Abstract
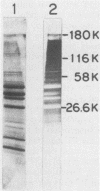
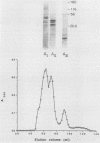
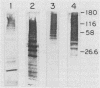
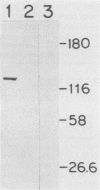
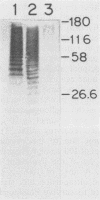
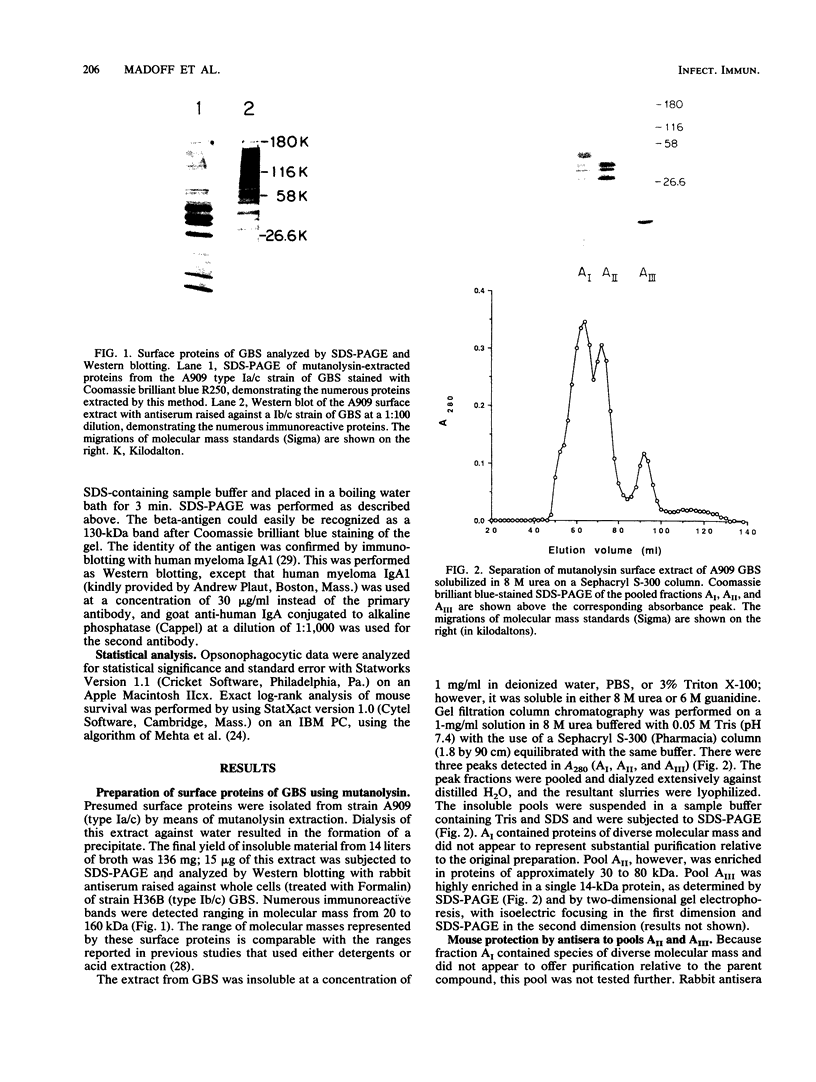
Group B streptococci (GBS) are the leading causes of neonatal sepsis and meningitis in the United States, with a high rate of fatality and serious morbidity despite appropriate therapy. The C-protein antigens of GBS appear to be important in immunity to experimental infection, yet these antigens remain incompletely characterized with respect to their number, structure, and function. None of these proteins has yet been purified to homogeneity. We have developed a novel method for extraction of surface proteins from the A909 (Ia/c) strain of GBS by using mutanolysin. Antibodies raised in rabbits against these partially purified proteins conferred passive protection to lethal GBS infection in mice challenged with a GBS strain expressing C proteins with a heterologous capsule type. In addition, mouse monoclonal antibodies were produced and identified by reactivity with the mutanolysin-extracted proteins. One of these monoclonal antibodies (4G8) identifies an epitope on the alpha-antigen of the GBS C proteins (identified by protease susceptibility and mouse protection). On sodium dodecyl sulfate-polyacrylamide gels, this epitope appears as a series of regularly spaced bands ranging in apparent molecular mass from 160,000 to 30,000 Da. The monoclonal antibody 4G8 induces opsonic killing of GBS and protects mice from lethal challenge with GBS. Thus, the 4G8 monoclonal antibody identifies a fully protective epitope on the C-protein alpha-antigen of GBS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J. Group B streptococcal infections. Adv Intern Med. 1980;25:475–501. [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Vecchitto J. Mouse protection test for group B Streptococcus type III. J Infect Dis. 1979 Jul;140(1):81–88. doi: 10.1093/infdis/140.1.81. [DOI] [PubMed] [Google Scholar]
- Bevanger L. Ibc proteins as serotype markers of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1983 Aug;91(4):231–234. doi: 10.1111/j.1699-0463.1983.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Bevanger L., Iversen J. The Ibc protein fraction of group B streptococci: characterization of protein antigens extracted by HCL. Acta Pathol Microbiol Scand B. 1981 Aug;89(4):205–209. doi: 10.1111/j.1699-0463.1981.tb00177_89b.x. [DOI] [PubMed] [Google Scholar]
- Bevanger L., Maeland J. A. Complete and incomplete Ibc protein fraction in group B streptococci. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):51–54. doi: 10.1111/j.1699-0463.1979.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Bevanger L., Naess A. I. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):121–124. doi: 10.1111/j.1699-0463.1985.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Daphtary U. D., Ayoub E. M., Boyle M. D. Two novel antigens associated with group B streptococci identified by a rapid two-stage radioimmunoassay. J Infect Dis. 1988 Nov;158(5):965–972. doi: 10.1093/infdis/158.5.965. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Yother J., McDaniel L. S. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S372–S374. doi: 10.1093/cid/10.supplement_2.s372. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Calandra G. B., Cole R. M. Lysis and protoplast formation of group B streptococci by mutanolysin. Infect Immun. 1980 Jun;28(3):1033–1037. doi: 10.1128/iai.28.3.1033-1037.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleat P. H., Timmis K. N. Cloning and expression in Escherichia coli of the Ibc protein genes of group B streptococci: binding of human immunoglobulin A to the beta antigen. Infect Immun. 1987 May;55(5):1151–1155. doi: 10.1128/iai.55.5.1151-1155.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Johansson I., Lundgren H. Chromatographic properties of Sephacryl S-300 Superfine. J Biochem Biophys Methods. 1979;1(1):37–44. doi: 10.1016/0165-022x(79)90044-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984 Apr;19(4):506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L. Bacterial capsule--old dogmas and new tricks. J Infect Dis. 1986 Mar;153(3):407–415. doi: 10.1093/infdis/153.3.407. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén V. Mouse-protective effect of rabbit anti-R-protein antibodies against group B streptococci type II carrying R-protein. Lack of effect on type III carrying R-protein. Acta Pathol Microbiol Immunol Scand B. 1983 Apr;91(2):145–151. doi: 10.1111/j.1699-0463.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Yother J., Vijayakumar M., McGarry L., Guild W. R., Briles D. E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med. 1987 Feb 1;165(2):381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta C. R., Patel N. R., Tsiatis A. A. Exact significance testing to establish treatment equivalence with ordered categorical data. Biometrics. 1984 Sep;40(3):819–825. [PubMed] [Google Scholar]
- Payne N. R., Ferrieri P. The relation of the Ibc protein antigen to the opsonization differences between strains of type II group B streptococci. J Infect Dis. 1985 Apr;151(4):672–681. doi: 10.1093/infdis/151.4.672. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Kim Y. K., Ferrieri P. Effect of differences in antibody and complement requirements on phagocytic uptake and intracellular killing of "c" protein-positive and -negative strains of type II group B streptococci. Infect Immun. 1987 May;55(5):1243–1251. doi: 10.1128/iai.55.5.1243-1251.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones G. J., Gotschlich E. C., Blake M. S. A surface receptor specific for human IgA on group B streptococci possessing the Ibc protein antigen. J Exp Med. 1984 Nov 1;160(5):1467–1475. doi: 10.1084/jem.160.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones G. J., Gotschlich E. C. Identification of protein antigens of group B streptococci, with special reference to the Ibc antigens. J Exp Med. 1984 Nov 1;160(5):1476–1484. doi: 10.1084/jem.160.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen M. V., Kasper D. L., Levy N. J. Isolation of a C (Ibc) protein from group B Streptococcus which elicits mouse protective antibody. Microb Pathog. 1986 Apr;1(2):191–204. doi: 10.1016/0882-4010(86)90021-5. [DOI] [PubMed] [Google Scholar]
- Walsh J. A., Hutchins S. Group B streptococcal disease: its importance in the developing world and prospect for prevention with vaccines. Pediatr Infect Dis J. 1989 May;8(5):271–277. [PubMed] [Google Scholar]
- Wilkinson H. W., Eagon R. G. Type-specific antigens of group B type Ic streptococci. Infect Immun. 1971 Nov;4(5):596–604. doi: 10.1128/iai.4.5.596-604.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K., Mattingly S. J. Biosynthesis of cell wall peptidoglycan and polysaccharide antigens by protoplasts of type III group B Streptococcus. J Bacteriol. 1983 Apr;154(1):211–220. doi: 10.1128/jb.154.1.211-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]