Abstract
Oligogalacturonides (OGs) are endogenous elicitors of defense responses released after partial degradation of pectin in the plant cell wall. We have previously shown that, in Arabidopsis (Arabidopsis thaliana), OGs induce the expression of PHYTOALEXIN DEFICIENT3 (PAD3) and increase resistance to the necrotrophic fungal pathogen Botrytis cinerea independently of signaling pathways mediated by jasmonate, salicylic acid, and ethylene. Here, we illustrate that the rapid induction of the expression of a variety of genes by OGs is also independent of salicylic acid, ethylene, and jasmonate. OGs elicit a robust extracellular oxidative burst that is generated by the NADPH oxidase AtrbohD. This burst is not required for the expression of OG-responsive genes or for OG-induced resistance to B. cinerea, whereas callose accumulation requires a functional AtrbohD. OG-induced resistance to B. cinerea is also unaffected in powdery mildew resistant4, despite the fact that callose accumulation was almost abolished in this mutant. These results indicate that the OG-induced oxidative burst is not required for the activation of defense responses effective against B. cinerea, leaving open the question of the role of reactive oxygen species in elicitor-mediated defense.
Plants need to recognize invading pathogens in a timely manner to mount appropriate defense responses. Specific molecules associated with different microbial pathogens can be perceived by plant cells at early stages of infection and trigger inducible defenses that include phytoalexin accumulation, expression of pathogenesis-related proteins, production of reactive oxygen species (ROS), and, at least in some cases, programmed cell death. Many of these molecules, traditionally called general elicitors, are secreted or are present on the surface of all strains of a given microbial taxonomic group and activate defense responses effective against a wide range of pathogens (Nurnberger et al., 2004). For this reason, they are also referred to as microbe-associated molecular patterns or pathogen-associated molecular patterns (PAMPs; Parker, 2003; He et al., 2007). PAMPs (for review, see Nurnberger and Brunner, 2002) are often structural components of the pathogen cell wall (e.g. chitin, glucan) or other macromolecular structures (e.g. bacterial flagellin).
Hahn and colleagues (1981) first showed that structural components of the plant cell wall, released during pathogen infection as a consequence of microbial enzymatic activities, can also induce defense responses. In particular, oligogalacturonides (OGs) with a degree of polymerization (DP) between 10 and 15 can accumulate when fungal polygalacturonases (PGs) degrade the homogalacturonan component of plant pectin (Hahn et al., 1981). OGs elicit a variety of defense responses, including accumulation of phytoalexins (Davis et al., 1986), glucanase, and chitinase (Davis and Hahlbrock, 1987; Broekaert and Pneumas, 1988). Exogenous treatment with OGs protects grapevine (Vitis vinifera) and Arabidopsis (Arabidopsis thaliana) leaves against infection with the necrotrophic fungus Botrytis cinerea (Aziz et al., 2004; Ferrari et al., 2007), suggesting that production of this elicitor at the site of infection, where large amounts of PGs are secreted by the fungus, may contribute to activate defenses responses. For these reasons, OGs can be considered as danger signals derived from an altered self (host-associated molecular patterns).
A prominent feature of the plant defense response is the oxidative burst, a common early response of plant cells to pathogen attack and elicitor treatment (Lamb and Dixon, 1997). ROS such as superoxide anion (O2−) and hydrogen peroxide (H2O2) are toxic intermediates resulting from reduction of molecular O2. ROS are important signals for defense responses and phytoalexin accumulation in several species. It is generally thought that ROS contribute to plant resistance by directly exerting a cytotoxic effect against pathogens, by participating in cell wall reinforcement (cross-linking of structural protein and lignin polymers), or by inducing hypersensitive cell death, expression of defense genes, or the accumulation of antimicrobial compounds (Levine et al., 1994). Generation of ROS can be induced by a variety of elicitors (Apostol et al., 1989; Legendre et al., 1993; Bolwell et al., 2002; Aziz et al., 2003; Kasparovsky et al., 2004; Pauw et al., 2004; Xu et al., 2005) and in many plant systems ROS production is biphasic (e.g. Dorey et al., 1999; Yoshioka et al., 2001).
O2−-generating NADPH oxidases are generally considered to be a major enzymatic source of ROS in the oxidative burst of plant cells challenged with pathogens or elicitors (Torres and Dangl, 2005; Torres et al., 2006). Two different NADPH oxidase genes in potato (Solanum tuberosum) are responsible for the elicitor-induced biphasic oxidative burst (Yoshioka et al., 2001). In Arabidopsis, several genes encoding proteins with high similarity to the mammalian NADPH oxidase gp91phox subunit have been characterized. Among them, AtrbohD is required for the production of ROS during infection with different bacterial and fungal pathogens, including B. cinerea (Torres et al., 2002, 2005). Besides NADPH oxidases, other enzymes appear to be important in the elicitor-mediated oxidative burst, including apoplastic oxidases, such as oxalate oxidase (Dumas et al., 1993), amine oxidase (Allan and Fluhr, 1997), and pH-dependent apoplastic peroxidases (Bolwell et al., 1995; Frahry and Schopfer, 1998), which generate either O2− or H2O2.
We have recently shown that OGs and an unrelated elicitor, the synthetic 22-amino acid peptide flg22 derived from bacterial flagellin (Felix et al., 1999), activate defense responses against B. cinerea both in wild-type Arabidopsis and in mutants impaired in salicylic acid (SA), jasmonate (JA)-, or ethylene (ET)-mediated signaling (Ferrari et al., 2007). Elicitor-induced protection against B. cinerea requires the PHYTOALEXIN DEFICIENT3 (PAD3) gene (Ferrari et al., 2007). PAD3 encodes the cytochrome P450 CYP71B15, which catalyzes the last step of the biosynthesis of the phytoalexin camalexin (Schuhegger et al., 2006). Camalexin is known to contribute to Arabidopsis basal resistance to B. cinerea (Ferrari et al., 2003a; Kliebenstein et al., 2005). Notably, the expression of PAD3, as well as that of another defense-related gene, AtPGIP1, which encodes a PG-inhibiting protein effective against B. cinerea, is induced by OGs independently of SA-, JA-, and ET-mediated signaling (Ferrari et al., 2003b, 2007). It is therefore likely that multiple defense responses are induced by OGs independently of SA, ET, and JA.
Transient accumulation of extracellular H2O2 was previously observed in tobacco (Nicotiana tabacum) leaf explants and grapevine cells treated with OGs (Bellincampi et al., 1996; Aziz et al., 2004). Because PAD3 expression and camalexin accumulation can be induced by chemicals that generate oxidative stress (Zhao et al., 1998; Denby et al., 2005), we have investigated the hypothesis that H2O2 mediates the induction of defense responses effective against B. cinerea in Arabidopsis plants treated with OGs. Here, we show that OGs induce an oxidative burst in Arabidopsis that is AtrbohD-dependent; however, we also show that H2O2-dependent responses are not required for OG-induced resistance against B. cinerea.
RESULTS
Early Activation of Genes in Response to General Elicitors Is Independent of SA, ET, and JA Signaling
To establish the degree of specificity of early gene expression in response to OGs and other general elicitors, we monitored the expression of AtPGIP1, PAD3, and several other early elicitor-induced genes (Ferrari et al., 2007; Denoux et al., 2008) in response to a pool of OGs with a DP between 10 and 15 (hereafter referred to as OGs), to purified oligodecagalacturonic acid (DP10), to flg22, and to a β-glucan elicitor from Phytophthora megasperma f. sp. Glya (Cheong et al., 1991). In addition to AtPGIP1 and PAD3, we tested the expression of AtWRKY40 (At1g80840), encoding a transcription factor that acts as a negative regulator of basal defense (Xu et al., 2006; Shen et al., 2007); CYP81F2 (At5g57220), encoding a cytochrome P450 with unknown function; and RetOx (At1g26380), encoding a protein with homology to reticuline oxidases, a class of enzymes involved in secondary metabolism and in defense against pathogens (Dittrich and Kutchan, 1991; Carter and Thornburg, 2004). These genes were selected because they are rapidly and strongly up-regulated upon exposure to elicitors, as previously demonstrated by whole-genome transcript profiling and real-time quantitative PCR analyses (Ferrari et al., 2007; Denoux et al., 2008). As negative controls, we treated seedlings with water or α-1,4-trigalacturonic acid (DP3; Hahn et al., 1981; Cervone et al., 1989; Bellincampi et al., 2000; Navazio et al., 2002).
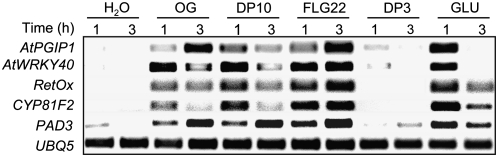
As shown in Figure 1, OGs, DP10, flg22, and β-glucan activated the expression of all tested genes in Arabidopsis seedlings, whereas water and DP3 failed to induce the expression of any of the genes analyzed. The expression of PAD3, RetOx, CYP81F2, AtWRKY40, and AtPGIP1 was also compared across a set of 322 publicly available Arabidopsis microarray datasets using the Arabidopsis Coexpression Tool (Manfield et al., 2006). The Pearson correlation coefficient between PAD3 and RetOx expression was the highest (r = 0.78) among the tested genes (Supplemental Fig. S1), followed by RetOx and CYP81F2 (r = 0.71). The AtWRKY40 expression pattern appeared to correlate moderately with that of PAD3 and RetOx (r = 0.58 in both cases), whereas no significant correlation between AtPGIP1 and any of the other genes was observed, suggesting that the expression of this gene is regulated differently from that of PAD3, RetOx, and CYP81F2. Despite the fact that AtPGIP1 does not significantly correlate with any other analyzed gene, it was included in subsequent analyses because of its established role in plant defense (Ferrari et al., 2003b, 2006). Transient expression of PAD3, RetOx, CYP81F2, and AtWRKY40 was also observed in rosette leaves infiltrated with OGs (Supplemental Fig. S2) with kinetics comparable to those occurring in seedlings, indicating that these genes can be considered markers of early elicitor-induced responses both in seedlings and in adult plants.
Figure 1.
Expression analysis of marker genes in response to elicitors. Arabidopsis seedlings were treated at the indicated time (h) with water (H2O), OGs, purified oligodecagalacturonic acid (DP10), flg22, trigalacturonic acid (DP3), or β-glucan (GLU). Expression of the indicated genes was analyzed by semiquantitative RT-PCR, using the UBQ5 gene as internal standard. This experiment was repeated twice with similar results.
To determine whether RetOx, CYP81F2, and AtWRKY40 are expressed after elicitor treatment independently of SA, ET, or JA, as previously shown for AtPGIP1 and PAD3 (Ferrari et al., 2003b, 2007), we analyzed their expression in the npr1ein2jar1 (nej) genetic background harboring mutations in the NON-PR1 EXPRESSOR1 (NPR1; Cao et al., 1997), JASMONATE RESISTANT1 (JAR1; Staswick et al., 1992), and ETHYLENE INSENSITIVE2 (EIN2; Guzman and Ecker, 1990) genes, and therefore impaired in all three signaling pathways (Clarke et al., 2000). No major difference in expression of RetOx, CYP81F2, and AtWRKY40 was observed, either in terms of kinetics of induction or in transcript levels, in wild-type or nej plants treated with OGs (Fig. 2, A–C), or in npr1, ein2, and jar1 single mutants (Supplemental Fig. S3A). Expression of AtPGIP1, that was previously shown to be independent of JAR1, EIN2 or NPR1, based on data obtained with single mutants (Ferrari et al., 2003b), was also unaffected in the triple mutant (Fig. 2D).
Figure 2.
Expression of elicitor-responsive genes in the nej triple mutant. Arabidopsis wild-type (white bars) or nej triple mutant (black bars) seedlings were treated with water (control) or OGs for 1, 3, or 6 h. Expression of RetOx (A), CYP81F2 (B), AtWRKY40 (C), and AtPGIP1 (D) was analyzed by real-time quantitative PCR and normalized using the expression of the UBQ5 gene. Bars indicate average expression ± sd of three replicates. This experiment was repeated three times with similar results.
Because some reports have suggested that the jar1-1 mutation is leaky (Staswick et al., 1998; Kariola et al., 2003), we also analyzed the coronatine insensitive1 (coi1) mutant, which is severely impaired in JA-mediated responses (Xie et al., 1998). Induction of RetOx and CYP81F2 by OGs in wild-type and coi1 seedlings was indistinguishable, whereas AtWRKY40 expression was slightly reduced in coi1 (Supplemental Fig. S3B), in accordance with a previous report indicating that AtWRKY40 gene can be induced by JA in a COI1-dependent manner (Wang et al., 2008). Similarly, to further rule out an effect of SA on OG-induced gene expression, we treated sid2-2 seedlings, which carry a mutation in the isochorismate synthase gene ICS1 required for pathogen-activated biosynthesis of SA (Wildermuth et al., 2001). Also, in this case, no significant reduction of OG-induced gene expression was observed compared to the wild type (Supplemental Fig. S3C). These results indicate that expression of the OG-induced marker genes tested is independent of SA, ET, and JA.
Production of H2O2, But Not Gene Expression, in Response to OGs Is Mediated by AtrbohD
Analysis of the publicly available expression data using Genevestigator (https://www.genevestigator.ethz.ch) indicates that PAD3, RetOx, AtWRKY40, and CYP81F2 transcript levels increase after treatment with H2O2, suggesting that their expression may be mediated by ROS (data not shown). Transient accumulation of extracellular H2O2 was previously observed in tobacco leaf explants and grapevine cells treated with OGs (Bellincampi et al., 1996; Aziz et al., 2004). To investigate whether OGs are also able to induce an apoplastic oxidative burst in Arabidopsis, we measured the release of H2O2 in the culture medium of seedlings treated with these elicitors. A significant oxidative burst was observed in response to OGs and DP10, whereas H2O2 accumulated to a much smaller extent in response to flg22, β-glucan, or DP3 (Fig. 3A).
Figure 3.
Oxidative burst and gene expression in response to elicitors. A, Arabidopsis seedlings were treated with water (H2O), OGs alone, or in the presence of catalase (OG + CAT), purified oligodecagalacturonic acid (DP10), flg22 (FLG), trigalacturonic acid (DP3), or β-glucan (GLU). H2O2 accumulation in the culture medium, expressed as μm g−1 fresh weight, was measured after 1 (white bars) or 3 h (black bars). This experiment was repeated twice with similar results. B, Arabidopsis seedlings were treated for 1 or 3 h with water (H2O) or with OGs alone or in presence of catalase (OG + CAT). Expression of the indicated genes was analyzed by semiquantitative RT-PCR, using the UBQ5 gene as internal standard. This experiment was repeated twice with similar results.
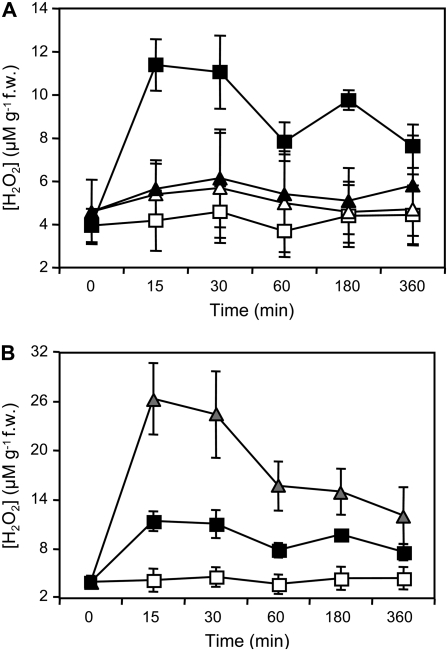
We then investigated the source of H2O2 generated after treatment with OGs. Previous reports suggest that the oxidative burst observed after inoculation with virulent and avirulent pathogens is generated in Arabidopsis by the NADPH oxidase AtrbohD (Torres et al., 2005). To determine whether this enzyme is also the source of the extracellular burst observed in response to OGs, we analyzed an Arabidopsis knockout (KO) line containing a T-DNA insertion in the AtrbohD gene (Torres et al., 2002). This line failed to accumulate extracellular H2O2 after elicitation (Fig. 4A), indicating that AtrbohD is necessary for the OG-induced oxidative burst.
Figure 4.
Accumulation of extracellular H2O2 in response to OGs or G/GO in Arabidopsis seedlings. A, Arabidopsis wild-type and atrbohD seedlings were treated with water (H2O) or OGs for the indicated time (min). Arabidopsis wild-type (squares) and atrbohD (triangles) seedlings were treated with water (white symbols) or OGs (black symbols). B, Arabidopsis seedlings were treated with water (H2O, white squares), OGs (black squares), or G/GO (gray triangles). H2O2 accumulation in the culture medium, expressed as μm g−1 fresh weight, was measured at the indicated times (min). Values are means of three samples ± sd.
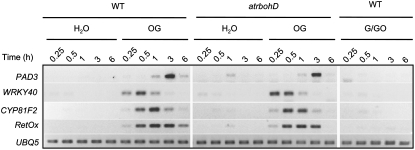
To determine the role of the oxidative burst in OG-triggered early gene expression, we analyzed the expression of PAD3, RetOx, CYP81F2, and AtWRKY40 in elicited wild-type and atrbohD mutant seedlings. Strikingly, despite the absence of a functional AtrbohD gene and of an oxidative burst, no significant differences in the mRNA levels of all tested marker genes could be detected (Fig. 5). Similar results were obtained in wild-type and atrbohD adult plants infiltrated with OGs (Supplemental Fig. S2). To conclusively rule out a role of NADPH oxidases in OG-induced marker gene expression, before application of OGs, we treated seedlings with diphenylene iodonium (DPI), which, at low concentrations, specifically inhibits this class of enzymes (Bolwell et al., 1995; Frahry and Schopfer, 1998). DPI completely blocked the OG-induced oxidative burst (Fig. 6A), but had no effect on the expression of PAD3, RetOx, CYP81F2, and AtWRKY40 (Fig. 6B), confirming that NADPH oxidases are not required for early OG-induced transcriptional changes.
Figure 5.
Effects of endogenous and exogenous H2O2 on OG-responsive genes. Arabidopsis wild-type and atrbohD seedlings were treated with water (H2O) or OGs for the indicated time (h). Wild-type seedlings were also treated with G/GO. Expression of the indicated genes was analyzed by semiquantitative RT-PCR, using the UBQ5 gene as internal standard. This experiment was repeated twice with similar results.
Figure 6.
Effect of DPI on the expression of elicitor-responsive genes. A, Arabidopsis seedlings were treated with water (control, white bars), OGs (light gray bars), DPI (dark gray bars), or OGs + DPI (black bars). H2O2 accumulation in the culture medium, expressed as μm g−1 fresh weight, was measured at the indicated times (min). Values are means of three samples ± sd. Asterisks indicate statistically significant differences between control and OG-treated seedlings, according to Student's t test (*, P < 0.05; ***, P < 0.01). The experiment was repeated twice with similar results. B, Arabidopsis seedlings were treated with DPI, OGs alone, or in the presence of DPI (OG + DPI) for the indicated time (min). Gene expression was analyzed by semiquantitative RT-PCR, using the UBQ5 gene as internal standard. This experiment was repeated twice with similar results.
To conclusively demonstrate that extracellular H2O2 is not involved in OG-induced gene expression, we elicited Arabidopsis seedlings in the presence of catalase at a concentration that almost completely abolished the oxidative burst (Fig. 3A). Coincubation of OGs with catalase had no significant effect on the expression of PAD3, AtPGIP1, RetOx, CYP81F2, and AtWRKY40 (Fig. 3B), confirming that H2O2 is not required for OG-induced marker gene expression. Furthermore, treatment of seedlings with Glc and Glc oxidase (G/GO) at concentrations that induced H2O2 levels in the same order of magnitude observed after OG treatments (Fig. 4B), failed to induce the expression of the same set of genes (Fig. 5).
Taken together, our results indicate that OG-mediated early gene expression is independent of the extracellular oxidative burst.
Basal and OG-Induced Resistance to B. cinerea Infection Are Independent of AtrbohD and of PMR4/GSL5
To determine whether defense responses that occur relatively late after treatment with OGs are also independent of H2O2, we analyzed callose deposition and induced resistance in wild-type and atrbohD KO plants. Callose is a high-Mr β-1,3-glucan deposited at the site of infection by pathogens, probably acting as a physical barrier against colonization of the intercellular space (Ryals et al., 1996; Donofrio and Delaney, 2001). It was previously shown that flg22 induces callose deposition in Arabidopsis seedlings (Gomez-Gomez et al., 1999) and that callose accumulation induced by flg22 is impaired in leaf strips of atrbohD KO plants (Zhang et al., 2007). Similarly, infiltration of OGs in wild-type rosette leaves resulted in a significant accumulation of callose (Denoux et al., 2008), which was reduced of about 50% in atrbohD leaves (Fig. 7A), indicating that the oxidative burst contributes to callose synthesis also in response to OGs. As expected, infiltration of leaves of the powdery mildew resistant4 (pmr4) mutant, which has a mutation in the callose synthase gene GLUCAN SYNTHASE-LIKE5 (GSL5; Nishimura et al., 2003), resulted in a dramatic decrease of callose deposition (Fig. 7B).
Figure 7.
Callose accumulation in atrbohD and pmr4 plants. Arabidopsis wild-type and atrbohD (A) or pmr4 (B) leaves were infiltrated with water (control, left) or OGs (right) for 24 h and stained with aniline blue for callose visualization. The number below each image indicates the average number of callose deposits ± se of eight different leaf samples from at least five independent plants (three microscopic fields of 0.1 mm2 for each leaf). Images show representative leaves for each treatment. All images are at the same scale; scale bar = 1 mm (10× magnification). This experiment was repeated twice with similar results.
We have previously observed that OGs induce protection of Arabidopsis plants against B. cinerea and that this protection requires PAD3 expression (Ferrari et al., 2007). To determine the role of AtrbohD in induced resistance, we treated wild-type, atrbohD, and, as a negative control, pad3 plants with OGs, and subsequently inoculated them with B. cinerea. As expected, pad3 plants showed increased basal susceptibility, and OG pretreatment did not reduce lesion development (Fig. 8A). In contrast, no significant difference in basal susceptibility and in OG-induced resistance between wild-type and atrbohD plants was observed either in detached leaves (Fig. 8A) or in intact plants (Fig. 9). This indicates that OG-induced activation of defense responses effective against B. cinerea does not require AtrbohD.
Figure 8.
OG-induced resistance to B. cinerea is independent of AtrbohD and PMR4. A, Arabidopsis Col-0 (wild type), atrbohD, and pad3 plants were treated with a control solution (white bars) or OGs (black bars) and inoculated with B. cinerea 24 h after treatment. B, Arabidopsis Col-0 (wild type) and pmr4 plants were treated with a control solution (white bars) or OGs (black bars) and inoculated with B. cinerea 24 h after treatment. Lesion areas were measured 48 h after inoculation. Values are means ± se of at least 14 lesions. Asterisks indicate statistically significant differences between control and OG-treated plants, according to Student's t test (*, P < 0.05; ***, P < 0.01). Numbers above bars represent the average reduction of lesion size (%) of OG-treated plants with respect to control-treated plants. The experiments were repeated at least twice with similar results.
Figure 9.
Basal and OG-induced resistance to B. cinerea in whole plants. Arabidopsis Col-0 (wild type) and atrbohD plants were treated with a control solution (white bars) or OGs (black bars) and leaves were inoculated with B. cinerea 24 h after treatment. Lesion areas were measured 48 h after inoculation. Values are means ± se of at least 12 lesions. Asterisks indicate statistically significant differences between control and OG-treated plants, according to Student's t test (***, P < 0.01). Numbers above bars represent the average reduction of lesion size (%) of OG-treated plants with respect to control-treated plants. The experiments were repeated at least twice with similar results.
Furthermore, we investigated the role of callose in OG-elicited resistance to B. cinerea. As shown in Figure 8B, lesion development in pmr4 plants inoculated with B. cinerea was unaffected or, in some experiments, slightly reduced, compared to wild-type plants. Moreover, OG treatment of the pmr4 mutant resulted in protection against B. cinerea infection (Fig. 8B), indicating that callose does not play a major role in either basal or elicitor-induced resistance against this pathogen.
Finally, we infiltrated adult rosette leaves with G/GO at concentrations that in seedlings induced production of H2O2 levels in the same order of magnitude observed after OG treatments. G/GO caused significant accumulation of H2O2 in infiltrated tissues (Fig. 10A), but did not alter basal resistance to B. cinerea (Fig. 10B). These data indicate that a moderate extracellular oxidative burst, comparable to that observed after OG treatment, is not sufficient to induce defense responses effective against B. cinerea.
Figure 10.
Basal resistance to B. cinerea after treatment with G/GO. A, Arabidopsis wild-type plants were infiltrated with Glc (G) alone or G/GO and, 24 h after treatment, stained with 3,3′-diaminobenzidine (DAB) for in vivo H2O2 visualization. B, Arabidopsis wild-type plants were infiltrated with Glc or G/GO and inoculated with B. cinerea 24 h after treatment. Lesion areas were measured 48 h after inoculation. Values are means ± se of at least 12 lesions. No statistically significant differences between Glc- and G/GO-treated plants were observed, according to Student's t test (P > 0.7).
DISCUSSION
One of the earliest responses observed in plants inoculated with a pathogen or treated with an elicitor is the oxidative burst, characterized by a rapid and transient production of ROS. OGs induce a strong extracellular oxidative burst, initially suggesting that ROS might play an important role in mediating responses to OGs. We therefore adopted both pharmacological and genetic approaches to investigate both the genesis and the role of the oxidative burst elicited by OGs in Arabidopsis plants.
There are a number of potential sources of ROS generated upon pathogen or elicitor perception. Increasing evidence points to superoxide-generating NADPH oxidases as the main sources of extracellular ROS produced during pathogen infection or elicitation (Yoshioka et al., 2001, 2003; Torres et al., 2002; Kobayashi et al., 2006; Nuhse et al., 2007). O2− generated by NADPH oxidases is rapidly dismutated into H2O2, which is much more stable and can accumulate in tissues. Extracellular H2O2 can also be generated by other sources, most notably apoplastic peroxidases (Bolwell et al., 2002), making it sometimes difficult to discern the involvement of specific sources of ROS in the oxidative burst. The data presented here clearly indicate that the NADPH oxidase AtrbohD is necessary for the extracellular burst induced in Arabidopsis by OGs, as previously shown for flg22 (Nuhse et al., 2007). H2O2 produced after OG treatment is therefore likely released by dismutation of O2− directly generated by AtrbohD in accordance with the observation that OGs induce the accumulation of O2− in Arabidopsis leaves (Song et al., 2006). In addition to the extracellular oxidative burst, protoplastic sources of ROS emanating from mitochondrial, chloroplastic, or peroxisomal generating systems have also been documented (Bolwell et al., 2002). However, intracellular generation of ROS has mainly been studied in relation to abiotic stress (Asada, 1999; del Río et al., 2002). There are reports of intracellular accumulation of ROS in response to elicitors, such as cryptogein (Ashtamker et al. 2007), although its role in plant defense response has not been assessed.
OGs activate a very strong extracellular oxidative burst; surprisingly, however, this burst has a minor, if any, role in several downstream responses, based on the following evidence: (1) under our experimental conditions, there is significantly less H2O2 accumulation in response to flg22 and β-glucan than in response to OGs, but the effect of flg22 and β-glucan on the expression of early molecular marker genes is comparable to that observed with OGs; (2) H2O2 generated by G/GO at levels comparable to those observed in OG-treated plants fails to activate the expression of elicitor-activated marker genes or to induce resistance to B. cinerea; (3) scavenging of H2O2 accumulation by catalase or inhibition of the OG-induced oxidative burst either by DPI or by the atrbohD mutation did not affect early gene expression. Taken together, these results indicate that early changes in gene expression activated by OGs independently of SA, ET, and JA do not require the oxidative burst generated by AtrbohD. Furthermore, OG-triggered resistance against B. cinerea, which is also independent of SA, ET, and JA, occurs in the absence of AtrbohD.
In contrast to OGs, flg22 and β-glucan elicited very low levels of H2O2 under our experimental conditions. An extracellular oxidative burst, peaking at about 10 to 15 min, was previously observed using a H2O2-dependent luminescence assay in Arabidopsis leaf explants treated with 1 μm flg22 (Gomez-Gomez et al., 1999). It is possible that the xylenol orange-based system used here is not sensitive enough to detect the burst induced by flg22, although previous work indicates the equivalence of this xylenol orange and the luminescence assays (Bindschedler et al., 2001). It is possible that the different levels of H2O2 that we observed after treatment with OGs or flg22 could be ascribed to different concentrations of the elicitors. However, at the doses used in this work, flg22 induced the expression of marker genes to levels comparable to OGs, indicating that the gene-activation response does not directly correlate to H2O2 accumulation. The observation that catalase, DPI treatments, or the atrbohD mutation block the oxidative burst, but have no significant impact on the expression of the early marker genes, confirms that the induction of these genes is uncoupled to ROS production.
The fact that none of the analyzed marker genes changed expression in response to H2O2 generated by G/GO was unexpected. Previous work showed that PAD3 expression and camalexin accumulation can be up-regulated by ROS-generating chemicals (Zhao et al., 1998; Denby et al., 2005) and the expression of CYP81F2, RetOx, and AtWRKY40 has been shown to be induced by millimolar concentrations of H2O2 (Davletova et al., 2005). However, the concentration of H2O2 measured in our experiments with G/GO was in the same order of magnitude as the concentration measured after elicitation with OGs (in the range of 10–30 μm g−1 fresh weight), which is comparable to the concentrations measured in leaves of different plant species under natural conditions (Cheeseman, 2006). This suggests that the relatively high concentrations of H2O2 used in previous expression analyses might be nonphysiological. Similarly, basal resistance to B. cinerea was not affected by treatment with G/GO at the same concentrations used in the seedling experiments. This result is apparently in contrast with a previous report indicating that G/GO infiltration of Arabidopsis leaves increases susceptibility to this pathogen (Govrin and Levine, 2000). However, the concentration of GO used by Govrin and Levine was 104-fold higher than in our work, suggesting that only very high levels of H2O2, which are not normally induced by elicitors, can affect basal resistance to B. cinerea.
Whereas OG-induced early gene expression and protection against B. cinerea occur independently of AtrbohD, callose accumulation is reduced in atrbohD KO plants. A similar result was obtained in atrbohD leaf strips treated with flg22 (Zhang et al., 2007). Callose deposition is required for β-amino butyric acid-induced resistance against the necrotrophic fungi Alternaria brassicicola and Plectosphaerella cucumerina (Ton and Mauch-Mani, 2004). Our observation that induced resistance to B. cinerea is unaffected in atrbohD plants, despite a reduction in callose accumulation, suggests that callose contributes only marginally to restrict B. cinerea in Arabidopsis. This hypothesis is confirmed by the observation that both basal and OG-induced resistance against B. cinerea are not impaired in the pmr4 mutant, which accumulates very little callose.
Besides callose accumulation, other responses induced by OGs and other elicitors may be dependent on the oxidative burst. Previous reports suggest the existence of both oxidative burst-dependent and independent signaling pathways linking elicitor perception to downstream responses. Treatment of parsley (Petroselinum crispum) cells with DPI blocked both Pep-13-induced phytoalexin production and accumulation of transcripts encoding enzymes involved in their synthesis. In contrast, DPI had no effect on Pep-13-induced PR gene expression (Kroj et al., 2003). In grapevine, the expression of six out of nine defense-related genes responsive to OGs is blocked by DPI (Aziz et al., 2004), and in Arabidopsis Landsberg erecta seedlings treated with OGs, DPI blocks the expression of several defense genes (Hu et al., 2004). It is possible that the activation of a subset of late, secondary responses to elicitors is dependent, or at least is amplified by the earlier production of ROS.
CONCLUSION
In this work, we investigated the role of the extracellular oxidative burst in the induction of early and late responses to OGs in Arabidopsis plants. Our results indicate that OGs induce a transient, but robust, production of H2O2 that is dependent on the NADPH oxidase AtrbohD. This oxidative burst does not have a major role in the induction of several early OG-responsive marker genes and in the induced protection against B. cinerea. It was previously observed that early gene expression, in contrast to callose deposition, in response to the bacterial PAMP flg22, is independent of AtrbohD (Zhang et al., 2007). Here, we show that OGs, which are host-associated molecular patterns of a completely different chemical nature, behave in a similar fashion. However, we have demonstrated that defense responses that require the oxidative burst, such as callose deposition, are not involved in OG-induced resistance to B. cinerea. In contrast, flg22-induced resistance against Pseudomonas syringae infection is dependent on the NADPH oxidase AtrbohD (Zhang et al., 2007). Taken together, these results indicate that the signaling pathway activated by elicitors bifurcates: activation of one branch requires the oxidative burst and is important against bacterial pathogens, whereas the oxidative burst-independent branch regulates defense responses effective against necrotroph fungi.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) Columbia-0 (Col-0) wild-type seeds were purchased from Lehle Seeds. pad3-1 (Glazebrook and Ausubel, 1994) and eds16-1/sid2-2 (Wildermuth et al., 2001) mutant lines were previously described. Seeds of ein2-1 and jar1-1 were obtained from the Arabidopsis Biological Resource Center. The npr1-1 line and the triple mutant nej were a kind gift from Xinnian Dong (Duke University). Heterozygous coi1-1/COI1-1 seeds were a kind gift from John Turner (University of East Anglia). The atrbohD KO line was kindly provided by Jonathan G.D. Jones (Sainsbury Laboratory, John Innes Centre). The pmr4-1 mutant line was kindly provided by Shauna C. Somerville (Carnegie Institution). All mutant lines used in this work are in the Col-0 background.
Growth Conditions and Plant Treatments
Plants were grown on a 3:1 mixture of soil (Einheitserde) and sand (Compo Agricoltura) at 22°C and 70% relative humidity under a 16-h light/8-h dark cycle (approximately 120 μmol m−2 s−1). For OG treatments, leaves from 4-week-old plants were infiltrated with water or 200 μg mL−1 OGs using a needleless syringe and harvested at the indicated times. Generation of H2O2 in adult plants was obtained by infiltrating rosette leaves of 4-week-old plants with 0.25 mm Glc and 0.01 unit mL−1 Glc oxidase (Sigma). As a negative control, plants were infiltrated with 0.25 mm Glc alone.
For seedling treatments, seeds were surface sterilized and germinated in multiwell plates (approximately 10 seeds/well) containing 1 mL per well of Murashige and Skoog medium (Sigma; Murashige and Skoog, 1962) supplemented with 0.5% Suc. Plates were incubated at 22°C with a 12-h light/12-h dark cycle and a light intensity of 120 μmol m−2 s−1. After 8 d, the medium was replaced and treatments were performed after two additional days. For treatment of coi1 seedlings, heterozygous coi1/COI1 seeds were first germinated on agar plates containing 30 μm methyl jasmonate, and, after 8 d of growth, homozygous JA-resistant seedlings were transferred to liquid Murashige and Skoog medium and treated with OGs 2 d later. As a control, wild-type seedlings were grown for 8 d on agar plates and then transferred to liquid Murashige and Skoog medium.
OG pools with an average DP of 10 to 15 (OGs) and purified decagalacturonic acid (DP 10) were kindly prepared by Gianni Salvi (Università di Roma “La Sapienza”) as previously described (Bellincampi et al., 2000). Trigalacturonic acid (DP3) was purchased from Sigma. Matrix-assisted laser desorption/ionization time-of-flight MS was used to verify the DP of OG preparations. Phytophthora megasperma f. sp. Glya β-glucan elicitor was a kind gift of Michael G. Hahn (Complex Carbohydrate Research Center, University of Georgia). The flg22 peptide was synthesized by Maria Eugenia Schininà (Università di Roma “La Sapienza”). Lyophilized elicitors or chemicals were dissolved in double-distilled water and added to the culture medium at the following final concentrations: 100 μg mL−1 (approximately 54 μm) OG; 52 μg mL−1 (approximately 54 μm) DP10; 29 μg mL−1 (approximately 54 μm) DP3, 1 μm flg22 and 50 μg mL−1 β-glucan. H2O2 was removed from the culture medium by adding bovine catalase (Sigma) at the same time as OG at a final concentration of 600 units mL−1. Generation of H2O2 was obtained by adding 0.25 mm Glc and 0.01 unit mL−1 Glc oxidase (Sigma) to the culture medium. DPI was prepared as a 1 mm stock in 20% dimethyl sulfoxide. DPI was added to the seedling growth medium at a final concentration of 10 μm, 15 min before OG treatment. As a control, dimethyl sulfoxide was added to the medium at a final concentration of 0.2%.
Botrytis cinerea growth and protection assays on detached leaves were performed as previously described (Ferrari et al., 2007). Infection of intact plants was performed by inoculating about three leaves per plant (at least four plants per genotype) with two 5-μl droplets of a B. cinerea spore suspension. Plants were subsequently covered with a plastic dome to keep humidity high as previously described (Ferrari et al., 2003a).
Determination of H2O2
The H2O2 concentration in the incubation medium of treated seedlings (about 100–120 mg in 1 mL of medium) was measured by the FOX1 method (Jiang et al., 1990), based on the peroxide-mediated oxidation of Fe2+, followed by the reaction of Fe3+ with xylenol orange dye (o-cresolsulfonephthalein 3′,3″-bis[methylimino] diacetic acid, sodium salt; Sigma). This method is extremely sensitive and used to measure low levels of water-soluble H2O2 present in the aqueous phase. To determine H2O2 concentration, 500 μL of the incubation medium were added to 500 μL of assay reagent (500 μm ammonium ferrous sulfate, 50 mm H2SO4, 200 μm xylenol orange, and 200 mm sorbitol). Absorbance of the Fe3+-xylenol orange complex (A560) was detected after 45 min of incubation. The specificity for H2O2 was tested by eliminating H2O2 in the reaction mixture with catalase. Standard curves of H2O2 were obtained for each independent experiment. Data were normalized and expressed as micromolar H2O2/g fresh weight of seedlings.
For in vivo H2O2 visualization, leaves were cut from infiltrated adult plants using a razor blade and dipped for 12 h in a solution containing 1 mg mL−1 of 3,3′-diaminobenzidine-HCl, pH 5.0. Chlorophyll was extracted for 10 min with boiling ethanol and for 2 h with ethanol at room temperature prior to photography (Orozco-Cardenas and Ryan, 1999).
Gene Expression Analysis
Treated seedlings or leaves were frozen in liquid nitrogen, homogenized with a mortar and pestle, and total RNA was extracted with Tri-Reagent (Sigma) according to the manufacturer's protocol. RNA was treated with RQ1 DNase (Promega) and first-strand cDNA was synthesized using ImProm-II reverse transcriptase (Promega) according to the manufacturer's instructions. Real-time quantitative PCR analysis was performed using an I-Cycler (Bio-Rad). Two microliters of a 1:5 dilution of cDNA (corresponding to 20 ng of total RNA) were amplified in a 30-μL reaction mix containing 1× IQ SYBR Green Supermix (Bio-Rad) and 0.4 μm of each primer. Expression levels of each gene, relative to UBQ5, were determined using a modification of the Pfaffl method (Pfaffl, 2001) as previously described (Ferrari et al., 2006). Semiquantitative reverse transcription (RT)-PCR analysis was performed in a 50-μL reaction mix containing 1 μL of cDNA, 1× buffer (Bioline), 3 mm MgCl2, 100 μm of each dNTP, 0.5 μm of each specific primer, and 1 unit Taq DNA Polymerase (Bioline). Twenty-five, 30, and 35 PCR cycles were performed for each primer pair to verify linearity of the amplification. Primer sequences are shown in Supplemental Table S1. PCR products were separated by agarose gel electrophoresis and stained with ethidium bromide.
Pearson correlation coefficients between the expression pattern of selected genes in 322 Affymetrix ATH1 microarray datasets obtained from different Arabidopsis tissues and after different treatments and available in the Genomic Arabidopsis Resource Network/Nottingham Arabidopsis Stock Centre microarray database (Craigon et al., 2004) and scatter plots of the correlation coefficient values were obtained using the Arabidopsis Coexpression Tool (http://www.arabidopsis.leeds.ac.uk/act/index.php; Manfield et al., 2006). The scatter plot allows users to visualize the correlation of all probe sets against two selected probe sets simultaneously. Every probe set is plotted on a scatter graph, where the two axes are the Pearson correlation coefficients against two different query probe sets. Analysis of the expression of single genes in publicly available microarray experiments was performed using Genevestigator (https://www.genevestigator.ethz.ch; Zimmermann et al., 2004).
Callose Deposition
Leaves from 4-week-old plants were infiltrated with water or 200 μg mL−1 OGs using a needleless syringe. After 24 h, for each treatment, about eight leaves, from at least five independent plants, were cleared and dehydrated with 100% ethanol. Leaves were fixed in an acetic acid:ethanol (1:3) solution for 2 h, sequentially incubated for 15 min in 75% ethanol, in 50% ethanol, and in 150 mm phosphate buffer, pH 8.0, and then stained for 1 h at 25°C in 150 mm phosphate buffer, pH 8.0, containing 0.01% (w/v) aniline blue. After staining, leaves were mounted in 50% glycerol and examined by UV epifluorescence using an Axioskop 2 plus microscope (Zeiss). Images were taken with a ProgRes C10 3.3 MegaPixel digital color camera (Jenoptik). Callose quantification was performed by using ImageJ software.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Co-correlation between expression pattern of PAD3 and RetOx.
Supplemental Figure S2. Expression of elicitor-responsive genes in adult Arabidopsis wild-type and atrbohD plants.
Supplemental Figure S3. Expression of selected marker genes in mutants impaired in SA, JA, and ET signaling.
Supplemental Table S1. Primers used in this article.
Supplementary Material
Acknowledgments
We would like to thank Massimiliano Sassi (Istituto di Biologia e Patologia Molecolari, Consiglio Nazionale delle Ricerche, Rome) for technical assistance with the epifluorescence microscope, Daniela Pontiggia (Università di Roma “La Sapienza,” Rome) for MS analysis of elicitor preparations, and Gianni Salvi (Università di Roma “La Sapienza,” Rome) for purification of DP10.
This work was supported by the Ministero dell'Università e della Ricerca (grant no. PRIN2006), by the European Union (grant no. 23044 [“Nutra-Snacks”] to S.F.), by the Ministero dell'Università e della Ricerca (grant no. PRIN 2005) and ERA-NET Plant Genomics (grant no. RBER063SN4) to G.D.L., and by the National Institutes of Health (grant no. GM48707) and the National Science Foundation (grant no. DBI–0114783) to F.M.A.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Simone Ferrari (simone.ferrari@uniroma1.it).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9 1559–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I, Heinstein PF, Low PS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiol 90 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50 601–639 [DOI] [PubMed] [Google Scholar]
- Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R (2007) Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol 143 1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz A, Heyraud A, Lambert B (2004) Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 218 767–774 [DOI] [PubMed] [Google Scholar]
- Aziz A, Poinssot B, Daire X, Adrian M, Bezier A, Lambert B, Joubert JM, Pugin A (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant Microbe Interact 16 1118–1128 [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Cardarelli M, Zaghi D, Serino G, Salvi G, Gatz C, Cervone F, Altamura MM, Costantino P, De Lorenzo G (1996) Oligogalacturonides prevent rhizogenesis in rolB-transformed tobacco explants by inhibiting auxin-induced expression of the rolB Gene. Plant Cell 8 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschedler LV, Minibayeva F, Gardber SL, Gerrish C, Davies DR, Bolwell GP (2001) Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol 151 185–194 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53 1367–1376 [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23 517–532 [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Pneumas WJ (1988) Pectic polysaccharides elicit chitinase accumulation in tobacco. Physiol Plant 74 740–744 [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 57–63 [DOI] [PubMed] [Google Scholar]
- Carter CJ, Thornburg RW (2004) Tobacco nectarin V is a flavin-containing berberine bridge enzyme-like protein with glucose oxidase activity. Plant Physiol 134 460–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone F, Hahn MG, De Lorenzo G, Darvill A, Albersheim P (1989) Host-pathogen interactions XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol 90 542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57 2435–2444 [DOI] [PubMed] [Google Scholar]
- Cheong JJ, Birberg W, Fugedi P, Pilotti A, Garegg PJ, Hong N, Ogawa T, Hahn MG (1991) Structure-activity relationships of oligo-beta-glucoside elicitors of phytoalexin accumulation in soybean. Plant Cell 3 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigon DJ, James N, Okyere J, Higgins J, Jotham J, May S (2004) NASCArrays: a repository for microarray data generated by NASC's transcriptomics service. Nucleic Acids Res 32 D575–D577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KR, Darvill AG, Albersheim P, Dell A (1986) Host-pathogen interactions: XXIX. Oligogalacturonides released from sodium polypectate by endopolygalacturonic acid lyase are elicitors of phytoalexins in soybean. Plant Physiol 80 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KR, Hahlbrock K (1987) Induction of defense responses in cultured parsley cells by plant cell wall fragments. Plant Physiol 85 1286–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu J, Mittler R (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Corpas FJ, Sandalio LM, Palma JM, Gómez M, Barroso JB (2002) Reactive oxygen species, antioxidant systems and nitric oxide in peroxisome. J Exp Bot 53 1255–1272 [PubMed] [Google Scholar]
- Denby KJ, Jason LJ, Murray SL, Last RL (2005) ups1, an Arabidopsis thaliana camalexin accumulation mutant defective in multiple defense signaling pathways. Plant J 41 673–684 [DOI] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck G, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J (2008) Activation of defense response pathways by OGs and flg22 elicitors in Arabidopsis seedlings. Mol Plant 1 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich H, Kutchan TM (1991) Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc Natl Acad Sci USA 88 9969–9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio NM, Delaney TP (2001) Abnormal callose response phenotype and hypersusceptibility to Peronospoara parasitica in defense-compromised Arabidopsis nim1-1 and salicylate hydroxylase-expressing plants. Mol Plant Microbe Interact 14 439–450 [DOI] [PubMed] [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S (1999) Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol 121 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas B, Sailland A, Cheviet JP, Freyssinet G, Pallett K (1993) Identification of barley oxalate oxidase as a germin-like protein. C R Acad Sci III 316 793–798 [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 265–276 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene or jasmonate signaling but requires PAD3. Plant Physiol 144 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Vairo D, Cervone F, De Lorenzo G (2006) Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol Plant Microbe Interact 19 931–936 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003. a) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35 193–205 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Vairo D, Ausubel FM, Cervone F, De Lorenzo G (2003. b) Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15 93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahry G, Schopfer P (1998) Inhibition of O2-reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry 48 223–227 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM (1994) Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA 91 8955–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Felix G, Boller T (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18 277–284 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 751–757 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MG, Darvill AG, Albersheim P (1981) Host-pathogen interactions: XIX. The endogenous elicitor, a fragment of plant cell wall polysaccharide that elicits phytoalexin accumulation in soy beans. Plant Physiol 68 1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Shan L, Sheen J (2007) Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol 9 1385–1396 [DOI] [PubMed] [Google Scholar]
- Hu XY, Neill SJ, Cai WM, Tang ZC (2004) Induction of defense gene expression by oligogalacturonic acid requires increases in both cytosolic calcium and hydrogen peroxide in Arabidopsis thaliana. Cell Res 14 234–240 [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Woollard AC, Wolff SP (1990) Hydrogen peroxide production during experimental protein glycation. FEBS Lett 268 69–71 [DOI] [PubMed] [Google Scholar]
- Kariola T, Palomaki TA, Brader G, Palva ET (2003) Erwinia carotovora subsp. carotovora and Erwinia-derived elicitors HrpN and PehA trigger distinct but interacting defense responses and cell death in Arabidopsis. Mol Plant Microbe Interact 16 179–187 [DOI] [PubMed] [Google Scholar]
- Kasparovsky T, Blein JP, Mikes V (2004) Ergosterol elicits oxidative burst in tobacco cells via phospholipase A2 and protein kinase C signal pathway. Plant Physiol Biochem 42 429–435 [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Rowe HC, Denby KJ (2005) Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J 44 25–36 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kawakita K, Maeshima M, Doke N, Yoshioka H (2006) Subcellular localization of Strboh proteins and NADPH-dependent O2−-generating activity in potato tuber tissues. J Exp Bot 57 1373–1379 [DOI] [PubMed] [Google Scholar]
- Kroj T, Rudd JJ, Nurnberger T, Gabler Y, Lee J, Scheel D (2003) Mitogen-activated protein kinases play an essential role in oxidative burst-independent expression of pathogenesis-related genes in parsley. J Biol Chem 278 2256–2264 [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48 251–275 [DOI] [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS (1993) Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol 102 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593 [DOI] [PubMed] [Google Scholar]
- Manfield IW, Jen CH, Pinney JW, Michalopoulos I, Bradford JR, Gilmartin PM, Westhead DR (2006) Arabidopsis Co-expression Tool (ACT): web server tools for microarray-based gene expression analysis. Nucleic Acids Res 34 W504–W509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) Revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15 437–479 [Google Scholar]
- Navazio L, Moscatiello R, Bellincampi D, Baldan B, Meggio F, Brini M, Bowler C, Mariani P (2002) The role of calcium in oligogalacturonide-activated signaling in soybean cells. Planta 215 596–605 [DOI] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301 969–972 [DOI] [PubMed] [Google Scholar]
- Nuhse TS, Bottrill AR, Jones AM, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger T, Brunner F (2002) Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol 5 318–324 [DOI] [PubMed] [Google Scholar]
- Nurnberger T, Brunner F, Kemmerling B, Piater L (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198 249–266 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JE (2003) Plant recognition of microbial patterns. Trends Plant Sci 8 245–247 [DOI] [PubMed] [Google Scholar]
- Pauw B, van Duijn B, Kijne JW, Memelink J (2004) Activation of the oxidative burst by yeast elicitor in Catharanthus roseus cells occurs independently of the activation of genes involved in alkaloid biosynthesis. Plant Mol Biol 55 797–805 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatos A, Halkier BA, Glawischnig E (2006) CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol 141 1248–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen QH, Saijo Y, Mauch S, Biskup C, Bieri S, Keller B, Seki H, Ulker B, Somssich IE, Schulze-Lefert P (2007) Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315 1098–1103 [DOI] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol 140 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15 747–54 [DOI] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38 119–130 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37 1130–1134 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cao G, Wang X, Miao J, Liu X, Chen Z, Qu LJ, Gu H (2008) Identification and characterization of COI1-dependent transcription factor genes involved in JA-mediated response to wounding in Arabidopsis plants. Plant Cell Rep 27 125–135 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Arabidopsis defense against pathogens requires salicylic acid synthesized via isochorismate synthase. Nature 414 562–565 [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hu X, Neill SJ, Fang J, Cai W (2005) Fungal elicitor induces singlet oxygen generation, ethylene release and saponin synthesis in cultured cells of Panax ginseng C. A. Meyer. Plant Cell Physiol 46 947–954 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Sugie K, Park HJ, Maeda H, Tsuda N, Kawakita K, Doke N (2001) Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol Plant Microbe Interact 14 725–736 [DOI] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1 175–185 [DOI] [PubMed] [Google Scholar]
- Zhao J, Williams CC, Last RL (1998) Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell 10 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.