Abstract
A reverse genetic approach was used to investigate the functions of three members of the cellulose synthase superfamily in Arabidopsis (Arabidopsis thaliana), CELLULOSE SYNTHASE-LIKE D1 (CSLD1), CSLD2, and CSLD4. CSLD2 is required for normal root hair growth but has a different role from that previously described for CSLD3 (KOJAK). CSLD2 is required during a later stage of hair development than CSLD3, and CSLD2 mutants produce root hairs with a range of abnormalities, with many root hairs rupturing late in development. Remarkably, though, it was often the case that in CSLD2 mutants, tip growth would resume after rupturing of root hairs. In silico, semiquantitative reverse transcription-polymerase chain reaction, and promoter-reporter construct analyses indicated that the expression of both CSLD2 and CSLD3 is elevated at reduced temperatures, and the phenotypes of mutants homozygous for insertions in these genes were partially rescued by reduced temperature growth. However, this was not the case for a double mutant homozygous for insertions in both CSLD2 and CSLD3, suggesting that there may be partial redundancy in the functions of these genes. Mutants in CSLD1 and CSLD4 had a defect in male transmission, and plants heterozygous for insertions in CSLD1 or CSLD4 were defective in their ability to produce pollen tubes, although the number and morphology of pollen grains was normal. We propose that the CSLD family of putative glycosyltransferases synthesize a polysaccharide that has a specialized structural role in the cell walls of tip-growing cells.
Almost all plant cells are surrounded by a carbohydrate-rich cell wall (Carpita and Gibeaut, 1993; Fry, 2004). These highly complex fiber composite structures provide support and defense for the plant body and also have important roles in signaling, cell fate, and cell-to-cell adhesion (Bacic et al., 1988; O'Neill et al., 1990; Carpita and Gibeaut, 1993; Ridley et al., 2001). Plant cell walls also affect human society, and particularly health and industry, in numerous ways. They are the largest source of biomass on earth and are an important renewable resource of high-value biopolymers with numerous industrial applications, including as feedstocks for biofuels, functional food ingredients, and industrial fibers (Kim and Triplett, 2001; Willats et al., 2006; Himmel et al., 2007; Reyna-Villasmil et al., 2007). Cell walls are constructed by the coordinated activity of a large number of biosynthetic enzymes, including glycosyl transferase (GT) enzymes that catalyze the formation of glycoside bonds in cell wall glycan polymers (Reiter, 2002; Scheible and Pauly, 2004). In Arabidopsis (Arabidopsis thaliana), it is thought that at least 200 GTs may be involved in cell wall biosynthesis, and steady progress has been made in identifying and characterizing these enzymes (Reiter, 2002; Scheible and Pauly, 2004). In the primary walls of growing plant tissues, the three major classes of polymer are cellulose, hemicelluloses, and pectins, and these form a coextensive network in most cells (Carpita and Gibeaut, 1993; Fry, 2004; Cosgrove, 2005). Cellulose biosynthesis is performed by cellulose synthase (CESA) GTs that are grouped in complexes at the plasma membrane. Ten CESAs have been identified in Arabidopsis, and their roles in the synthesis of primary and secondary walls have been extensively characterized (Taylor et al., 2003; Paredez et al., 2006; Desprez et al., 2007; Persson et al., 2007). In contrast, only a small fraction of the at least 54 GTs involved in pectin biosynthesis have been identified so far (Ridley et al., 2001; Sterling et al., 2006). Hemicelluloses are a family of polysaccharides that typically have roles in providing structural support by cross-linking cellulose microfibrils. Unlike cellulose, which is made exclusively from Glc, hemicelluloses are structurally diverse, and most consist of linear backbone domains with short side chains (Fry, 2004). Backbone domains can consist of (1–4)-β-d-linked mannan, xylan, glucan, and, in the case of the Poales and Equisetum spp., (1→3)(1→4)-β-d-glucan (Fry, 2004; Sørensen et al., 2008). Recently, several GTs involved in the synthesis of the backbones of hemicelluloses were described, and all are products of genes in the CELLULOSE SYNTHASE-LIKE (CSL) family (Dhugga et al., 2004; Liepman et al., 2005; Burton et al., 2006, 2008; Cocuron et al., 2007).
CSL genes were first identified in Arabidopsis by their similarity to the CESA genes, and on the basis of predicted protein sequences, these CSL genes were grouped into six families: CSLA, CSLB, CSLC, CSLD, CSLE, and CSLG (Richmond and Somerville, 2000). All CSLs appear to be integral membrane proteins that contain features that are characteristic of processive GTs (Richmond and Somerville, 2001; Dhugga et al., 2004; Liepman et al., 2005). Also, all predicted CSL gene products contain the D,D,D,Q/RXXRW motif, which, together with topological features, indicates that they are involved in the synthesis of β-linked glycan polymers (Dhugga et al., 2004; Liepman et al., 2005). So far, this is borne out by in vitro activity assays of several heterologously expressed CSLs, all of which have been shown to synthesize (1→4)-β-linked products. For example, the CSLA family includes mannan synthases, while the CSLC family includes glucan synthases that are probably responsible for the construction of the backbone of xyloglucan (Dhugga et al., 2004; Liepman et al., 2005; Burton et al., 2006; Cocuron et al., 2007). Furthermore, CSLF genes in rice (Oryza sativa) were demonstrated to mediate the synthesis of (1→3),(1→4)-β-d-glucans (Burton et al., 2006). As expression systems and assays are optimized, it is likely that the products of the remaining CSLs will emerge. Possible products could include the backbones of xylan, noncrystalline cellulose, and the galactan side chains of pectin or arabinogalactan proteins (Liepman et al., 2005). Several Arabidopsis mutants with lesions in CSL genes have been described. The kojak mutant revealed that CSLD3 is required for root hair growth, while CSLA7 is required for pollen tube growth and embryogenesis (Favery et al., 2001; Wang et al., 2001; Goubet et al., 2003). In the rat4 mutant, disruption of CSLA9 results in the inhibition of Agrobacterium tumefaciens-mediated root transformation (Zhu et al., 2003). CSLD5 is required for normal growth of the plant (Bernal et al., 2007). However, it has also been reported that for many CSLs, knockout plants do not have any obvious phenotypical defects (Richmond and Somerville, 2001). One reason for this may be functional redundancy within CSL families. However, it may also be the case that the activities of some CSLs may be spatially or temporally restricted, so that phenotypes that arise from disrupting their function are only apparent in particular cells or during a limited phase of development. Data from large-scale transcriptomics databases are providing detailed new insights into CSL expression and providing clues to where best to focus the analysis of CSL knockout plants. In this work, we have focused on the CSLD family, the closest one to the CESA genes, with 35% identity at the amino acid level (Richmond and Somerville, 2001). This family includes genes CSLD1 through CSLD6, and most members of this family have highly distinctive expression profiles. We report here on the roles of CSLD genes in pollen tube and root hair development and discuss our findings in the wider context of the roles of CSLD genes in tip growth.
RESULTS
In Silico and Semiquantitative Reverse Transcription-PCR Expression Profiling of CSLD Genes
The meta-analyzer Genevestigator database, which collates data from Affymetrix ATH1 Arabidopsis arrays (https://www.genevestigator.ethz.ch/), was used to obtain an overview of the expression of the CSLD gene family (Supplemental Fig. S1). Four broad patterns of expression were observed. (1) CSLD1 and CSLD4 expression was very high in pollen, high in stamens, and moderate in flowers. Expression in all other tissues/organs was very low. (2) CSLD2 and CSLD3 expression was generally highest in root tissues but low in root tips. Both genes were also expressed at low or moderate levels in most other tissues/organs. (3) CSLD5 was expressed at highest levels in hypocotyls and shoot apices but was also expressed at moderate levels in a range of other organs/tissues, including flowers, roots, and leaves. (4) CSLD6 was expressed at near background levels in all tissues/organs. The expression of CSLD genes in response to environmental stress factors was analyzed using the “response-viewer” function of Genevestigator. This analysis revealed that the expression levels of CSLD2 and CSLD3 were increased by growth at reduced temperatures (data not shown). Furthermore, a more detailed analysis of the expression of CSLD1 and CSLD4 was obtained by interrogating a transcriptome database of pollen development (Honys and Twell, 2004). Neither CSLD1 nor CSLD4 was expressed above background levels during early pollen development (microspore stage). However, expression of both genes increased during pollen development and peaked in mature pollen (data not shown). Taken together, these data suggested that like CSLD3, CSLD2 was likely to be involved in root development, while CSLD1 and CSLD4 were likely to be involved in an aspect of late pollen development.
To extend the in silico analysis, we conducted semiquantitative reverse transcription (RT)-PCR of CSLD1, CSLD2, CSLD3, and CSLD4 in wild-type Columbia (Col-0) plants (Supplemental Fig. S2). In agreement with in silico analysis, CSLD2 and CSLD3 were expressed to some extent in all plant organs examined, although the expression of CSLD2 was somewhat lower than that of CSLD3. However, the expression of both of these genes was not notably higher in roots. The RT-PCR analyses of CSLD1 and CSLD4 were in broad agreement with the in silico analyses, and both genes were expressed at highest levels in flowers. However, CSLD1 expression was also detected in roots and stems.
We further analyzed the cellular expression patterns of CSLD2 and CSLD3 in young seedlings by the use of plants expressing constructs containing a nucleus-targeted GFP:GUS fusion protein driven by the promoters of CSLD2 or CSLD3 (Supplemental Fig. S3). Confocal microscopy of 5-d-old seedlings carrying these constructs showed that both genes were expressed during the early and late stages of root hair development (Supplemental Fig. S3, A−D). However, CSLD3 expression was generally much stronger than that of CSLD2 in the distal portion of roots in trichoblasts at an early stage of root hair development (Supplemental Fig. S3, E−G).
Generation of CSLD Knockout Mutants
Insertional mutants of CSLD1 (csld1-1), CSLD2 (csld2-1), CSLD3 (csld3-2), and CSLD4 (csld4-1) were obtained from the SALK collection (Supplemental Fig. S4). The csld3-2 mutant was so named to distinguish it from csld3-1, another previously described mutant with a lesion in CSLD3 (Wang et al., 2001). In a separate forward genetic screen of T-DNA-mutagenized Arabidopsis seedlings, we identified a mutant with root hairs characterized by swollen bases and ruptured tips. Thermal asymmetric interlaced PCR confirmed a T-DNA insertion located in the first exon of CSLD2. This mutant was designated csld2-2 (Supplemental Fig. S4). All mutants were generated in the Col-0 background, apart from csld4-1, which had a quartet (qrt) background. Homozygous lines were generated for all of the CSLD2 and CSLD3 mutants, and zygosity was confirmed by PCR (data not shown). Loss of expression of CSLD2 and CSLD3 in csld2-1, csld2-2, and csld3-2 was confirmed by RT-PCR, as shown in Supplemental Figure S4F. A double homozygote mutant with insertions in both CSLD2 and CSLD3 (designated csld2/3) was also produced by crossing csld2-2 and csld3-2. However, of more than 150 F1 selfed plants, no homozygous mutants of CSLD1 or CSLD4 were found. Therefore, both the csld1-1 and csld4-1 lines were maintained as heterozygous for insertions in these genes.
Mutants with Lesions in CSLD2 Have a Defective Root Hair Phenotype That Is Distinct from Mutants with Lesions in CSLD3
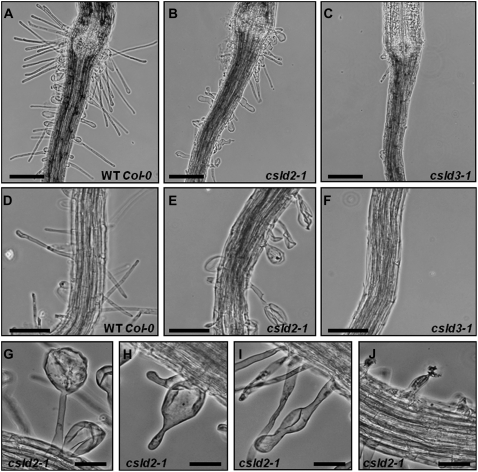
Under the conditions we used, no aerial phenotype was observed for any of the mutants in CSLD2 or CSLD3. The roots of 4-d-old wild-type Col-0 seedlings produced root hairs of varying lengths, as shown in Figure 1, A and D. In contrast, the root hairs of csld2-1 seedlings were shorter than those of wild-type controls, and most had obvious visible deformities (Fig. 1, B and E). csld2-2 had an identical defective root hair phenotype to csld2-1 (data not shown). For comparison, three mutants with lesions in CSLD3 were also examined, our own csld3-2 and the previously described mutants csld3-1 (Wang et al., 2001) and kojak (Favery et al., 2001). All three CSLD3 mutants had identical root hair phenotypes, and the csld3-1 phenotype is shown in Figure 1, C and F. In contrast to csld2-1 and csld2-2, the CSLD3 mutants produced few root hairs, and those hairs that were produced were usually ruptured at the tip soon after initiation. This was also the case for the double mutant csld2/3, which had an identical root hair phenotype to csld3-2, csld3-1, and kojak (Fig. 5E). The root hairs of csld2-1 and csld2-2 had deformities of various types, and representative examples are shown (Fig. 1, G–J). Some hairs appeared normal for much of their length but had severely bulged tip regions (Fig. 1G), whereas in others, the base of the root hair was bulged and the tip appeared normal (Fig. 1H). Other common phenotypes included multiple bulges along the length of the root hair (Fig. 1I) and root hair rupturing (Fig. 1J).
Figure 1.
csld2-1 has a defective root hair phenotype that is different from that of mutants with lesions in CSLD3. A to C, Images showing the root hypocotyl junction region of wild-type Col-0 (A), csld2-1 (B), and csld3-1 (C). D to F, Images of the middle portion of roots of wild-type Col-0 (D), csld2-1 (E), and csld3-1 (F). G to J, High-magnification images showing the range of aberrant root hair phenotypes observed in csld2-1. All images are of roots from 10-d-old csld2-1 seedlings. Bars = 50 μm (A–F) and 25 μm (G–J).
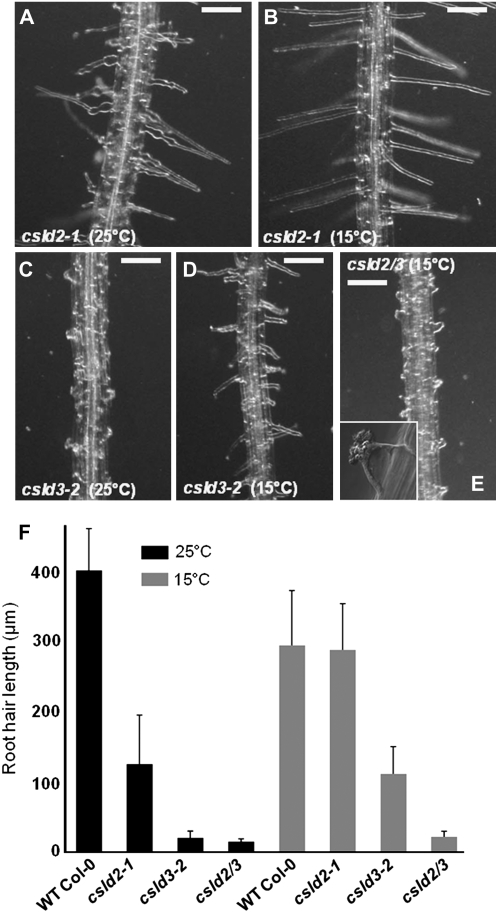
Figure 5.
The altered root hair phenotypes in csld2-1 and csld3-2 are partially rescued by growth at reduced temperature. A to E, csld2-1 (A and B) and csld3-2 (C and D) were grown at 25°C (A and C) or 15°C (B and D). Growth at 15°C resulted in partial rescue of root hair phenotypes in both csld2-1 and csld3-2. However, the phenotype of a double mutant with lesions in CSLD2 and CSLD3 (csld2/3) was not rescued by growth at 15°C (E). The inset in E shows a high-magnification image of a ruptured root hair of the csld2/3 double mutant. F, Quantification of root hair length in csld2-1, csld3-2, and csld2/3. The graph shows average root hair length of 10 seedlings. WT, Wild type. Error bars indicate se. Bars in A to E = 100 μm.
Detailed Analysis of Root Hair Rupturing in csld2-1 and csld3-2
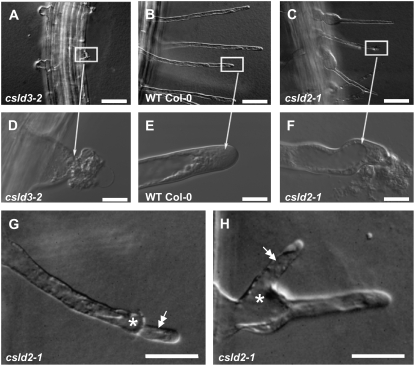
The rupture of csld2-1 and csld3-2 root hairs was analyzed in more detail using differential interference contrast (DIC) microscopy (Fig. 2). In both mutants, rupture was characterized by the loss of material that was presumably cytoplasm from the inside of cells. In csld3-2, rupture occurred soon after the initiation, as reported previously for csld3-1 and kojak (Fig. 2, A and D). In contrast, if rupturing occurred in csld2-1 root hairs, it was most commonly at a later stage when considerable hair growth had already occurred (Fig. 2, C and F). An additional notable difference between csld2-1 and csld3-2 was that in some cases, it appeared that root hair growth in csld2-1 resumed after rupturing (Fig. 2, G and H). In some instances, the new growth apparently emanated from the tip of a previously ruptured hair, although it was not clear if this was a resumption of growth of the ruptured root hair or the growth of a new hair through the body of the original one (Fig. 2G). Where rupturing occurred on the flank of a root hair, new growth sometimes resumed at the basal region of the original one (Fig. 2H). In order to investigate this observation in more detail, hair growth in csld2-1 and csld3-2 was examined using time-lapse microscopy of living root hairs (Supplemental Movies S1–S5). In csld2-1, relatively mild ruptures, as evident by the appearance of a lesion at the surface of the hair cell, would sometimes be followed by temporary arrest of tip growth. During this period, cytoplasmic streaming in the root hair persisted, and in some cases a gradual swelling of the basal region of the root hair also occurred. Nevertheless, apparently normal tip growth was resumed (Supplemental Movie S1). In other cases, rupturing appeared to be more severe and material that was presumably cytoplasm was expelled from the tip of the root hair, as seen in the middle two root hairs in Supplemental Movie S2. However, even after these more severe ruptures, tip growth was still observed to resume after a period of growth arrest (Supplemental Movie S2). When rupturing was very severe, as was evident by massive cytoplasmic discharge, cytoplasmic streaming ceased and tip growth did not recover (Supplemental Movie S3). csld2-2, which we isolated from a forward genetic screen, displayed identical root hair swelling and rupturing phenotypes as csld2-1 (data not shown). As with csld2-1, the severity of rupturing of csld3-2 hairs appeared to vary. For example, in some cases, relatively little cytoplasm was released and cytoplasmic streaming continued (Supplemental Movie S4). In other cases, more cytoplasm was apparently released and cytoplasmic streaming ceased altogether (Supplemental Movie S5).
Figure 2.
Tip rupture in csld2-1 and csld3-2 and regrowth in csld2-1. A to F, DIC images of root hairs from csld3-2 (A and D), wild-type Col-0 (B and E), and csld2-1 (C and F). The rupturing of root hair tips and loss of cytoplasm are visible in both csld3-2 (D) and csld2-1 (F). G and H, In csld2-1, new root hair growth was apparent after rupturing of hairs both at the tip (G) and flank (H) regions. Double arrows indicate new root hair growth. Asterisks indicate the area of rupture. Bars = 50 μm (A–C), 10 μm (D–F), and 20 μm (G and H).
We measured the frequency of root hair growth resumption after tip rupture from several time-lapse movies of growing root hairs located at 500 to 700 μm from the primary root tips of 3- to 4-d-old csld2-1 seedlings. Of a total of 157 root hairs from 15 independent time-lapse sequences spanning 1 h of growth, we observed that 16% of the root hairs ruptured, but rupturing was soon followed by tip growth resumption. Irrespective of the severity of rupturing, and in contrast to csld2-1, resumption of tip growth was never observed in csld3-2.
csld2-1 and csld2-2 Root Hairs Have Altered Wall Ultrastructure But Normal Cellular Patterning in Roots
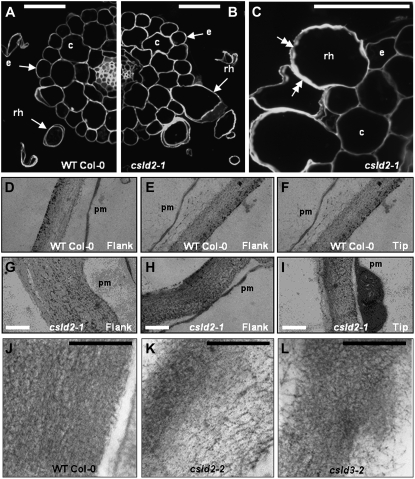
Expression data indicated that both CSLD2 and CSLD3 are expressed to some extent throughout roots. However, the CSLD3 mutants kojak and csld3-1 have been reported to have no abnormalities in cell types other than root hairs (Favery et al., 2001; Wang et al., 2001). In order to investigate if this was also the case for csld2-1, sections were taken through roots of 10-d-old csld2-1 seedlings and equivalent wild-type Col-0 seedlings (Fig. 3, A–C). Cell number and cellular patterning in the cortex and epidermis were normal in csld2-1, nor were defects observed in the stele. Excessive bulging was apparent in many of the csld2-1 root hairs sectioned, but other regions of csld2-1 trichoblasts appeared normal, and bulging did not intrude into other epidermal or cortical cells (Fig. 3B). At higher magnification, it was apparent that in many cases, the cell walls of csld2-1 root hairs appeared variable in thickness (Fig. 3C).
Figure 3.
The cellular anatomy of csld2 is normal apart from the altered root hair phenotype. A and B, Images showing resin-embedded roots from 12-d-old wild-type Col-0 (A) and csld2-1 (B) seedlings. Overall cellular patterning was normal in csld2-1 roots, and apart from root hairs (rh), all cells had normal morphologies, including atrichoblast epidermal cells (e) and cortical cells (c). C, High-magnification image of a section through a root of csld2-1 showing root hair, atrichoblast epidermal cells, and cortical cells. Double arrows indicate the region of root hair walls that are thickened or of variable thickness. D to L, Transmission electron microscopy of cell walls from wild-type Col-0 (D–F and J), csld2-1 (G–I), csld2-2 (K), and csld3-2 (L). Images were taken of equivalent flank (D, E, G, and H) and tip (F and I) regions of wild-type Col-0 and csld2-1 root hairs. In J to L, note the diffuse appearance of csld2-2 and csld3-2 walls compared with wild-type Col-0 walls. Bars = 50 μm (A–C), 0.2 μm (D–I), and 0.1 μm (J–L).
In order to investigate cell wall ultrastructure in more detail, sections through csld2-1, csld2-2, csld3-2, and wild-type Col-0 root hairs were examined using transmission electron microscopy. Walls were examined at various positions along root hairs from base to tip, and representative examples are shown in Figure 3, D to L. The walls of wild-type Col-0 hairs had a dense fibrillar appearance and had a consistent thickness of approximately 0.25 μm along the length of root hairs (Fig. 3, D–F). In contrast, the walls of csld2-1 root hairs varied in thickness from approximately 0.2 to 0.6 μm (Fig. 3, G–I). In regions where csld2-1 root hair walls were thickened, they also had a more diffuse appearance, as shown in Figure 3G. This variability in thickness and more diffuse fibrillar network were also apparent in the walls of csld2-2 and csld3-2 root hairs (Fig. 3, K and L).
csld2-1 Root Hairs Have a Disrupted Cytoskeletal Organization
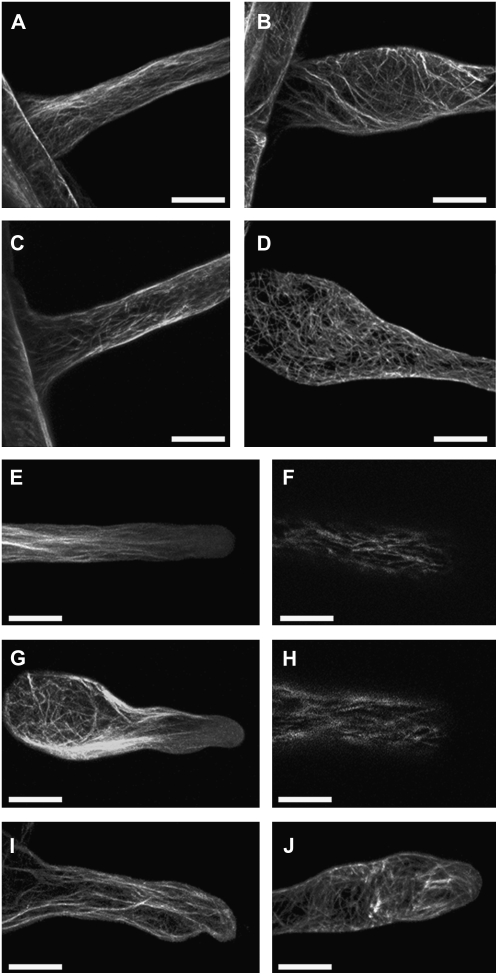
It is known that there is an intimate relationship between cell wall formation and cytoskeletal organization (Wasteneys, 2004; Paredez et al., 2006). Given the disrupted cell wall architecture and severe morphological defects in root hairs of csld2-1, we were interested to see if cytoskeletal organization was also affected. csld2-1 was crossed with Arabidopsis plants expressing GFP reporters that bind to microtubules (35S∷GFP-MBD; Marc et al., 1998) or actin filaments (35S∷GFP-ABD2-GFP; Wang et al., 2008). We observed that the organization of both components of the cytoskeleton was disrupted in csld2-1 root hairs but that the severity of disruption depended on the growth status and shape of the root hair. For example, in the base of wild-type root hairs, F-actin and microtubules were arranged in a predominantly longitudinal fashion (Fig. 4, A and C). In contrast, in the swollen bases of csld2-1 root hairs, the orientation of F-actin and microtubules was less organized (Fig. 4, B and D). Tip regions of wild-type root hairs were characterized by longitudinal F-actin arrays that did not extend to the apex of hairs (Fig. 4E). In csld2-1, although F-actin was disorganized in bulged root hair bases, in tip regions with normal morphologies F-actin had a similar predominantly longitudinal orientation as wild-type Col-0; also like wild-type Col-0, the F-actin arrays did not extend to the apex of hairs (Fig. 4G). However, in cases where the tips of csld2-1 root hairs were swollen or had ruptured, the F-actin arrays were disorganized and did extend to the root hair apices (Fig. 4I). Similar observations were also made for microtubules: in wild-type and nonruptured csld2-1 hairs, microtubules had a predominantly longitudinal orientation and did not extend to root hair apices (Fig. 4, F–H), while in swollen or ruptured csld2-1 hairs, microtubules had a less organized orientation and did extend to the hair apex (Fig. 4J). The bases of some root hairs of csld2 did not bulge. In these nonbulged bases, the organization of F-actin and microtubules resembled that of wild-type roots (data not shown).
Figure 4.
Microtubule and F-actin organization is disrupted in living csld2-1 root hairs. A to D, F-actin (A and B) and microtubules (C and D) in the base of wild-type Col-0 (A and C) and csld2-1 (B and D). E to J, F-actin organization in the tip regions of wild-type Col-0 (E) and csld2-1 (G and I), and microtubule organization in the tip regions of wild-type Col-0 (F) and csld2-1 (H and J). F-actin and microtubules were visualized by GFP-ABD2-GFP and GFP-MBD2 fusions, respectively. Bars = 20 μm.
The Defective Root Hair Phenotypes of csld2-1 and csld3-2 Are Partially Rescued by Growth at Reduced Temperature
In silico expression analysis had revealed that the expression of both CSLD2 and CSLD3 is increased by growth at a reduced temperature, and we were interested to see if this increased expression had an effect on the root hair phenotypes of csld2-1 and csld3-2. Root hair morphologies (Fig. 5, A–E) and length (Fig. 5F) were compared for seedlings grown at 25°C and 15°C. At 25°C, csld2-1 root hairs displayed the characteristic phenotype described previously, including swelling at the hair base and tip rupturing (Fig. 5A). However, when csld2-1 was germinated and grown at 15°C for 6 to 9 d, these defects were largely reduced: swelling of hair bases and tip rupturing were rare, and root hairs grew to a similar length as wild-type controls (Fig. 5, B and F). The phenotype of csld3-2 was also partially rescued by growth at 15°C, although to a lesser extent than for csld2-1. At 25°C, the usual severe defects in csld3-2 root hairs were observed (Fig. 5C), but when grown at 15°C, rupturing of csld3-2 hairs was delayed such that many root hairs grew to approximately half the length of wild-type controls (Fig. 5, D and F). In all previous analyses, the double mutant csld2/3 appeared to have an identical defective root hair phenotype as csld3-2 and no additional phenotypical changes. Interestingly, though, unlike csld3-2, the root hair defects in csld2/3 were not rescued by growth at 15°C (Fig. 5, C, E, and F).
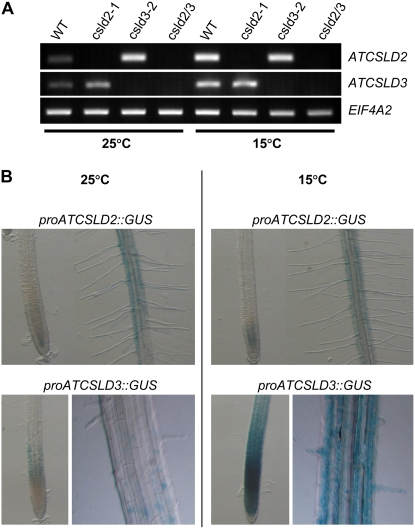
As noted, in silico analysis showed that CSLD2 and CSLD3 were induced by low temperatures, and this could explain the partial suppression of the csld2 and csld3 root hair phenotypes when grown at 15°C. To verify the in silico data, we conducted semiquantitative RT-PCR of CSLD2 and CSLD3 expression in 6-d-old wild-type, csld2-1, csld3-2, and double csld2/3 mutant roots grown at 25°C and 15°C (Fig. 6A). Consistent with the in silico analysis, expression of CSLD2 and CSLD3 was higher in roots of wild-type plants grown at 15°C compared with 25°C. Furthermore, CSLD3 expression was higher in csld2-1 at 15°C compared with 25°C. However, CSLD2 expression in csld3-2 mutants grown at 15°C was not increased in seedlings grown at 15°C. We also analyzed the effect of temperature on CSLD2 and CSLD3 expression by the use of plants expressing the GUS gene driven by the promoters of CSLD2 or CSLD3 (Fig. 6B). Using this approach, the expression of CSLD2 was not notably elevated at 15°C compared with 25°C, but the expression of CSLD3 was markedly higher at the lower temperature.
Figure 6.
Effect of reduced temperature on CSLD2 and CSLD3 expression. A, RT-PCR analysis of CSLD2 and CSLD3 genes in the wild type (WT), csld2-1, csld3-2, and csld2/3 mutants. RNA was isolated from roots of 6-d-old seedlings grown at 25°C or 15°C. Arabidopsis translation initiation factor EIF4A2 was used as a constitutive expression control. B, Histochemical analysis of GUS expression in roots of 6-d-old wild-type seedlings carrying constructs of GFP∷GUS driven by the CSLD2 or CSLD3 promoter and grown at 25°C or 15°C.
The csld-2 Root Hair Phenotype Is Rescued by Complementation with CSLD2 Driven by Its Own Promoter
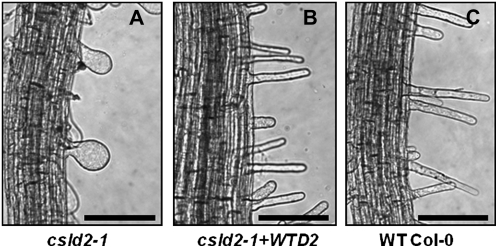
In order to determine if the defective root hair phenotype of csld2-1 was indeed due to the mutation of the CSLD2 gene, csld2-1 was transformed with a wild-type version of the CSLD2 gene driven by its own promoter. As shown in Figure 7, the root hairs of these complemented plants were identical in appearance and length to the root hairs of wild-type Col-0.
Figure 7.
The altered root hair phenotype of csld2-1 was restored by transformation with wild-type CSLD2 driven by its own promoter. Images show the root hair morphologies in csld2-1 (A), csld2-1 transformed with the wild-type CSLD2 gene driven by its own promoter (csld2-1+WTD2; B), and wild-type Col-0 (C). All images are of roots from 10-d-old seedlings. Bars = 50 μm.
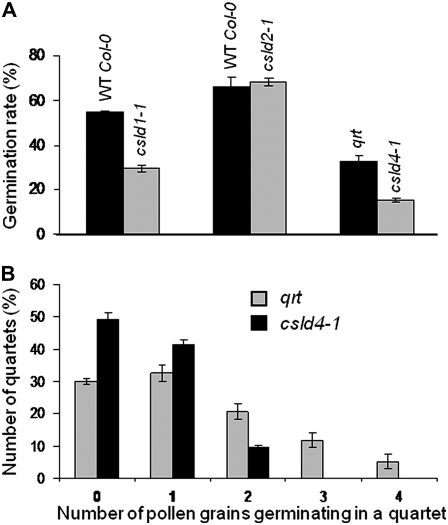
csld4-1 Has Normal Pollen Grain Morphology But Reduced Pollen Tube Germination
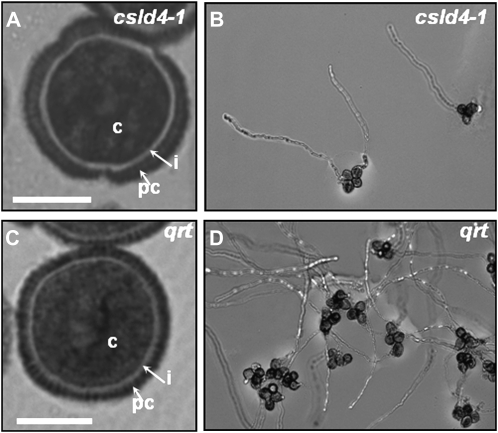
The facts that CSLD1 and CSLD4 were both expressed at high levels in pollen and that no homozygous plants were obtained in the F1 generation suggested that both genes may have roles in pollen development and/or pollen tube germination. In order to investigate if csld4-1 had defects in pollen grain morphology, sections through resin-embedded anthers from csld4-1 and qrt were stained with toluidine blue (Fig. 8, A and C). No differences were observed between csld4-1 and qrt with respect to the morphology and cellular anatomy of anthers, the number of pollen grains (data not shown), or the overall shape or size of pollen grains (Fig. 8, A and C). At higher magnifications of toluidine blue-stained sections, the pollen coat and intine were clearly visible, and again there were no obvious differences between csld4-1 and qrt (Fig. 8, A and C).
Figure 8.
csld4-1 pollen has a normal morphology but reduced pollen tube germination. A and C, Resin-embedded pollen from csld4-1 (A) and qrt (C) was sectioned and stained with toluidine blue. B and D, Images from in vitro germination assays showing quartets of pollen grains and pollen tubes from csld4-1 (B) and qrt (D). c, Cytoplasm; i, intine; pc, pollen coat. Bars in A and C = 1 μm.
Pollen tube germination in csld4-1 and qrt was assessed using in vitro germination assays (Fig. 8, B and D). The qrt mutant is defective in pectin degradation, and pollen grains in qrt adhere together rather than separating as usual after meiosis completion (Rhee and Somerville, 1998). By generating csld4-1 in a qrt background, we were able to examine pollen tube germination in relation to quartet genotype, since in each heterozygote csld4-1 quartet, two of the grains have a wild-type version of CSLD4 and two have the mutated version. By visual examination, it was clear that the overall extent of pollen tube germination was greater in qrt compared with csld4-1 (Fig. 8, B and D). Moreover, whereas up to four pollen tubes were observed to emerge from qrt quartets, a maximum of two were produced by csld4-1 quartets.
Quantification of Pollen Tube Germination in csld1-1, csld2-1, and csld4-1
Total pollen tube germination rates were determined for csld1-1, csld4-1, and csld2-1 (control) by scoring the in vitro germination assays (Fig. 9A). Germination was reduced by approximately 50% in csld1-1 and csld4-1 compared with wild-type Col-0 and qrt, respectively. However, the germination rate of pollen from csld2-1 did not significantly differ from that of wild-type Col-0 control pollen. The number of pollen tubes that germinated from individual quartets was also determined for qrt and csld4-1 (Fig. 9B). In both qrt and csld4-1, of the quartets that germinated, most produced one or two pollen tubes. However, whereas some qrt quartets produced three or four pollen tubes, csld4-1 quartets produced a maximum of two, confirming the visual examination shown in Figure 8. Moreover, the segregation of the insertional mutations in csld1-1 and csld4-1 was analyzed by a series of crosses between mutant and wild-type plants (Table I). The segregation ratio for self crosses of both csld1-1 and csld4-1 was approximately 1:1, suggesting a disorder in gametophytic transmission rather than a zygotic lethality of the mutation. We then further analyzed the transmission of the mutation in csld4-1 by performing reciprocal crosses with qrt and analyzed the progeny for BASTA resistance. Male transmission was abrogated by the mutation in csld4-1, whereas female transmission was not affected.
Figure 9.
Quantification of pollen tube germination rates in CSLD mutants. A, Quantification of the in vitro germination rate of pollen tubes from csld1-1, csld2-1, and csld4-1 compared with wild-type (WT) Col-0 and qrt. Note that pollen tube germination was normal in csld2-1. B, Quantification of the number of pollen tubes germinating from quartets. In csld4-1, the maximum number of pollen tubes germinating from a single quartet was two, whereas up to four tubes germinated from the qrt quartet. Error bars indicate se among three independent experiments with 80 samples each.
Table I.
Segregation analysis of mutations in CSLD1 and CSLD4
| Cross | Progeny with Insertiona | Progeny without Insertionb | Ratioc | χ2d | Pe |
|---|---|---|---|---|---|
| d4/D4fqrt/qrt♀ × d4/D4 qrt/qrt♂ | 88 | 93 | 1:1 | 0.138 | 0.710 |
| d1/D1♀ × d1/D1♂ | 86 | 103 | 1:1 | 1.529 | 0.216 |
| d4/D4 qrt/qrt♀ × D4/D4 qrt/qrt♂ | 27 | 30 | 1:1 | 0.158 | 0.691 |
| D4/D4 qrt/qrt♀ × d4/D4 qrt/qrt♂ | 0 | 120 | 0:1 | 0 | 1 |
| d4/D4 +WTD4♀ × d4/D4 +WTD4♂ | 59 | 20 | 3:1 | 0.004 | 0.948 |
BASTA-resistant plants in crosses with csld4-1 and progeny positive for the PCR analysis in crosses with csld1-1.
BASTA-susceptible plants in crosses with csld4-1 and progeny negative for the PCR analysis in crosses with csld1-1.
Segregation ratio for which χ2 values were calculated.
χ2 values calculated with expected/observed values for the segregation ratios shown in the previous column.
Probability associated to the χ2 value shown in the previous column with 1 degree of freedom. Probability values obtained indicate that no difference can be detected between observed and expected values in each experiment.
d4/D4, Plants heterozygous for the csld4-1 mutation.
In order to determine if the mutation of the CSLD4 gene was indeed responsible for the distorted transmission ratios we observed, segregation analysis was also performed on csld4-1 plants that had been transformed with the CSLD4 gene driven by its own promoter. The T1 progeny of a self-pollinated plant segregated in a 3:1 ratio for BASTA resistance, confirming that the mutation in CSLD4 was responsible for the abrogated male transmission (Table I).
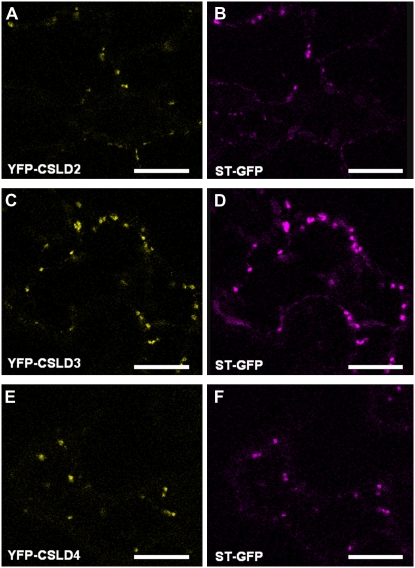
Subcellular Localization of CSLD2, CSLD3, and CSLD4
Previous work to determine the subcellular localization of CSLD3 (KOJAK), using a C-terminal fluorescent tag, concluded that this protein is localized in the endoplasmic reticulum (Favery et al., 2001). We found that CSLD5 also appeared to be endoplasmic reticulum localized when a C-terminal florescent tag was used. However, when CSLD3 or CSLD5 was tagged at the N terminus, both proteins were localized in the Golgi apparatus (Bernal et al., 2007). Proteomic LOPIT (for Localization of Organelle Proteins by Isotope Tagging) analysis recently demonstrated that CSLD2 and CSLD3 are in fact localized in the Golgi apparatus (Dunkley et al., 2006), and a considerable body of evidence indicates that most GTs involved in cell wall biosynthesis are located either at the plasma membrane (in the case of the CESAs) or in the Golgi apparatus. Together, these data suggest that N-terminal, rather than C-terminal, tagging is more likely to reliably report the location of CSLD proteins. With this in mind, we conducted subcellular localization experiments of CSLD2 and CSLD4 using yellow fluorescent protein (YFP) fused at the N terminus together with the STtmd-GFP Golgi apparatus marker for comparison (Fig. 10). The locations of YFP-CSLD2, YFP-CSLD3, and YFP-CSLD4 were similar to that of STtmd-GFP; therefore, these results confirmed the previous findings for CSLD2 and CSLD3 and indicate that CSLD4 is likewise localized in the Golgi apparatus. The functionality of the YFP-CSLD3 fusion was demonstrated by the fact that the csld3-2 phenotype was rescued by complementation with this construct (Supplemental Fig. S5).
Figure 10.
Subcellular localization of CSLD1 and CSLD4. Fluorescently tagged versions of CSLD2 (A), CSLD3 (C), and CSLD4 (E) in which YFP was fused to the N termini were expressed in Nicotiana benthamiana leaves and visualized using confocal laser-scanning microscopy. The Golgi apparatus marker STtmd-GFP (ST-GFP) was also expressed in N. benthamiana leaves (B, D, and F). Bars = 15 μm.
DISCUSSION
Tip growth is an important and highly specialized cellular growth process carried out in certain cells, including root hairs, pollen tubes, fungal hyphae, moss protonema, and fern rhizoids (Parton et al., 2000; Carol and Dolan, 2002; Cole and Fowler, 2006). In contrast to the growth of most plant cells, during tip growth secretory vesicles containing cell wall components are targeted exclusively to the growing apex of cells (Cole and Fowler, 2006). We have shown here that CSLD1 and CSDL4 are required for pollen tube development and that CSLD2 is required for normal root hair formation. The phenotypes observed for plants with mutations in these genes were broadly consistent with expression profiles determined by in silico, GFP, RT-PCR, and GUS analyses, and the results from complementation experiments also supported the direct involvement of these genes in pollen tube and root hair growth.
Although the in silico expression profiles of CSLD2 and CSLD3 were nearly identical, it was clear from more detailed analysis that they in fact have subtly different expression patterns, and this is reflected in the distinct phenotypes of mutants with lesions in the genes. While CSLD3 is required during the transition from root hair initiation to tip growth proper, CSLD2 appears to be required at a later stage of development during tip growth itself. This might be partly explained by the subtle differences in timing of the expression of these genes in root hairs, as indicated by GFP analysis that showed higher CSLD3 expression in the distal part of roots compared with CSLD2. It is possible that there are other differences in the expression of these genes that were not revealed by our experiments.
The observation that the csld2/3 homozygous double mutant has the same phenotype as csld3-2 under normal growth conditions indicates that CSLD3 is epistatic to CSLD2. Moreover, the partial rescue of the csld2-1 and csld3-2 phenotypes, when grown at 15°C, suggests that there may be partial functional redundancy between these genes. Thus, it may be the case that increased expression of CSLD3 at 15°C may be sufficient to partially compensate for the lack of the CSLD2 in csld2-1, and likewise for CSLD2 in csld3-2. This hypothesis is also supported by the fact that the phenotype of csld2/3, in which both genes are defective, was not rescued by growth at 15°C. While the csld2-1 phenotype was restored to nearly normal by growth at 15°C, the effect was less complete for csld3-2. This is consistent with the fact that although in silico expression indicated that CSLD2 expression was elevated by reduced temperature growth, GUS and RT-PCR analyses did not. This suggests that reduced temperature has only a slight effect on CSLD2 expression, which could account for the phenotypic compensation observed in csld3 mutants.
It is conceivable that the phenotypic recovery at reduced temperature resulted from altered physical properties of root hairs and/or growth medium at 15°C that rendered root hairs less susceptible to developing growth defects. However, if this were the case, then it would be expected that the csld2/3 double mutant would also show a similar phenotype at reduced temperature to csld3-2. A partial redundancy between CSLD2 and CSLD3 also seems plausible in the light of evidence that GTs within a given CSL family all appear to catalyze the synthesis of the same polysaccharide (Dhugga et al., 2004; Liepman et al., 2005; Cocuron et al., 2007). Both CSLD2 and CSLD3 are expressed to some extent in many Arabidopsis organs and tissues, but apart from root hair defects there were no obvious phenotypes in csld2-1, csld3-2, or csld2/3. This may suggest that another CSLD gene(s) can compensate for the loss of CSLD2 and CSLD3 in locations other than roots. If this is the case, then CSLD5 and CSLD1 are the most likely candidates, since their expression profiles overlap with those of CSLD2 and CSLD3 (Bernal et al., 2007; Supplemental Fig. S2).
In both csld1-1 and csld4-1, approximately half of the pollen grains failed to produce pollen tubes at all, indicating that CSLD1 and CSLD4 are required at a very early stage of pollen tube growth. However, it is important to add that the data presented here do not completely rule out the possibility that these mutations affect a pregermination, rather than a postgermination, process. CSLD1 and CSLD4 showed no signs of redundancy, since both single mutants showed abnormalities in the male gametophyte. This might be due to small differences in gene expression not detected with the experimental procedures used. Alternatively, it might be the case that functional cooperation is required by the GTs encoded by CSLD1 and CSLD4 in order to fulfill their functions.
One remarkable feature of csld2-1 that was revealed by time-lapse microscopy was that root hair growth could recover after hair rupturing, even after an apparent loss of cytoplasm. Several root hair mutants, for example, tip1 and cow1, have been reported in which multiple hairs develop from a single trichoblast (Grierson et al., 1997; Böhme et al., 2004). However, in these mutants, rupturing was not observed and the multiple hairs were proposed to be the result of defects in the mechanisms that regulate hair number (Grierson et al., 1997). Root hair number was normal in csld2-1, and hair regrowth appeared to be a repair mechanism. In most plant cells, rupture of the tonoplast to the extent that cytoplasm is released results in cell death. Indeed, in some cells, tonoplast rupture is the first event in programmed cell death (Gunawardena et al., 2004). One notable example of where recovery does occur after rupturing is in fern gametophyte rhizoids (Parton et al., 2000). These tip-growing cells are very similar in appearance to angiosperm root hairs, and many aspects of their cytology during tip growth are also similar. A detailed study of Dryopteris affinis rhizoids showed that new tip growth occurred after mechanically induced rupturing and loss of cellular material (Parton et al., 2000). In both D. affinis rhizoids and csld2-1 root hairs, recovery was observed in cells that had already undergone active tip growth. Since regrowth after rupture did not occur in csld3-2, this may indicate that the ability to recover is linked to a certain developmental phase in tip-growing cells. Rhizoids and root hairs are both adapted to anchorage and nutrient uptake, and it may be the case that a capacity for recovery is the result of convergent evolution in response to common environmental challenges. It has also been suggested that there may be an ancient evolutionary link between sporophyte root hairs and gametophyte rhizoids, and their shared ability to recover after rupture may reflect this (Parton et al., 2000).
Our results add to a compelling body of evidence that implicates CSLD genes in tip growth across the plant kingdom. The rice CSLD1 gene appears to be a functional ortholog of CSLD3, and root hair growth is impaired when CSLD1 is disrupted in rice (Kim et al., 2007). The CSLD1 gene is abundantly expressed in Nicotiana alata pollen tubes, and CSLD expression is high in tip-growing moss protonema cells. In contrast to higher plants, in which tip grow is limited to relatively few cells, in moss it is a prominent cellular growth mechanism of the plant body (Roberts and Bushoven, 2007). It is significant, therefore, that in Physcomitrella patens, 46% of all CESA superfamily ESTs are CSLDs, compared with only 10% in Arabidopsis (Roberts and Bushoven, 2007). Intriguingly, CSLD5 does not obviously fit this pattern. CSLD5 is expressed at highest levels during stem growth, and the csld5-1 mutant has reduced stem growth but no visible defects in tip-growing cells (Bernal et al., 2007). It may be the case that CSLD5 is not involved in tip growth, but it is worth noting that GUS expression analysis of CSLD5 indicated a role in xylem development, and xylem fibers grow by invasive tip growth (Mellerowicz et al., 2001; Samuga and Joshi, 2004). In this regard, it may also be significant that the CSLD2 gene in poplar (Populus spp.) that shares 90% similarity with CSLD3 is expressed in developing xylem, and it has been proposed to be involved in the control of fiber length (Samuga and Joshi, 2004).
The most straightforward explanation of the phenotypes we observed is that the product of CSLD GTs is a structural component of cell walls, and disrupting or reducing the level of this component leads to wall weakening, basal swelling, and tip rupture. The transmission electron microscopy images shown in Figure 3 indicate that one aspect of reduced wall integrity may be a reduction in the cohesion of the mesh-like wall matrix that leads to a less dense appearance. It is worth noting that the LXR1 and LXR2 Leu-rich repeat extensins, which probably have structural roles in cell walls, are also involved in maintaining normal root hair growth. Similar to csld2-1 and csld2-2, root hairs of lxr1 mutants rupture at the tip and exhibit basal swelling (Baumberger et al., 2001). Root hairs of double lxr1/lxr2 mutants also have irregular wall thickness and density, which resemble the cell wall architecture of csld2-1 and csld2-2 (Baumberger et al., 2001). Recently, plants that are deficient in xyloglucans resulting from disruptions in genes encoding xylosyltransferases produced root hairs with swollen bases that resembled csld2 mutants (Cavalier et al., 2008). It is highly likely that all CSLD GTs are involved in the synthesis of a β-linked polysaccharide, and although we do not know which polysaccharide this is, the evidence to date suggests a β-glucan. One clue comes from the similarity of the CSLDs to other GT genes that are known to catalyze β-glucan synthesis. Of all the CSLs, the D family members are the most similar to the CESAs (Richmond and Somerville, 2000). Also, phylogenetic analysis of CSL genes in rice suggests that the CSLF genes that are involved in (1→3),(1→4)-β-d-glucan synthesis may have diverged from the CSLD family (Buckeridge et al., 2004; Keegstra and Walton, 2006). Additional evidence comes from analysis of N. alata pollen tubes that contain (1→3)-linked and (1→4)-linked β-glucans and α-linked pectic polymers (Doblin et al., 2001). The presence of the (1→3)-β-glucan can be accounted for by an abundantly expressed putative callose synthase, NaGsl1, but no significant expression of any CESA genes was detected. However, a CSLD gene, NaCslD1, is abundantly expressed and is likely to be involved in the synthesis of the (1→4)-β-glucan (Doblin et al., 2001). We have shown here that CSLD2, CSLD3, and CSLD4 proteins are localized in the Golgi apparatus, and this is also the case for CSLD5 (Bernal et al., 2007). Moreover, it was recently shown that CSLD2 is an integral Golgi membrane protein (Zeng and Keegstra, 2008). With this in mind, it seems that if CSLD GTs do synthesize a β-glucan, it is distinct from crystalline cellulose, which is made by the plasma membrane-bound CESAs. It is possible that CSLDs synthesize another, possibly noncrystalline, form of β-glucan. Support for this possibility comes from Arabidopsis suspension cultured cells that were habituated to grow in the herbicide isoxaben that specifically disrupts CESA activity. CSLD5 was highly up-regulated in these cells, and their walls contained an apparently amorphous and glucanase-sensitive β-glucan (Manfield et al., 2004).
The extensive distortion to the cytoskeleton we observed in csld2-1 and csld3-2 root hairs suggests that disruption of CSLD function also has secondary effects. There is substantial evidence of an intimate and reciprocal link between the cortical cytoskeleton and cellulose synthesis (Wasteneys, 2004; Emons et al., 2007). This relationship is manifest both in cellulose synthase mutants that have disrupted cytoskeletal organization and in cytoskeleton mutants that have disrupted cellulose deposition (Burk and Ye, 2002; Chu et al., 2007; Paredez et al., 2008). However, this relationship does not involve absolute mutual dependence. For example, in mor1-1 root cells, cellulose microfibrils are correctly deposited even after disruption of cortical microtubules (Sugimoto et al., 2003; Wasteneys, 2004). One explanation of our results, given that CSLD2 and CSLD3 are very unlikely to be directly involved in the synthesis of either crystalline cellulose or the cytoskeleton, is that the glycan product of the GTs encoded by these genes is associated directly or indirectly with cellulose microfibrils. If so, then disruption of this glycan may also disrupt microfibril deposition and, hence, cytoskeletal stability and alignment. However, a more subtle explanation can be suggested based on the fact that microtubule polymer status is also known to be related to adaptive responses. For example, many environmental triggers, such as cold and osmotic stress, are associated with the reorganization of the cytoskeleton (Blancaflor and Hasenstein, 1995; Sangwan et al., 2001; Wojtaszek et al., 2007). The recent discovery that a receptor-like kinase may act as a cell wall integrity sensor suggests that internal as well as external stresses could be an aspect of the adaptive response (Hématy et al., 2007). It is possible, therefore, that detection of a change in cell wall status caused by loss of the CSLD2 and CSLD3 product affects cytoskeletal organization, which could in turn also affect cellulose microfibril deposition and amplify the phenotypic effect. Alternatively, given that cytoskeletal reorganization was mostly associated with bulges and previously ruptured root hairs, the remodeling of the cytoskeleton we observed here could be an indirect result of these morphological disturbances rather than direct sensing of changes in cell wall integrity. However, it was shown recently that general disturbances to cell wall structure that lead to morphological changes in the cell do not always lead to altered cortical microtubule organization. Thus, the genetic disruption of cell wall properties may be more strongly correlated to cytoskeletal organization than to cell morphology per se (Paredez et al., 2008).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) T-DNA knockout lines csld1-1 (SALK_043260), csld2-1 (SALK_119808), csld3-1 (CS899; Wang et al., 2001), csld3-2 (SALK_112105), and csld4-1 (SAIL_13_H04) were obtained from the Nottingham Arabidopsis Stock Centre. csld2-2 was obtained from a forward genetic screen for mutants with abnormal root hairs. All of the insertional mutants were in the Col-0 background, except for csld3-1, which was in the Wassilewskija background (Wang et al., 2001), and csld4-1, which was in the qrt background. The correct insertion sites were confirmed by PCR using oligonucleotides specific for each insertion line. All work presented here for CSLD2 and CSLD3 was carried out in homozygous lines, and the homozygosity was confirmed in two successive generations. csld1-1 and csld4-1 were confirmed to be heterozygous using PCR and PCR plus BASTA selection, respectively. Seeds were surface sterilized, sown on plates containing Murashige and Skoog medium (1× Murashige and Skoog salts, 8 g L−1 agar, 1× B5 vitamins, and 10.8 g L−1 Suc), and incubated for 48 h at 4°C in the dark. For root growth and root hair microscopy, plates were incubated vertically for 5 d at 21°C with a 16-h photoperiod. For all other purposes, plates were incubated horizontally for 1 week and seedlings were transferred to soil at 19°C with a 16-h photoperiod. For time-lapse microscopy and DIC experiments, seeds were germinated directly on 0.5% phyta-agar supplemented with 0.5× Murashige and Skoog salts layered onto 62- × 48-mm sterile glass coverslips as described previously (Blancaflor et al., 2003). For complementation experiments, 7-week-old csld2-1 and csld4-1 plants were vacuum infiltrated with suspensions of the Agrobacterium tumefaciens strain GV3101 bearing constructs containing the corresponding genomic fragment, including the promoter region, using previously described procedures (Bernal et al., 2007).
Semiquantitative RT-PCR Analysis
Total RNA was extracted from roots, leaves, stems, and flowers of wild-type Arabidopsis plants using the RNeasy Plant Mini Kit (Qiagen). For removal of contaminating genomic DNA, RNA samples were incubated in RNase-free DNaseI (Invitrogen). RT-PCR was carried out using 500 ng of total RNA with the Omniscript RT kit (Qiagen) following the manufacturer's protocol. Primers used for CSLD1 were CSDL1-RT-F (5′-CAATTCTCACCTTTGAAGCTATG-3′) and CSLD1-RT-R (5′-GGCACTTTGCTCATGATCTG-3′) to generate a 445-bp product; primers used for CSLD4 were CSLD4-RT-F (5′-ACGTGATGCCATGTGAATGC-3′) and CSLD4-RT-R (5′-CTTCATCCATATCATCGC-3′) to generate a 327-bp product; primers used for CSLD2 were CSLD2-RT-f (5′-GATGGAGATGGTGACGGTATG-3′) and CSLD2-RT-r (5′-AGCTCACAAACCACAGACATTC-3′) to generate a 221-bp product; primers used for CSLD3 were CSLD3-RT-f (5′-AGTCTGGTTTGATGAGGAGTC-3′) and CSLD3-RT-r (5′-ACAACAATTCGGATAAGTATC-3′) to generate a 255-bp product. Arabidopsis translation initiation factor EIF4A2 forward (5′-GAATCTTCTTAGGGGTATCTATGC-3′) and reverse (5′-CTATGACATATTCCAGCTTCTCCC-3′) primers were used as a control.
Histochemical Staining for GUS Activity
Histochemical staining of whole plant seedlings for GUS activity was performed as described by Jefferson et al. (1987). Briefly, seedlings growing under agar medium were soaked in GUS substrate solution of 100 mm sodium phosphate, pH 7.0, 0.2% (v/v) Triton X-100, 2.5 mm potassium ferricyanide, 2.5 mm potassium ferrocyanide, and 2 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronide and then incubated at room temperature for 24 h. Samples were then washed with 100 mm sodium phosphate, pH 7.0, three to five times to remove the background stain. GUS-positive samples were examined with a Nikon Microphot-FX compound microscope equipped with DIC optics, and images of roots were captured using a Nikon DXM 1200 camera.
Pollen Microscopy
For staining of pollen grains with toluidine blue, anthers were collected from 8-week-old plants and fixed, dehydrated, and resin embedded as described by Orfila et al. (2001). Sections were stained with toluidine blue as described previously (Manfield et al., 2004; Bernal et al., 2007) and observed using a light microscope (BH2; Olympus; http://www.olympusglobal.com), and images were collected using a 36-bit digital camera (Coolsnap; Media Cybernetics; http://www.mediacy.com).
Time-Lapse Microscopy of Living Root Hairs
Coverslips containing 3- to 4-d-old seedlings were transferred directly onto the stage of an inverted Nikon TE300 compound microscope equipped with DIC optics. Images of growing root hairs were captured every 1 min over a 2-h time period using a Hamamatsu C2400-75i camera running on Metamorph 6.3 image-acquisition software (Molecular Devices).
Transmission Electron Microscopy
Three-day-old wild-type and csld2-1 and csld3-2 seedlings were fixed in 2.5% glutaraldehyde and postfixed in 1% OsO4 for 2 h at room temperature. Roots were dehydrated through a graded ethanol series and embedded in Spurr's epoxy resin (Electron Microscopy Science). Root hair longitudinal (oblique to the main root axis) sections were cut with an Ultracut E microtome (Reichert-Jung). Ultrathin sections (90–100 nm) were mounted onto 200-mesh copper grids and stained with 1% aqueous uranyl acetate and Reynolds lead citrate solution. The sections were examined and photographed at 80 kV with a Philips CM12 transmission electron microscope. All of the photographs were converted to digital images with a ScanMaker 8700 scanner (Microtek).
In Vitro Pollen Germination and Transmission Assays
Pollen germination assays were carried out using the procedures described by Boavida and McCormick (2007) with slight modifications. Anthers were extracted from flowers of 7- to 10-week-old plants using fine forceps. Pollen grains were evenly spread over a microscope slide containing pollen germination medium (Taylor et al., 1998) using a dissecting microscope. Slides were placed in a tip box with approximately 30 mL of water in the bottom and incubated at 25°C under constant light for 6 to 7 h. Pollen germination efficiency was assessed by counting germinated grains using a light microscope (BH2; Olympus). Images were collected using a digital camera. For the transmission analyses, all crosses were performed by emasculating flowers from 7- to 10-week-old plants and fertilizing with pollen from 7- to 13-week-old plants at 1 d after the emasculation. Seeds were allowed to set; they were carefully collected and grown directly in soil at 19°C with a 16-h photoperiod. BASTA was applied to seedlings from the crosses at 3 and 4 weeks after germination at a concentration of 250 mg L−1.
Cloning Procedures
All complementation experiments were carried out by transformation of the genomic fragments of CSLD2 and CSLD4, including the putative promoter region. The CSLD2 clone was generated in two steps. One fragment was amplified from bacterial artificial chromosome F20K13 (Arabidopsis Biological Resource Center) using oligonucleotides that annealed 1,950 bp upstream from the CSLD2 start codon (5′-GAGTTTTGGTTCGGGAATGA-3′) and 1,185 bp downstream of the stop codon (5′-TCACGTACCTGCACCCATAA-3′). This product was digested with EcoRI and BamHI, and the resulting fragment was cloned in the corresponding sites in pCAMBIA3300. The second fragment was amplified using oligonucleotides that annealed 1,950 bp upstream of the CSLD2 start codon (5′-GAGTTTTGGTTCGGGAATGA-3′) and 2,049 bp inside the predicted CSLD2 open reading frame (5′-HindIII-TCGCAGAAGCTCTGACTAAGG-3′). This was cloned into pGEM Teasy (Promega). The HindIII fragment from this clone was subcloned into the HindIII site from the plasmid generated in the first step. The orientation of the resulting clone was verified by restriction digestion and sequencing.
The CSLD4 clone for complementation was generated by amplification from the bacterial artificial chromosome F20D10 using oligonucleotides that annealed 1,573 bp upstream of the predicted CSLD4 start codon (5′-SalI-ACGCTGCGTTTCTTTCATT-3′) and 402 bp downstream of the stop codon (5′-SalI-CACGGAGGAGGAAGACAGAC-3′). The resulting fragment was cloned into pGEM Teasy and subcloned into the SalI site of pCAMBIA2300.
For subcellular localizations, recombinant proteins with fluorescent tags were constructed as follows. The genomic region of AtCSLD2 was amplified with primers 5′-ATGGCATCTAATAAGCATTTTG-3′ and 5′-TCATGGAAAACTGAAGTTTCC-3′. The genomic region of CSLD4 was amplified with primers 5′-ATGGCGTCCACGCCT-3′ and 5′-TTACGGGAATTGAAAACCAC-3′. The amplicons were obtained from genomic Arabidopsis DNA using Phusion polymerase (Finnzymes). The resulting PCR products were purified by the QIAquick PCR Purification Kit (Qiagen) and subsequently functioned as templates in the following USER clonings (Nour-Eldin et al., 2006). YFP-CSLD2 and YFP-CSLD4 were obtained by USER cloning of CSLD2 and CSLD4 into the unique USER site in pCAMBIA330035SuYFP in-frame with the downstream YFP (Bernal et al., 2007). In the case of CSLD2, primers 5′-GGCTTAAUATGGCATCTAATAAGCATTTTG-3′ and 5′-GGTTTAAUTCATGGAAAACTGAAGTTTCC-3′ were used, and in the case of AtCSLD4, primers 5′-GGCTTAAUATGGCGTCCACGCCT-3′ and 5′-GGTTTAAUTTACGGGAATTGAAAACCAC-3′ were used. Underlined oligonucleotides were engineered to provide USER cloning sites and in such a way that the reading frames of CSLD2 and CSLD4 were in frame with the upstream YFP of the N-terminal fusion vector (Nour-Eldin et al., 2006). The cloned PCR products and vector-insert junctions were subsequently verified by sequencing.
For the expression studies, the promoters of CSLD2 and CSLD3 were PCR amplified from genomic DNA using the primers 5′-AGTTAGGATCTAACTTGGC-3′ and 5′-ATCTGAAAACCTGAGACTGAG-3′ (CSLD2) and 5′-TGTCTAATAATAACACTATCCAC-3′ and 5′-GGTGGGAATACAAACCATG-3′ (CSLD3) using Phusion polymerase (Finnzymes). The resulting PCR products were subsequently cloned in pBGF-0 (Chytilova et al., 1999) using the USER method (Nour-Eldin et al., 2006) and the primers 5′-GGTTTAAUAGTTAGGATCTAACTTGGC-3′ and 5′-GGCTTAAUATCTGAAAACCTGAGACTGAG-3′ (CSLD2) and 5′-GGTTTAAUTGTCTAATAATAACACTATCCAC-3′ and 5′-GGCTTAAUGGTGGGAATACAAACCATG-3′ (CSLD3), resulting in proCSLD2∷GFP∷GUS and proCSLD3∷GFP∷GUS, respectively. Inserts and insert-vector junctions were subsequently verified by sequencing.
Plant Transformation
For complementation experiments, flowers from 7-week-old csld2-1 and csld4-1 plants were vacuum infiltrated with suspensions of the A. tumefaciens strain GV3101 bearing constructs containing the corresponding genomic fragment, including the promoter region, using previously described procedures (Clough and Bent, 1998). csld2-1 plants were transformed with pCAMBIA3300 bearing the genomic fragment of CSLD2. Transformants were selected for BASTA resistance and verified by PCR. csld4-1 plants were transformed with pCAMBIA2300 bearing the genomic fragment of CSLD4. Transformants were selected for kanamycin resistance and verified by PCR. Transformations for promoter studies were carried out in a similar manner, except that the plasmid pBGF-0 and the A. tumefaciens strain C58C1 pGV3850 were employed.
Subcellular Localization of CSLD2 and CSLD4
The vectors containing YFP-CSLD2 and YFP-CSLD4 were transformed into A. tumefaciens C58C1 pGV3850. The resulting strains were grown overnight in Luria-Bertani medium, harvested by centrifugation, resuspended in a buffer containing 10 mm MES, 10 mm MgCl2, and 100 μm acetosyringone (optical density at 600 nm = 0.05), and allowed to stand at room temperature for 2 to 3 h. Strains carrying the CSLD2 and CSLD4 fusions were mixed with a strain harboring the 35S:p19 construct for suppression of gene silencing (Voinnet et al., 2003) and with the Golgi marker STtmd-GFP (Boevink et al., 1998) or the endoplasmic reticulum marker GFP-HDEL (Boevink et al., 1996), where indicated. The bacterial mix was infiltrated into leaves of 2- to 4-week-old Nicotiana benthamiana plants. For comparison, similar experiments were done in parallel using YFP-CSLD3, which has been characterized previously (Bernal et al., 2007). Infiltrated leaves were observed at 24 to 36 h after infiltration by confocal laser-scanning microscopy (TCS SP2; Leica Microsystems). The GFP and YFP fluorescent signals were monitored by sequential line scanning (switching between detection of GFP and YFP signal) with eight line scans per line. The excitation and detection wavelengths for GFP and YFP were 488 and 514 nm for excitation, and 495 to 510 nm and 565 to 593 nm for detection, respectively.
Imaging Cytoskeletal Organization in Living Root Hairs
csld2-1 and csld3-2 mutants were crossed with wild-type Arabidopsis plants expressing GFP that binds to microtubules and F-actin. For microtubule labeling, mutants were crossed with plants expressing GFP fused to the microtubule-binding domain (MBD) of the MICROTUBULE-ASSOCIATED PROTEIN4 (Marc et al., 1998). For F-actin labeling, mutants were crossed with plants expressing GFP fused to both the N and C termini of the ACTIN-BINDING DOMAIN2 (ABD2) of Arabidopsis FIMBRIN1 (GFP-ABD2-GFP; Wang et al., 2008). Root hairs of 3-d-old seedlings expressing the fusion proteins were imaged using a Leica TCS SP2 AOBS confocal laser-scanning microscope. GFP was excited using the 488-nm line of the argon laser, and emission was detected at 510 nm.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. In silico expression profiling of the CSLD family.
Supplemental Figure S2. RT-PCR analysis of the CSLD family.
Supplemental Figure S3. Analysis of promoter activity for CSLD2 and CSLD3.
Supplemental Figure S4. Generation of CSLD knockout lines.
Supplemental Figure S5. Functional complementation of csld3-2 with YFP-CSLD3.
Supplemental Movie S1. Time-lapse microscopy of living csld2-1 root hairs.
Supplemental Movie S2. Time-lapse microscopy of living csld2-1 root hairs.
Supplemental Movie S3. Time-lapse microscopy of living csld2-1 root hairs.
Supplemental Movie S4. Time-lapse microscopy of living csld3-2 root hairs.
Supplemental Movie S5. Time-lapse microscopy of living csld3-2 root hairs.
Supplementary Material
Acknowledgments
We thank Paul Dupree for insightful comments. Dr. Chris Hawes is thanked for the clones for STtmd-GFP and GFP-HDEL. Thanks to Sonia Gutierrez and Nadia Morales for help with growing plants and PCRs.
This work was supported by the Danish Research Agency (grants to A.J.B., J.K.J., I.S., H.V.S., and W.G.T.W.), the National Science Foundation (grant no. DBI–0400580 to E.B.B.), and the Samuel Roberts Noble Foundation (grants to C.-M.Y. and E.B.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: William G.T. Willats (willats@bio.ku.dk).
The online version of this article contains Web-only data.
References
- Bacic A, Harris PJ, Stone BA (1988) Structure and function of plant cell walls. In J Preiss, ed, The Biochemistry of Plants, Vol 14. Academic Press, New York, pp 297–371
- Baumberger N, Ringli C, Keller B (2001) The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev 15 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal AJ, Jensen JK, Harholt J, Sørensen S, Moller I, Blaukopf C, Johansen B, de Lotto R, Pauly M, Schelle HV, et al (2007) Disruption of ATCSLD5 results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. Plant J 52 791–802 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH (1995) Growth and microtubule orientation of Zea mays roots subjected to osmotic stress. Int J Plant Sci 156 774–783 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hou G, Chapman KD (2003) Elevated levels of N-lauroethanolamine, an endogenous constituent of desiccated seeds, disrupt normal root development in Arabidopsis thaliana seedlings. Planta 217 206–217 [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52 570–582 [DOI] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15 441–447 [DOI] [PubMed] [Google Scholar]
- Boevink P, Santa Cruz S, Hawes C, Harris N, Oparka KJ (1996) Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J 10 935–941 [Google Scholar]
- Böhme K, Li Y, Charlot F, Grierson C, Marrocco K, Okada K, Laloue M, Nogué F (2004) The Arabidopsis COW1 gene encodes a phosphatidylinositol transfer protein essential for root hair tip growth. Plant J 40 686–698 [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Rayon C, Urbanowicz B, Tine MAS, Carpita NC (2004) Mixed linkage (1-3),(1-4)-β-D-glucans of grasses. Cereal Chem 81 115–127 [Google Scholar]
- Burk DH, Ye ZH (2002) Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell 14 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, Bacic A, Fincher GB (2008) The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiol 146: 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science 311 1940–1942 [DOI] [PubMed] [Google Scholar]
- Carol RJ, Dolan L (2002) Building a hair: tip growth in Arabidopsis thaliana root hairs. Philos Trans R Soc Lond B Biol Sci 357 815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3 1–30 [DOI] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, et al (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Chen H, Zhang Y, Zhang Z, Zheng N, Yin B, Yan H, Zhu L, Zhao X, Yuan M, et al (2007) Knockout of the AtCESA2 gene affects microtubule orientation and causes abnormal cell expansion in Arabidopsis. Plant Physiol 143 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytilova E, Macas J, Galbraith DW (1999) Green fluorescent protein targeted to the nucleus, a transgenic phenotype useful for studies in plant biology. Ann Bot (Lond) 83 645–654 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cocuron JC, Lerouxel O, Drakakaki G, Alonso AP, Liepman AH, Keegstra K, Raikhel N, Wilkerson CG (2007) A gene from the cellulose synthase-like C family encodes a beta-1,4 glucan synthase. Proc Natl Acad Sci USA 104 8550–8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RA, Fowler JE (2006) Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol 9 579–588 [DOI] [PubMed] [Google Scholar]
- Cosgrove D (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6 850–861 [DOI] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Höfte H, Gonneau M, Vernhettes S (2007) Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 104 15572–15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazebroek J, Randhawa GS, Dolan M, Kinney AJ, Tomes D, Nichols S, et al (2004) Guar seed beta-mannan synthase is a member of the cellulose synthase super gene family. Science 303 363–366 [DOI] [PubMed] [Google Scholar]
- Doblin MS, De Melis L, Newbigin E, Bacic A, Read SM (2001) Pollen tubes of Nicotiana alata express two genes from different beta-glucan synthase families. Plant Physiol 125 2040–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley TP, Hester S, Shadforth IP, Runions J, Weimar T, Hanton SL, Griffin JL, Bessant C, Brandizzi F, Hawes C, et al (2006) Mapping the Arabidopsis organelle proteome. Proc Natl Acad Sci USA 103 6518–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emons AMC, Hofte H, Mulder BM (2007) Microtubules and cellulose microfibrils: How intimate is their relationship? Trends Plant Sci 12 279–281 [DOI] [PubMed] [Google Scholar]
- Favery B, Ryan E, Foreman F, Linstead P, Boudonck K, Steer M, Shaw P, Dolan L (2001) KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev 15 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161 641–675 [DOI] [PubMed] [Google Scholar]
- Goubet F, Misrahi A, Park SK, Zhang Z, Twell D, Dupree P (2003) AtCSLA7, a cellulose synthase-like putative glycosyltransferase, is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol 131 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson CS, Roberts K, Feldmann KA, Dolan L (1997) The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol 115 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena AH, Greenwood JS, Dengler NG (2004) Programmed cell death remodels lace plant leaf shape during development. Plant Cell 16 60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17 R541–R542 [DOI] [PubMed] [Google Scholar]
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315 804–807 [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5 R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K, Walton J (2006) Beta-glucans: brewer's bane, dietician's delight. Science 311 1872–1873 [DOI] [PubMed] [Google Scholar]
- Kim CM, Park SH, Je BI, Park SH, Park SJ, Piao HL, Eun MY, Dolan L, Han CD (2007) OsCSLD1, a cellulose synthase-like D1 gene, is required for root hair morphogenesis in rice. Plant Physiol 143 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Triplett BA (2001) Cotton fiber growth in planta and in vitro: models for plant cell elongation and cell wall biogenesis. Plant Physiol 127 1361–1366 [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Wilkerson CG, Keegstra K (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, Gilmartin PM, Mikkelsen JD, Knox JP, Willats WG (2004) Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant J 40 260–275 [DOI] [PubMed] [Google Scholar]
- Marc J, Granger CL, Brincat J, Fisher DD, Kao TH, McCubbin AG, Cyr RJ (1998) A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10 1927–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W (2001) Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol 47 239–274 [PubMed] [Google Scholar]
- Nour-Eldin HH, Hansen BG, Norholm MHH, Jensen JK, Halkier BA (2006) Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res 34 e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M, Albersheim P, Darvill A (1990)The pectic polysaccharides of primary cell walls. In PM Dey, ed, Methods in Plant Biochemistry, Vol 2. Academic Press, London, pp 415–441
- Orfila C, Seymour GB, Willats WG, Huxham IM, Jarvis MC, Dover CJ, Thompson AJ, Knox JP (2001) Altered middle lamella homogalacturonan and disrupted deposition of (1-5)-α-L-arabinan in the pericarp of Cnr, a ripening mutant of tomato. Plant Physiol 126 210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Persson S, Ehrhardt DW, Somerville CR (2008) Genetic evidence that cellulose synthase activity influences microtubule cortical arrays activity. Plant Physiol 147: 1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312 1491–1495 [DOI] [PubMed] [Google Scholar]
- Parton RM, Dyer AF, Read ND, Trewava AJ (2000) Apical structure of actively growing fern rhizoids examined by DIC and confocal microscopy. Ann Bot (Lond) 85 233–245 [Google Scholar]
- Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, Hahn MG, Mohnen D, Somerville C (2007) The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell 19 237–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter WD (2002) Biosynthesis and properties of the plant cell wall. Curr Opin Plant Biol 5 536–542 [DOI] [PubMed] [Google Scholar]
- Reyna-Villasmil N, Bermúdez-Pirela V, Mengual-Moreno E, Arias N, Cano-Ponce C, Leal-Gonzalez E, Souki A, Inglett GE, Israili ZH, Hernández-Hernández R, et al (2007) Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am J Ther 14 203–212 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Somerville CR (1998) Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J 15 79–88 [DOI] [PubMed] [Google Scholar]
- Richmond TA, Somerville CR (2000) The cellulose synthase superfamily. Plant Physiol 124 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TA, Somerville CR (2001) Integrative approaches to determining Csl function. Plant Mol Biol 47 131–143 [PubMed] [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57 929–967 [DOI] [PubMed] [Google Scholar]
- Roberts AW, Bushoven JT (2007) The cellulose synthase (CESA) gene superfamily of the moss Physcomitrella patens. Plant Mol Biol 63 207–219 [DOI] [PubMed] [Google Scholar]
- Samuga A, Joshi CP (2004) Cloning and characterization of cellulose synthase-like gene, PtrCSLD2 from developing xylem of aspen trees. Physiol Plant 120 631–641 [DOI] [PubMed] [Google Scholar]
- Sangwan V, Foulds I, Singh J, Dhindsa RS (2001) Cold-induction of Brassica napus gene, BN115, is mediated by structural changes in the membrane and cytoskeleton and requires Ca2+ influx. Plant J 27 1–2 [DOI] [PubMed] [Google Scholar]
- Scheible WR, Pauly M (2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol 7 285–295 [DOI] [PubMed] [Google Scholar]
- Sørensen I, Pettolino FA, Wilson SM, Doblin MS, Johansen B, Bacic A, Willats WGT (2008) Mixed linkage (1→3),(1→4)-β-D-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. Plant J 54: 510–521 [DOI] [PubMed] [Google Scholar]
- Sterling JD, Atmodjo MA, Inwood SE, Kumar Kolli VS, Quigley HF, Hahn MG, Mohnen D (2006) Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proc Natl Acad Sci USA 103 5236–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Himmelspach R, Williamson RE, Wasteneys GO (2003) Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15 1414–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR (2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci USA 100 1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PE, Glover JA, Lavithis M, Craig S, Singh MB, Knox RB, Dennis ES, Chaudhury AM (1998) Genetic control of male fertility in Arabidopsis thaliana: structural analyses of postmeiotic developmental mutants. Planta 205 492–505 [DOI] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33 949–56 [DOI] [PubMed] [Google Scholar]
- Wang X, Cnops G, Vanderhaeghen R, De Block S, Van Montagu M, Van Lijsebettens M (2001) AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol 126 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YS, Yoo CM, Blancaflor EB (2008) Improved imaging of actin filaments in transgenic Arabidopsis plants expressing a green fluorescent protein fusion to the C and N terminus of the fimbrin actin binding domain 2. New Phytol 177 525–536 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO (2004) Progress in understanding the role of microtubules in plant cells. Curr Opin Plant Biol 7 651–660 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Knox JP, Mikkelsen JD (2006) Pectin: new insights into an old polymer are starting to gel. Trends Food Sci Technol 17 97–104 [Google Scholar]
- Wojtaszek P, Baluška F, Kasprowicz A, Luczak M, Volkmann D (2007) Mechanosensory transmission of cell wall disturbances to the actin cytoskeleton in maize root apex cells. Protoplasma 230 217–230 [DOI] [PubMed] [Google Scholar]
- Zeng W, Keegstra K (2008) AtCSLD2 is an integral Golgi membrane protein with its N-terminus facing the cytosol. Planta 228: 823–838 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Nam J, Carpita NC, Matthysse AG, Gelvin SB (2003) Agrobacterium-mediated root transformation is inhibited by mutation of an Arabidopsis cellulose synthase-like gene. Plant Physiol 133 1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.