and combined antisense inhibition resulted in some lines that were essentially devoid of PGM activity (Lytovchenko et al., 2002a). Therefore, two of the PGM bands resolved by native PAGE most likely represent modified forms of a single gene product.
Both elevated and lowered levels of cPGM activity affect the cytosolic heteroglycans (i.e. subfraction I), whereas the apoplastic water-soluble glycans (subfraction II) remain unchanged (Figs. 3 and 4). This result is consistent with the scheme presented in Figure 1, as subfraction I is linked, via a series of reversible reactions, with the cytosolic pools of Glc-6-P and Glc-1-P. Elevated levels of PGM activity (lines A and B) favor the interconversion of the two Glc monophosphates and result in an increased flux into Suc (Lytovchenko et al., 2005a). It should be noted that the biosynthesis of Suc utilizes both Glc-1-P (to form UDP-Glc) and Glc-6-P (to synthesize Fru-6-P); therefore, the cytosolic phosphorylase is likely to act mainly as a Glc-1-P-forming activity. Consequently, the glucosyl content of the cytosolic heteroglycans is diminished (Fig. 3). As in the overexpressing lines, the cPGM activity is permanently elevated, and this effect is observed in both illuminated and darkened leaves (Fig. 3). To some extent, this effect resembles the decreased content of glucosyl residues in subfraction I that is observed when the cytosolic phosphorylase activity is increased (Fettke et al., 2005b). Under in vivo conditions, Pho2 acts primarily as a heteroglycan-depolymerizing activity, utilizing the heteroglycans as donor and orthophosphate as acceptor of glucosyl residues (Fettke et al., 2005b). Furthermore, the increased flux toward Suc results in a more general decrease in the size distribution of subfraction I (Fig. 4). This effect concurs with the diminished pool size of various monosaccharides and disaccharides, such as maltose, isomaltose, Gal, and Ara, which has been observed previously in these lines (Lytovchenko et al., 2005a). By contrast, in the cPGM-underexpressing lines, the size distribution of subfraction I does not significantly differ from that of wild-type plants (Fig. 4). Similarly, the glucosyl content of the cytosolic heteroglycans is unchanged or even significantly increased if the cPGM activity is lowered (lines C and D; Fig. 3). In leaves of these transgenic lines, the photosynthetic Suc synthetic capacity is largely unaltered but a wide range of other metabolic pathways are changed (Lytovchenko et al., 2002b).
The other system used in this study is growing tubers, which constitute the most active sinks of potato plants. In tubers, parenchyma cells import source-derived sugars and direct most of the reduced carbon toward the amyloplasts to support a massive starch biosynthetic flux (Geigenberger et al., 2004). However, for the heterotrophic cells, very little is known about the occurrence, structure, and function of cytosolic heteroglycans. In studying the second system, growing tubers, evidence is presented that both subfractions I and II exist in heterotrophic tissues, and the principal features of both glycans (such as monomer pattern, size distribution, and the selective interaction with the β-glucosyl Yariv reagent) do not differ significantly from those of leaves. The labeling experiments performed with tuber discs that are incubated with 14C-labeled Glc indicate that the incorporation of the 14C label into the plastidial starch differs by more than 2 orders of magnitude from that into glucosyl residues from subfraction I or II (Table II). Recently, tubers from transgenic potato lines that overexpress or underexpress the cPGM activity were comprehensively analyzed. While antisense repression of this isozyme resulted in restriction of tuber growth and reserve starch biosynthesis (Fernie et al., 2002b), overexpression of cPGM led to a complex phenotype including a significant delay of tuber sprouting and changes in tuber morphology (Lytovchenko et al., 2005a). In the transgenic lines, the average tuber weight is increased by more than 50% but the number of tubers per plant is strongly diminished (Lytovchenko et al., 2005b). However, these changes are the result of long-term processes in the entire plant and, therefore, are difficult to explain at either the biochemical or the molecular level. Tubers from the transgenic lines contained lower levels of a wide range of amino acids and a significantly decreased ratio of Glc-6-P to Glc-1-P. Furthermore, short-term feeding experiments performed with 14C-labeled Glc and tuber discs clearly indicate that the overexpression of cPGM leads to an alteration of intracellular carbon metabolism, including an enhanced incorporation into starch (Lytovchenko et al., 2005b). As an extension of these studies, we incubated tuber discs from strong overexpressing and underexpressing lines in 14C-labeled Glc and monitored the distribution of label over a period of 4 h (Table II). Incorporation into starch and cell wall material was increased in the overexpressing line A. By contrast, the underexpressing line C strongly deviated from the wild-type control, with the total uptake of the labeled Glc and the labeling of organic acids, of proteins, and of cell wall material being diminished (Table II). However, in spite of these complex alterations and unlike the situation observed in potato leaves, we detected no significant changes in the amount, size, or monomer patterns of the heteroglycans from the tubers. Similarly, incorporation into both subfractions I and II and also into the various constituents of the two glycan fractions was low and did not differ significantly from that of the wild type (Fig. 5). In growing tubers, source-derived Suc is imported into the parenchyma cells and subsequently cleaved, by the action of the cytosolic Suc synthase, into Fru and UDP-Glc (Appeldoorn et al., 1997; Viola et al., 2001). The latter is converted to Glc-1-P (plus UDP), while the former enters the Glc-P metabolism via a reaction sequence catalyzed by fructokinase and phosphoglucoisomerase. The Glc-6-P resulting from these conversions is imported into the amyloplast in order to support plastidial biosynthesis. Following conversion to Glc-1-P, it forms the glucosyl donor of starch biosynthesis, ADP-Glc. Consequently, loss of the plastidial PGM activity strongly inhibits the formation of reserve starch within tuber amyloplasts (Tauberger et al., 2000). As revealed by the 14C-labeling experiments performed in this study, the Glc-metabolizing path is largely separated from the cytosolic heteroglycans. Consequently, no preferential labeling of the heteroglycan-related glucosyl residues occurs and any significant incorporation of 14C into any constituent of the glycans is detectable only after a prolonged incubation time (Fig. 5). Presumably, the labeling of Fuc, Ara, Gal, Glc, Xyl, and Man requires complex metabolic paths that are not directly affected by the cPGM activity.
The supply of Glc-1-P results in totally different carbon fluxes from those following the supply of Glc. Glc-1-P seems to be selectively taken up (Fig. 8) and then immediately enriches the cytosolic Glc-1-P pool, providing the cytosolic phosphorylase (Pho2) isozyme with an increased substrate supply. Given the reversibility of the Pho2-catalyzed glucosyl transfer, the label in Glc residues of subfraction I increases rapidly and massively (Fig. 6). This increase correlates, quantitatively, with the level of the cytosolic phosphorylase activity, as indicated by the data obtained with Pho2-overexpressing or -underexpressing lines (Fig. 7A). As would be anticipated from Figure 1, these effects are restricted to subfraction I, as the cytosolic phosphorylase does not possess immediate access to the apoplastic glycans. The conclusions drawn from the data shown in Figures 6 to 8 are summarized in Figure 9. By the action of Pho2, subfraction I is immediately linked to the cytosolic Glc-1-P pool. In the heterotrophic system used here, this reaction appears to be rapid and dominant. This is somewhat surprising, as, by the action of the UDP-Glc pyrophosphorylase, Glc-1-P can be converted into UDP-Glc, which is a widely used glucosyl donor for several pathways leading to the biosynthesis of Suc and most of the cell wall-related polysaccharides (Reiter, 2008). The data presented here clearly indicate that, at least within this heterotrophic system, the cytosolic pool of Glc is spatially distinct from that of supplied Glc-1-P, as reflected by the entirely different labeling patterns of the heteroglycans. Although the exact reason for this remains unclear in this study, this observation is not without precedent in plant primary metabolism. The commonly ascribed view that cytosolic Glc levels are vanishingly small was recently augmented by both nonaqueous fractionation studies within the potato tuber (Farre et al., 2001) and the use of metabolite-sensing technology in Arabidopsis roots (Deuschle et al., 2006). Moreover, increasing evidence is building, suggesting an important role for metabolite channeling within primary metabolism, including the pathway of glycolysis (Giege et al., 2003; Holtgrawe et al., 2005; Graham et al., 2007). At present, the transporter that actually imports external Glc-1-P into the cytosol has not been identified. Similarly, it is unknown whether or not the import of Glc-1-P is relevant for the carbon fluxes in the intact plants. Nevertheless, the data presented here clearly indicate that the rapid and massive uptake of Glc-1-P, as observed in tuber discs, is a valuable tool to study the intracellular carbon fluxes with a high time resolution.
Figure 9.
Proposed conversion of exogeneous Glc or Glc-1-P into constituents of the cytosolic heteroglycans. Glc-1-P taken up by the potato tuber cells acts as a glucosyl donor for the transfer to the cytosolic heteroglycans that is mediated by Pho2. This reaction results in a fast increase in the glucosyl content of the cytosolic heteroglycans (subfraction I), which quantitatively reflects the level of the cytosolic phosphorylase activity. Incorporation into the nonglucosyl constituents of subfraction I is much lower. By contrast, the metabolism of Glc results in a minor incorporation into the heteroglycan. Following an extended incubation period, the [U-14C]Glc-dependent incorporation of the constituents of the heteroglycans essentially reflects their relative abundance.
Our results indicate that Glc-1-P is taken up by an alternative route to Glc and that the differences observed in our experiments reflect the differential availability of these compounds within the cytosol. Intriguingly, however, despite this apparent separation, the Glc-1-P-dependent pathway remains capable of supporting a massive carbon flux into starch (Fig. 8). Interestingly, transgenic potato plants that contain low activities of both the cPGM and plastidial PGM unexpectedly exhibit a phenotype that is similar to that of wild-type plants (Fernie et al., 2002a). It is not inconceivable that under these conditions an intracellular carbon flux is dominant that resembles the Glc-1-P-dependent flux, as observed in this study. At present, experiments are being performed in our laboratories that should allow this hypothesis to be tested.
MATERIALS AND METHODS
Plant Materials
Potato plants (Solanum tuberosum ‘Desiree’) and Pho2 transgenic lines were grown under controlled conditions as described (Fettke et al., 2005b). Two transgenic lines that possess a decreased expression of the cPGM are described elsewhere (Lytovchenko et al., 2002a; line C, C-66; line D, C-44). Lines expressing the PGM from Escherichia coli in the cytosol (overexpressing plants) have been described (Lytovchenko et al., 2005a; line A, Ec-82; line B, Ec-75). However, for the [U-14C]Glc-labeling experiments, plants were grown in a greenhouse (Lytovchenko et al., 2005a). Potato leaves were harvested from nonflowering plants. Potato tubers were removed from the plant and were used immediately for the incubation experiments performed with tubers discs.
Extraction of Buffer-Soluble Proteins
Leaf material was frozen in liquid nitrogen and broken using a mortar. All subsequent steps were performed at 4°C. Per 1 g fresh weight, 1 mL of extraction buffer (100 mm HEPES-NaOH, pH 7.5, 1 mm EDTA, 2 mm dithioerythritol, 10% [v/v] glycerol, and 0.5 mm phenylmethylsulfonyl fluoride) was added to the powdered leaf material, and the resulting homogenate was centrifuged for 12 min at 20,000g. Tuber material (1–3 g fresh weight each) was homogenized in 5 mL of extraction buffer using an Ultra Turrax homogenizer. Following centrifugation (as above), the supernatant was passed through a nylon net (100-μm mesh) and the filtrate (designated the crude extract) was used for enzyme activity assays, protein quantification, and native PAGE. Protein was quantified using the microassay of Bradford (1976) with bovine serum albumin serving as a standard.
Native PAGE, Activity Staining, and SDS-PAGE
For native PAGE (7.5% [w/v] total monomer concentration), the Mini-Protean II apparatus (Bio-Rad) was used. Electrophoresis was performed at 4°C using a precooled electrode buffer that was continuously stirred during electrophoresis. PGM activity was resolved using the native discontinuous PAGE system originally described by Davis (1964). Following electrophoresis (3 h at 250 V), the slab gel was incubated for 10 min at room temperature in PGM staining buffer (50 mm HEPES-NaOH, pH 7.5, 3.3 mm MgCl2, and 0.9 mm EDTA) and was then transferred into the PGM staining solution (0.85 mm NADP+, 8.2 μm Glc-1,6-bisP, 33 μm PMS, 250 μm nitroblue tetrazolium, 3 mm Glc-1-P, and 1 nkat mL−1 Glc-6-P dehydrogenase, dissolved in PGM staining buffer; Kofler et al., 2000). To ensure the specificity of the staining procedure, a control mixture was included that lacks Glc-1-P. Following incubation at room temperature in darkness (30 min at most), PGM activity staining was terminated by washing with 10% (v/v) acetic acid. Both phosphorylase and DPE2 were separated using a modified native PAGE system that separates at a less alkaline pH (Steup, 1990). Staining of the two enzyme activities was performed as described elsewhere (Fettke et al., 2006). SDS-PAGE and mass spectrometry analysis were performed as described previously (Eckermann et al., 2002).
Isolation and Characterization of SHG
Leaves were quickly frozen in liquid nitrogen. Aliquots of the frozen leaf material (2 g each) were broken in a mortar, and the SHG was extracted in 10 mL of 20% (v/v) ethanol. The entire isolation and fractionation procedure is described elsewhere (Fettke et al., 2005a). As revealed by SDS-PAGE, both SHGS and SHGL did not contain any detectable protein contamination when up to 500 μg of Glc equivalents per lane was applied. For SHG isolation from tuber material, growing tubers were freshly harvested from potato plants. Tuber material (approximately 1–2 g fresh weight) was homogenized in 10 mL of precooled 20% (v/v) ethanol that contained 1 mm dithioerythritol using an Ultra Turrax homogenizer. Samples were processed as described for leaf material. SHGS from potato tubers did not contain any noticeable protein contamination. However, in the SHGL preparations, some contaminating proteins were generally observed, and these were removed by treatment with proteinase K (see “Results”; Supplemental Fig. S3). 14C-labeled SHGL was isolated using essentially the same procedure. However, subfractions I and II were separated by treatment with the β-glucosyl Yariv reagent (for details, see Fettke et al., 2004). Radioactivity was monitored using a liquid scintillation counter. Enzymatic glycan degradation with endo-α-1,5-arabinanase, acid hydrolysis of the glycans, HPAEC, and FFF were performed as described elsewhere (Fettke et al., 2005b).
Assay of the PGM Activity
For photometric determination of the PGM, an assay similar to that of the phosphorylase (Steup and Latzko, 1979) was used. However, the reaction mixture contained soluble starch (1 mg mL−1) and rabbit muscle phosphorylase (Sigma-Aldrich) but lacked any purified PGM. Using this assay, the substrate of PGM (i.e. Glc-1-P) is continuously formed by the phosphorolytic starch degradation. The rate of the Glc-6-P formation increased linearly with time and the volume of the PGM-containing extract.
Incubation of Potato Tuber Discs with [U-14C]Glc to Assess the Dominant Fluxes of Carbohydrate Metabolism in the Transgenic Potato Lines
Developing tubers were removed from 10-week-old plants. A longitudinal core (10 mm diameter) was taken, and the core was sliced into discs (1 mm thickness) that were washed three times in precooled buffer (10 mm MES-KOH, pH 6.5). Subsequently, the tuber discs (20 each) were placed into a 100-mL Erlenmeyer flask containing 10 mL of incubation medium consisting of 50 mm unlabeled Glc and 10 μCi [U-14C]Glc (specific radioactivity, 0.74 MBq mmol−1) in 10 mm MES-KOH, pH 6.5. The flasks were sealed with Parafilm, and the CO2 released was captured in a KOH trap for quantification by liquid scintillation counting. Incubation of the tuber discs was terminated by washing with cold water. Subsequently, discs were frozen in liquid nitrogen and were stored at −80°C until sample processing. Metabolites were extracted by a series of incubation steps (10 min each). The discs were extracted using a series of boiling solvents (2 mL each) consisting of 80% (v/v) ethanol, 50% (v/v) ethanol, 20% (v/v) ethanol, water, and, finally, 80% (v/v) ethanol. The supernatants were combined and dried under vacuum. Subsequently, the pellet was rapidly resuspended in water (2 mL) and an aliquot was used to monitor radioactivity by liquid scintillation counting. Hexoses and Suc from the ethanol-soluble fraction and starch, protein, and cell wall matter were separated by enzymatic degradation as described by Carrari et al. (2006) and by subsequent anion-exchange chromatography as detailed by Fernie et al. (2001).
Incubation of Potato Tuber Discs with Nonlabeled Sugars or Sugar Derivatives
Discs (8 mm diameter, approximately 2 mm thickness) were prepared from freshly harvested tubers and were incubated at room temperature in one of the following mixtures (10 discs each in 10 mL), all containing 10 mm citrate-NaOH, pH 6.5, as buffer: (1) buffer; (2) 25 mm Glc; (3) 25 mm Glc-1-P; (4) 25 mm Glc-6-P; (5) 25 mm Glc, 25 mm ATP, and 2.5 mm MgCl2; (6) 25 mm Suc. At intervals (0, 30, and 60 min), five discs each were withdrawn and frozen in liquid nitrogen. SHG were isolated as described above. Buffer-soluble proteins were extracted from five discs each and were subjected to native PAGE. Gels were stained for phosphorylase or DPE2 activity as described above.
Incubation of Potato Tuber Discs with [U-14C]Glc, [U-14C]Suc, or [U-14C]Glc-1-P
Using exactly the same experimental conditions as described above, tuber discs were incubated with 50 mm [U-14C]Glc, [U-14C]Suc, or 10 mm [U-14C]Glc-1-P in 50 mm citrate-NaOH, pH 6.5. In addition, 10 mm [U-14C]Glc-1-P and unlabeled 5 mm Glc-6-P and Gal-1-P, respectively were mixed. To each mixture, 74 kBq of radioactivity was included. After incubation at room temperature for 45 and 60 min, four discs each were withdrawn, washed with water, and frozen in liquid nitrogen. For sample processing, four discs were homogenized in 5 mL of precooled 20% (v/v) ethanol using an Ultra Turrax homogenizer. Subsequently, the samples were centrifuged (10 min at 10,000g, 4°C) and the supernatant was collected separately. The resulting pellets were washed with 1 mL of water and centrifuged as above. The 14C label in the pellet, representing predominantly starch, was monitored using a liquid scintillation counter. The supernatants were combined and 100% (v/v) ethanol was added to give a final concentration of 70% (v/v). Following incubation overnight at −20°C, the samples were centrifuged (as above), and in both the resulting pellet (designated the polysaccharides, essentially SHGL) and the supernatant (containing monosaccharides and oligosaccharides, SHGS, and other constituents soluble in 70% [v/v] ethanol), the 14C-label was monitored using a liquid scintillation counter.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phosphorylase patterns and DPE2 activity in the four transgenic potato lines.
Supplemental Figure S2. Monosaccharide patterns from the low molecular weight SHG fraction from leaves of cPGM-overexpressing (lines A and B) or cPGM-underexpressing (lines C and D) potato lines.
Supplemental Figure S3. Removal of contaminating proteins from SHGL by proteinase K treatment.
Supplemental Figure S4. Phosphorylase patterns and DPE2 activity in tuber discs following incubation with various sugars or sugar derivatives.
Supplementary Material
Acknowledgments
This work was supported by the Interdisciplinary Center for Advanced Protein Technologies of the University of Potsdam.
This work was supported by the Deutsche Forschungsgemeinschaft: Sonderforschungsbereich 429 “Molecular Physiology, Energetics, and Regulation of Primary Metabolism in Plants,” Teilprojekte A11 (A.R.F.), and B2 (J.F. and M. Steup).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Martin Steup (msteup@rz.uni-potsdam.de).
The online version of this article contains Web-only data.
References
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas LHW (1997) Developmental changes of enzymes involved in conversion of sucrose to hexose-phosphate during early tuberisation of potato. Planta 202 220–226 [Google Scholar]
- Bajorath J, Hinrichs W, Saenger W (1988) The enzymatic activity of proteinase K is controlled by calcium. Eur J Biochem 176 441–447 [DOI] [PubMed] [Google Scholar]
- Benfey PN, Chua NH (1990) The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 16 959–966 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor MI, Nunes-Nesi A, Nikiforova V, Centero D, Ratzka A, Pauly M, et al (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol 142 1380–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starch-less mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia T, Thorneycroft SM, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004) A cytosolic glucosyltransferase is required for the conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37 853–863 [DOI] [PubMed] [Google Scholar]
- Chritchley JH, Zeeman SC, Tahaka T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutant in Arabidopsis. Plant J 26 89–100 [DOI] [PubMed] [Google Scholar]
- Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann N Y Acad Sci 121 404–427 [DOI] [PubMed] [Google Scholar]
- Deschamps P, Colleoni C, Nakamura Y, Suzuki E, Putaux JL, Buléon A, Haebel S, Ritte G, Steup M, Falcón LJ, et al (2008) Metabolic symbiosis and the birth of the plant kingdom. Mol Biol Evol 25 536–548 [DOI] [PubMed] [Google Scholar]
- Deuschle K, Chaudhuri B, Okumoto S, Lager I, Lalonde S, Frommer WB (2006) Rapid metabolism of glucose detected with FRET glucose nanosensors in epidermal cells and intact roots of Arabidopsis RNA-silencing mutants. Plant Cell 18 2314–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckermann N, Fettke J, Steup M (2002) Identification of polysaccharide binding proteins by affinity electrophoresis in inhomogeneous polyacrylamide gels and subsequent SDS-PAGE/matrix-assisted laser desorption ionization-time of flight analysis. Anal Biochem 304 180–192 [DOI] [PubMed] [Google Scholar]
- Edner C, Li J, Albrecht T, Mahlow S, Hejazi M, Hussein H, Kaplan FI, Guy C, Smith SM, Steup M, et al (2007) Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial beta-amylases. Plant Physiol 145 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol 127 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Roessner U, Geigenberger P (2001) The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers (Solanum tuberosum). Plant Physiol 125 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Swiedrych A, Tauberger E, Lytovchenko A, Trethewey RN, Willmitzer L (2002. a) Potato plants exhibiting combined antisense repression of cytosolic and plastidial isoforms of phosphoglucomutase surprisingly approximate wild type with respect to the rate of starch synthesis. Plant Physiol Biochem 40 921–927 [Google Scholar]
- Fernie AR, Tauberger E, Lytovchenko A, Roessner U, Willmitzer L, Trethewey RN (2002. b) Antisense repression of cytosolic phosphoglucomutase in potato (Solanum tuberosum L.) results in severe growth retardation, reduction in tuber number and altered carbon metabolism. Planta 214 510–520 [DOI] [PubMed] [Google Scholar]
- Fettke J, Chia T, Eckermann N, Smith AM, Steup M (2006) A transglucosidase necessary for starch degradation and maltose metabolism in leaves acts on cytosolic heteroglycans (SHG). Plant J 46 668–684 [DOI] [PubMed] [Google Scholar]
- Fettke J, Eckermann N, Kötting O, Ritte G, Steup M (2007) Novel starch-related enzymes and carbohydrates. Cell Mol Biol 52 OL883–OL904 [PubMed] [Google Scholar]
- Fettke J, Eckermann N, Poeste S, Pauly M, Steup M (2004) The glycan substrate of the cytosolic (Pho 2) phosphorylase isoform from Pisum sativum L.: identification, linkage analysis and subcellular localization. Plant J 39 933–946 [DOI] [PubMed] [Google Scholar]
- Fettke J, Eckermann N, Tiessen A, Geigenberger P, Steup M (2005. a) Identification, subcellular localization and biochemical characterization of water-soluble heteroglycans (SHG) in the leaves of Arabidopsis thaliana L.: distinct SHG reside in the cytosol and in the apoplast. Plant J 43 568–585 [DOI] [PubMed] [Google Scholar]
- Fettke J, Poeste S, Eckermann N, Tiessen A, Pauly M, Geigenberger P, Steup M (2005. b) Analysis of cytosolic heteroglycans from leaves of transgenic potato (Solanum tuberosum L.) plants that under- or overexpress the Pho 2 phosphorylase isozyme. Plant Cell Physiol 46 1987–2004 [DOI] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, Vaughan CK, Li J, Francisco P, Gil M, Reinhold H, Eicke S, Messerli G, et al (2008) β-Amylase4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell 20 1040–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M, Fernie AR (2004) Metabolic control analysis and regulation of the conversions of sucrose to starch in growing potato tubers. Plant Cell Environ 27 655–673 [Google Scholar]
- Geiger M, Stitt M, Geigenberger P (1998) Metabolism in slices from growing potato tubers responds differently to addition of sucrose and glucose. Planta 206 234–244 [Google Scholar]
- Giege P, Heazlewood JL, Roessner-Tunali U, Millar AH, Fernie AR, Leaver CJ, Sweetlove LJ (2003) Enzymes of glycolysis are functionally associated with the mitochondrion in Arabidopsis cells. Plant Cell 15 2140–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW, Williams TC, Morgan M, Fernie AR, Ratcliffe RG, Sweetlove LJ (2007) Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell 19 3723–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi M, Fettke J, Haebel S, Edner C, Paris O, Frohberg C, Steup M, Ritte G (2008) Glucan, water dikinases phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J 55 323–334 [DOI] [PubMed] [Google Scholar]
- Holtgrawe D, Scholz A, Altmann B, Scheibe R (2005) Cytoskeleton-associated, carbohydrate-metabolizing enzymes in maize identified by yeast two-hybrid screening. Physiol Plant 125 141–156 [Google Scholar]
- Kofler H, Häusler RE, Schulz B, Gröner F, Flügge UI, Weber A (2000) Molecular characterisation of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis, and complementation of the mutant with the wild-type cDNA. Mol Gen Genet 263 978–986 [DOI] [PubMed] [Google Scholar]
- Kosegarten H, Mengel K (1994) Evidence for a glucose 1-phosphate translocator in storage tissue amyloplasts of potato (Solanum tuberosum) suspension-cultured cells. Physiol Plant 91 111–120 [Google Scholar]
- Lu Y, Sharkey TD (2004) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 207 271–274 [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Bieberich K, Willmitzer L, Fernie AR (2002. a) Carbon assimilation and metabolism in potato leaves deficient in plastidial phosphoglucomutase. Planta 215 802–811 [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Hajirezaei M, Eickmeier I, Mittendorf V, Sonnewald U, Willmitzer L, Fernie AR (2005. a) Expression of an Escherichia coli phosphoglucomutase in potato (Solanum tuberosum L.) results in minor changes in tuber metabolism and a considerable delay in tuber sprouting. Planta 221 915–927 [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Schauer N, Willmitzer L, Fernie AR (2005. b) Tuber-specific cytosolic expression of a bacterial phosphoglucomutase in potato (Solanum tuberosum L.) dramatically alters carbon partitioning. Plant Cell Physiol 46 588–597 [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Sweetlove LJ, Pauly M, Fernie AR (2002. b) The influence of cytosolic phosphoglucomutase on photosynthetic carbohydrate metabolism. Planta 215 1013–1021 [DOI] [PubMed] [Google Scholar]
- Naeem M, Tetlow IJ, Emes MJ (1997) Starch synthesis in amyloplasts purified from developing potato tubers. Plant J 11 1095–1103 [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303 87–89 [DOI] [PubMed] [Google Scholar]
- Nothnagel EA (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174 195–291 [DOI] [PubMed] [Google Scholar]
- Reiter WD (2008) Biochemical genetics of nucleotide sugar interconversion reactions. Curr Opin Plant Biol 11 236–243 [DOI] [PubMed] [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch-related R1 protein is an α-glucan, water dikinase. Proc Natl Acad Sci USA 99 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Pauly M (2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol 7 285–295 [DOI] [PubMed] [Google Scholar]
- Scheidig A, Fröhlich A, Schulze S, Lloyd JR, Kossmann J (2002) Down regulation of a chloroplast-targeted β-amylase leads to a starch-excess phenotype in leaves. Plant J 30 581–591 [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30 1126–1149 [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56 73–97 [DOI] [PubMed] [Google Scholar]
- Steichen JM, Petty RV, Sharkey TD (2008) Domain characterization of a 4-α-glucanotransferase essential for maltose metabolism in photosynthetic leaves. J Biol Chem 283 20797–20804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M (1990) Starch degrading enzymes. In PJ Lea, ed, Methods in Plant Biochemistry, Vol 3. Academic Press, London, pp 103–128
- Steup M, Latzko E (1979) Intracellular localization of phosphorylases in spinach and pea leaves. Planta 145 69–75 [DOI] [PubMed] [Google Scholar]
- Takamiya S, Fukui T (1978) Purification and multiple forms of phosphoglucomutase from potato tubers. Plant Cell Physiol 19 759–768 [Google Scholar]
- Tauberger E, Fernie AR, Emmermann M, Renz A, Kossmann J, Willmitzer L, Trethewey RN (2000) Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J 23 43–53 [DOI] [PubMed] [Google Scholar]
- Viola R, Roberts AG, Haupt S, Gazzani S, Hancock RD, Marmiroli N, Machray GC, Oparka KJ (2001) Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell 13 385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge I (2000) Identification, purification, and molecular cloning of a putative plastidic glucose transporter. Plant Cell 12 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Weber APM, Sharkey TD (2004) Maltose is the major form of carbon exported from chloroplasts at night. Planta 218 474–482 [DOI] [PubMed] [Google Scholar]
- Yariv J, Rapport M, Graf L (1962) The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycoside. Biochem J 85 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Delatte T, Messerli G, Umhang M, Stettler M, Mettler T, Streb S, Reinhold H, Kötting O (2007. a) Starch breakdown: recent discoveries suggest distinct pathways and novel mechanisms. Funct Plant Biol 34 465–473 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM (2007. b) The diurnal metabolism of leaf starch. Biochem J 401 13–28 [DOI] [PubMed] [Google Scholar]
- Zhang L, Häusler RE, Greiten C, Hajirezaei MR, Haferkamp I, Neuhaus HE, Flügge UI, Ludewig F (2008) Overriding the co-limiting import of carbon and energy into tuber amyloplasts increases the starch content and yield of transgenic potato plants. Plant Biotechnol J 6 453–464 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.