Abstract
The effects of reductions in cell wall lignin content, manifested by RNA interference suppression of coumaroyl 3′-hydroxylase, on plant growth, water transport, gas exchange, and photosynthesis were evaluated in hybrid poplar trees (Populus alba × grandidentata). The growth characteristics of the reduced lignin trees were significantly impaired, resulting in smaller stems and reduced root biomass when compared to wild-type trees, as well as altered leaf morphology and architecture. The severe inhibition of cell wall lignification produced trees with a collapsed xylem phenotype, resulting in compromised vascular integrity, and displayed reduced hydraulic conductivity and a greater susceptibility to wall failure and cavitation. In the reduced lignin trees, photosynthetic carbon assimilation and stomatal conductance were also greatly reduced, however, shoot xylem pressure potential and carbon isotope discrimination were higher and water-use efficiency was lower, inconsistent with water stress. Reductions in assimilation rate could not be ascribed to increased stomatal limitation. Starch and soluble sugars analysis of leaves revealed that photosynthate was accumulating to high levels, suggesting that the trees with substantially reduced cell wall lignin were not carbon limited and that reductions in sink strength were, instead, limiting photosynthesis.
Lignin has been shown to be essential to cell wall integrity (Boyce et al., 2004). This complex macromolecule is assembled via the free radical coupling of monolignol precursors derived from p-hydroxycinnamyl alcohol, resulting in varying proportions of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) lignin subunits according to plant species, tissue type, and response to environmental stress (Campbell and Sederoff, 1996). The genes and enzymes responsible for the biosynthesis of this complex molecule have been extensively characterized (Humphreys and Chapple, 2002; Boerjan et al., 2003).
Lignified secondary cell walls provide significant compressive strength (up to 40 MPa) relative to nonlignified walls. Cells that have compromised lignin are more prone to cavitation than to collapse, as evidenced by the inherent rarity of tracheary element collapse in trees undergoing normal growth (Sperry, 2003). Changes in lignin content and monomer composition resulting from altered monolignol biosynthesis have been well documented in the literature (Dwivedi et al., 1994; Kajita et al., 1997; Lee et al., 1997; Sewalt et al., 1997; Piquemal et al., 1998; Franke et al., 2000; Chabannes and Barakate, 2001; Huntley et al., 2003; Abdulrazzak et al., 2006; Coleman et al., 2008), however, information describing the effect(s) of altered lignin quantity and structure on physiological state of trees and its effect on development is limited (Hancock et al., 2007). Numerous studies have demonstrated the effect(s) of reduced enzyme activity on monolignol biosynthesis and plant cell wall lignification, as well as the effects of changes in monolignol composition on plant morphology. However, few have investigated the effect of reduced lignin on the vasculature of plants (Anterola and Lewis, 2002). Reductions in Phe ammonia-lyase (PAL), cinnamic acid 4-hydroxylase, and coumaroyl 3′-hydroxylase (C3′H), three rate-limiting steps in lignification, have resulted in decreased lignin and alterations in the monomer (S:G:H) ratio (Elkind et al., 1990; Sewalt et al., 1997; Blount et al., 2000; Franke et al., 2002a; Coleman et al., 2008). In addition to the suppression of rate-limiting enzymes involved in lignification, misregulation of other key enzymes has led to similar effects (Li et al., 2003).
The irregular xylem phenotype associated with decreased lignification on water transport has been suggested to be the result of the plants' inability to produce cell walls that are strong enough to withstand the substantive xylem water tensions produced by transpiration, making the vessels susceptible to cavitation or collapse. This is especially true in trees, where the long distance transport of water can generate significant gradients in water potential. Despite a good understanding of the importance of xylem anatomy in water transport (Tyree and Zimmermann, 2002), the effects of altered lignin composition on xylem function have received very little attention. Earlier studies have attempted to correlate alterations in lignin biosynthesis with xylem anatomy. For example, Amrhein and Gödeke (1977), using l-α-aminooxy-β-phenylpropionic acid, an inhibitor of PAL, found that a reduction in PAL activity not only affected the production of soluble metabolites but caused a decrease in lignin biosynthesis, resulting in the collapse of xylem vessels. Similarly, Elkind et al. (1990), using sense and antisense inhibition of PAL, identified compromised vascular integrity in tobacco (Nicotiana tabacum) plants with reduced lignin. In addition, altered vascular morphology has been documented in the irregular xylem (irx4) Arabidopsis (Arabidopsis thaliana) mutants, which were shown to be defective in the cinnamoyl-CoA reductase (Jones et al., 2001).
One of the key biosynthetic steps in phenylpropanoid metabolism is the meta-hydroxylation step catalyzed by the CYP98A gene, as demonstrated by the phenotype of the Arabidopsis ref8 mutant, which was identified by the lack of sinapoylmalate in leaf tissues (Schoch et al., 2001; Franke et al., 2002b). Lignin analysis of the ref8 mutant showed a 20% to 40% reduction in lignin compared to wild-type cell walls, and a large shift in monomer composition (Franke et al., 2002a). A C3′H T-DNA insertion mutant was further shown to have similar reduced lignin properties, with only trace amounts of G- and S-derived lignin subunits in the stem, and also exhibited a drastic inhibition in plant development and inhibition of cell growth (Abdulrazzak et al., 2006). However, the roots were shown to be ectopically lignified, with a substantial amount of G and S lignin, suggesting a CYP98A3-independent pathway of 3′-hydroxylation in root tissue. The plants also showed a gradation of developmental changes and changes in lignin content and structure based on the extent of suppression of C3′H (Abdulrazzak et al., 2006).
RNA interference (RNAi) suppression of C3′H in hybrid poplar (Populus alba × grandidentata) yielded similar results, generating trees with reduced (up to 55%) lignin (Coleman et al., 2008). The most significantly suppressed C3′H trees also demonstrated altered S:G:H lignin monomer ratios, resulting from a reduction in the rate of G-monomers synthesis (S lignin remained relatively constant). The decrease in G monomers was compensated for by an increase in H units. Ectopic lignification of the shoots, with irregular patterning of lignin deposition throughout the stem cross section was evident, but lignification was most notably reduced around vessel elements.
In this investigation trees with the most dramatically reduced lignin composition were employed to evaluate the impact of altered lignin composition on tree physiology and growth. This study examines the C3′H-suppressed trees in terms of biomass production, gas exchange capabilities, water use efficiency (WUE), hydraulic conductivity, and function of the vascular system. Despite decreased hydraulic conductivity and susceptibility to collapse, water transport in support of photosynthesis is not the limiting factor influencing growth at this stage of development.
RESULTS
Growth and Morphology Are Decreased in C3′H-RNAi Trees
RNA transcript abundance, lignin content, and monolignol composition (Table I), as well as spatial and temporal collapse of xylem tissue of wild-type and C3′H-RNAi transgenic poplar were described previously (Coleman et al., 2008). Following harvest, trees were measured for changes in biomass and growth rates. The most highly C3′H RNAi-suppressed trees were consistently smaller than wild-type trees, demonstrating a reduction in total tree volume of the bole from 57.1 cm3 in wild type to 15.6 cm3 in the C3′H-RNAi trees (Table II). This decrease was due to a decrease in both height and diameter (as measured by caliper). Over a 4-month, greenhouse-grown assessment period, the average height growth was 167.7 cm in the wild-type trees and 129.1 cm in the C3′H-RNAi trees. Concurrently, diameter was 11.3 mm in wild type compared to 6.6 mm in C3′H-RNAi trees. In addition to total stem biomass, the transgenic poplar showed reductions in both leaf dry weight (DW; decreased by 44%) and total leaf area (decreased by 50%). Leaf mass per area was also significantly lower in the C3′H-RNAi trees (0.49 mg DW cm−2) relative to wild-type trees at 0.80 mg DW cm−2 (Fig. 1; Table II).
Table I.
Lignin content and monomer composition of C3′H-RNAi and wild-type hybrid poplar (from Coleman et al., 2008)
Bold denotes significance at P = 0.1.
| Tree Line | Lignin | H | G | S |
|---|---|---|---|---|
| % | % | % | % | |
| C3′H RNAi | 10.5 (0.19) | 16.9 (0.8) | 13.0 (0.5) | 70.1 (1.3) |
| Wild type | 23.8 (0.32) | 0.1 (0.0) | 28.6 (1.1) | 71.3 (1.0) |
Table II.
Biomass measurements (±se) of wild-type and C3′H-RNAi hybrid poplar
Bold denotes significance at P = 0.1.
| Tree Line | Height | Caliper | Internode | Leaf Area | Root DW | Stem DW | Root-to-Shoot Ratio | Volume | FW/Area | DW/Area |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | mm | cm | cm2 | g | g | cm3 | mg/cm2 | mg/cm2 | ||
| C3′H RNAi | 129.1 (8.37) | 6.6 (0.22) | 4.1 (0.23) | 84.0 (2.23) | 1.5 (0.23) | 4.9 (0.84) | 0.33 (0.03) | 15.6 (1.72) | 2.10 (0.06) | 0.49 (0.01) |
| Wild type | 167.7 (3.82) | 11.3 (0.34) | 3.6 (0.27) | 166.2 (16.79) | 13.9 (3.75) | 16.8 (3.60) | 0.81 (0.08) | 57.1 (4.29) | 2.53 (0.17) | 0.80 (0.07) |
Figure 1.
Images depicting common differences in tree morphology between C3′H-RNAi and wild-type hybrid poplar. A, Comparison of wild-type (left) and C3′H-RNAi (right) height growth. B, Leaf morphology and axial shooting of C3′H-RNAi poplar. C, Root biomass and architecture of wild-type poplar. D, Root biomass and architecture of C3′H-RNAi poplar. E, Leaf morphology of wild-type poplar.
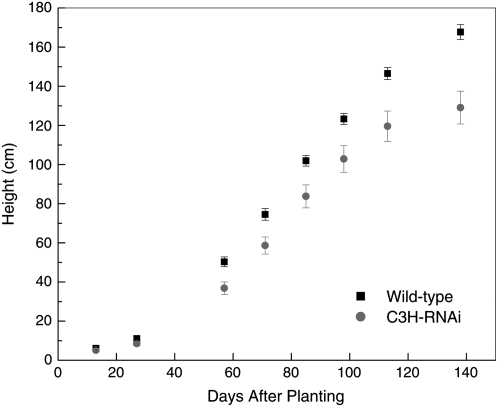
During the first 6 weeks of growth following transfer to soil there were no observable abnormalities in growth characteristics associated with the reduced lignin trees. However, after this time the growth rate for lignin-compromised trees declined dramatically (Fig. 2). Not only was the aboveground biomass affected, but also root biomass in C3′H RNAi-suppressed trees was also substantially less, only 11% of that in the wild-type trees. This reduction was greater than the reduction in stem mass, with C3′H-RNAi trees only accruing 29% stem mass of wild-type trees, which translates into a decrease in root-to-shoot ratio of 0.81 in wild type to 0.33 in C3′H-RNAi trees (Table II).
Figure 2.
Cumulative height growth of C3′H-RNAi and wild-type hybrid poplar over a 4-month period under greenhouse conditions.
In addition to these quantifiable changes, the C3′H-RNAi trees also exhibited axial shoots and altered leaf morphology (Fig. 1). The leaves were not only smaller in area, but were lighter in color and curled gently under at the edges. As the transgenic trees reached heights greater than approximately 50 cm, they showed signs of foliar stress that appeared to be similar to trees subject to water deficiency, including leaf tip necrosis (but no wilting). The basal half of the leaf, nearest the petiole, remained green and healthy in appearance and did not abscise (Fig. 1). Furthermore, the onset of observable changes in leaf morphology corresponded closely with the retardation of relative growth rate in the C3′H-RNAi trees.
Soluble Sugars and Starch Increase in C3′H-RNAi Trees
Leaf soluble sugar and starch content were examined to determine whether photosynthetic assimilation was affected by alterations in the transport of photoassimilate. An analysis of total soluble sugars in leaf tissue showed an increase of 50% in C3′H-RNAi trees, from 66.9 mg g−1 DW in the wild-type trees to 101 mg g−1 DW in the C3′H-RNAi trees. This increase was due to significant pooling of both Glc and Fru, increasing 7.8- and 5.4-fold, respectively, while Suc availability decreased in the C3′H-RNAi trees to 72% that of wild-type trees (Table III). Similarly, leaf starch content was elevated to 1.8% (w/w) of the leaf DW in the transgenic trees, compared to 1.2% in the wild-type trees (Table III).
Table III.
Soluble carbohydrates and starch (±se) composition of leaf tissue (leaf tissue represents leaves from PI = 5) of wild-type and C3′H-RNAi hybrid poplar
Bold denotes significance at P = 0.1.
| Tree Line | Glu | Fru | Suc | Total Sugars | Starch |
|---|---|---|---|---|---|
| mg/g | mg/g | mg/g | mg/g | % | |
| C3′H RNAi | 31.4 (0.3) | 27.3 (0.3) | 42.2 (0.2) | 100.9 (0.3) | 1.8 (0.11) |
| Wild type | 4.2 (0.1) | 4.9 (0.1) | 57.8 (0.3) | 66.9 (0.3) | 1.2 (0.14) |
C3′H-RNAi Trees Have Dramatic Reductions in Gas Exchange Accompanied by a Reduction in WUE
There was a substantial reduction in photosynthetic assimilation rate (A), with transgenic trees having less than 11% the assimilation rate of wild-type trees (Table IV). There was also a significant decrease in transpiration (E) rates in the C3′H-RNAi trees, at only 25% the rate of wild type. These reductions translate into a decline in WUE (A/E) of 2.3 μmol CO2 mmol−1 water in wild type to 1.2 μmol CO2 mmol−1 water. These findings concur with the δ13C values in which wild-type trees were significantly (P = 0.05) less negative at −25.5‰ than the C3′H-RNAi (−28.2‰) trees. More negative δ13C (i.e. increased isotope discrimination) is indicative of higher intercellular CO2 concentration (Ci; Table IV) as would result from a reduced stomatal limitation to photosynthesis.
Table IV.
Gas-exchange values and δ13C (±se) of wild-type and C3′H-RNAi hybrid poplar
Bold denotes significance at P = 0.1.
| Tree Line | A | E | WUE | δ13C | Stomatal Conductance | Ci |
|---|---|---|---|---|---|---|
| μmol CO2 m−2 s−1 | mmol water m−2 s−1 | μmol CO2 mmol−1 water | ‰ | mol m−2 s−1 | μmol CO2 mol−1 air | |
| C3′H RNAi | 1.5 (0.36) | 1.4 (0.02) | 1.2 (0.43) | −28.2 (0.64) | 0.086 (0.02) | 313.30 (18.60) |
| Wild type | 13.9 (0.76) | 6.0 (0.05) | 2.3 (0.08) | −25.5 (0.24) | 0.544 (0.05) | 291.98 (2.47) |
Table V.
Foliar nitrogen and carbon contents, conductivity, water potential, and relative water content (±se) of wild-type and C3′H-RNAi hybrid poplar
Bold denotes significance at P = 0.1.
| Tree Line | Nitrogen | Carbon | Initial Conductivity | P50 | Water Potential | Relative Water Content |
|---|---|---|---|---|---|---|
| % | % | mg cm−2 min−1 | MPa | % | ||
| C3′H RNAi | 2.6 (0.25) | 34.47 (3.37) | 0.037 (0.01) | −0.67 (0.07) | −0.87 (0.04) | 59.17 (2.85) |
| Wild type | 4.6 (0.09) | 44.85 (1.30) | 0.580 (0.17) | −1.72 (0.11) | −1.25 (0.06) | 55.01 (2.35) |
Leaf elemental analysis showed a decrease in both carbon and nitrogen in the C3′H-RNAi trees. Carbon content decreased from 44.8% in wild-type trees to 34.5% in C3′H-RNAi trees, while nitrogen content decreased from 4.6% to 2.6%, respectively. As such, the carbon-to-nitrogen ratio in the wild-type and transgenic poplar was altered from 9.8 to 13.6, respectively. The substantial reduction in leaf carbon content can be accounted for by the perturbed incorporation of lignin in the cell walls, while the reduction in foliar nitrogen is ascribed to a reduction in photosynthetic protein (Table V).
C3′H-RNAi Trees Have Higher Water Potential and Lower Dry and Fresh Weights
Although the C3′H-RNAi trees had both lower leaf dry and fresh weight (FW) per area than wild-type trees, the relative water content of the leaves was not significantly different. The average midday water potential of wild-type trees was −1.25 MPa, while the C3′H-RNAi trees had an average midday potential of −0.87 MPa, indicating a greater xylem tension in wild-type trees than in the transgenics with reduced cell wall lignin synthesis.
Trees with Altered Lignin Had Reduced Hydraulic Conductivity and Susceptibility to Xylem Cavitation
Hydraulic conductivity of the C3′H-RNAi trees was also greatly reduced. The initial conductivity of the transgenic trees was less than 7% that observed in the wild-type trees. As a general rule, sections that have no evidence of previous collapse should be used for cavitation studies, however, in the case of the C3′H-RNAi trees, it was extremely difficult to find sections that had not already experienced some degree of collapse. In the C3′H-RNAi trees, flow staining of safranin dye was without exception intermittent through the xylem, and clearly indicated reduced conductivity.
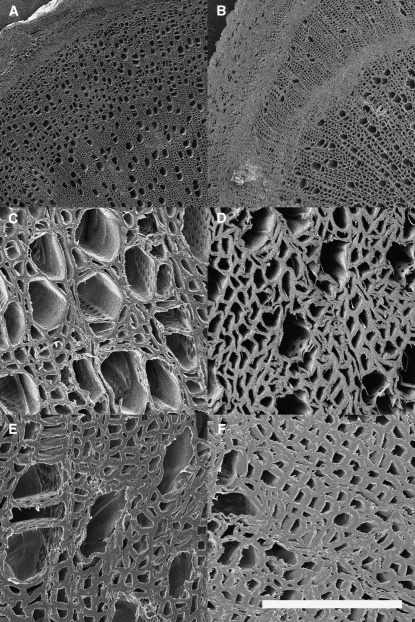
The observed increased collapse also directly corresponded to the transgenic trees being more susceptible to cavitation, as the C3′H-RNAi trees experienced xylem cavitation at applied pressures of between 1.0 to 1.5 MPa, while parallel wild-type trees required pressures of 3.0 to 3.5 MPa to induce cavitation in stem sections (Table V). Scanning electron microscopy (SEM) analysis of the stems prior to and immediately after cavitation revealed clear similarities between precavitation C3′H-RNAi trees and postcavitation wild-type trees. Large regions of the C3′H-RNAi xylem were compressed and the vessels exhibited irregular shapes prior to cavitation. These regions were observed in a number of patterns, including patches of collapse and banding of collapsed xylem. Postcavitation C3′H-RNAi xylem appeared similar to collapsed postcavitation wild-type xylem (Fig. 3).
Figure 3.
SEM images showing changes in xylem morphology. A and C, Wild-type precavitation. B and D, C3′H-RNAi precavitation. E, Wild-type postcollapse. F, C3′H-RNAi postcollapse. Scale bar (in white) represents 100 μm.
DISCUSSION
The majority of previous studies investigating plants with genetically manipulated lignin content have focused their evaluation on the morphological and chemical phenotypes. Numerous studies have demonstrated the effects of reduced enzyme activity on monolignol biosynthesis and plant cell wall lignification, as well as the effects of changes in monolignol composition on plant morphology. Reductions in PAL, cinnamic acid 4-hydroxylase, and C3′H activities all have decreased cell wall lignification and altered lignin monomer ratio (Elkind et al., 1990; Sewalt et al., 1997; Blount et al., 2000; Franke et al., 2002a; Coleman et al., 2008). In contrast, this study documents the physiological consequences, at the whole tree level, of down-regulation of phenylpropanoid metabolism and consequently lignification.
The initial analysis of C3′H-RNAi poplar trees grown under greenhouse conditions showed visual effects consistent with water stress, including stunted growth and leaf tip necrosis, despite being well watered and maintained in the same fashion as the wild-type trees. A microscopic evaluation of the xylem vasculature by SEM was also suggestive of water stress as the xylem showed a distinct banding pattern, localized lignification, and collapse. More specifically, lignification of the vessel elements was not apparent and they were commonly collapsed, likely contributing to restriction of water flow through the stem. Similar lignin banding patterns have previously been reported in Pinus radiata trees subject to drought stress (Donaldson, 2002).
Hydraulic conductivity and susceptibility to cavitation were measured to further elucidate the limitations of decreased cell wall lignin content on water transport. Conductivity was shown to be significantly reduced in the C3′H-RNAi trees, achieving only 7% of wild-type poplar. Cavitation of the C3′H-RNAi trees also occurs at significantly lower applied pressures than those observed in wild-type trees and there was evidence of collapse prior to the application of pressure. Plants with larger vessel lumens may be more susceptible to cavitation (Gullo et al., 1995), however, lignin content may have an even greater influence on cell wall architecture and integrity as the C3′H-RNAi trees had smaller lumens, which would imply reduced susceptibility to cavitation.
Gas-exchange measurements were conducted to examine differences in photosynthetic rates and stomatal conductance to further elucidate the effects of altered lignification on growth characteristics and water transport. Rates of both carbon assimilation and transpiration were significantly affected by inhibition of lignin biosynthesis. The impact on photosynthesis, however, was greater than the impact on transpiration, causing the instantaneous WUE of the lignin-compromised trees to be decreased relative to wild type. Ci values were increased, consistent with the decrease in carbon isotope discrimination indicated by the lower (more negative) δ13C, a proxy measure of intrinsic WUE. Consequently, stomatal conductance to CO2 diffusion did not impose an increased limitation on photosynthesis in the lignin-compromised trees. In fact, despite reductions in the ability of the xylem to transport water, low lignin trees were replete with carbon and actually had higher midday water potentials than the corresponding wild-type trees.
Although low stomatal conductance is not the limiting factor influencing growth rates of the C3′H-RNAi trees, it is reduced. The accumulation of starch and soluble sugars can have a negative impact on photosynthetic rates, as they affect the rate at which Pi is recycled through the reactions of photosynthesis (Paul and Foyer, 2001; McCormick et al., 2006). As such, the nonstructural carbohydrate constituents (starch, Glc, and Fru) of leaf tissue were found to be significantly increased in the C3′H-RNAi trees. Starch content directly affects the production of photosynthetic proteins such as Rubisco, and also decreases ribulose-bisP carboxylation and regeneration capacity (Araya et al., 2006). Furthermore, high leaf Glc content affects photosynthetic rates by regulating gene expression through the action of hexokinase (Jang et al., 1997). In this study, the apparent decrease in photosynthesis, based on accumulating leaf carbohydrate content (soluble and storage), is supported by the elemental analysis that showed an increased carbon-to-nitrogen ratio in C3′H-RNAi trees. Similarly, low nitrogen levels have been shown to inhibit growth, increase carbohydrate accumulation, and subsequently decrease photosynthesis (Paul and Foyer, 2001). As radial stem growth is one of the most important carbon sinks in trees (Cannell and Dewar, 1994), we propose that the inhibition of lignin deposition resulting from the silencing of C3′H reduces carbon sink strength and results in an accumulation of photosynthate as starch and soluble sugars in the leaves, ultimately resulting in decreased rates of photosynthesis (Araya et al., 2006), leading to increased Ci and accompanying reductions in stomatal conductance (gs; Krapp and Stitt, 1995).
Barring direct effects of genetic manipulation on root-to-shoot partitioning, the reduction in root-to-shoot ratio is also inconsistent with water availability as the limiting factor. In this scenario, the altered root-to-shoot ratio is most influenced by a greater reduction in root weight relative to wild-type trees, as compared to the observed differences in shoot DW. Plants suffering from water stress normally partition more carbon to root development to promote water uptake (Hsiao and Xu, 2000). Additionally, there is no change in the relative water content of the leaves, suggesting that the plant water status of the transgenics is similar to that of the wild-type trees.
In summary, we propose that despite a significant reduction in water-transport capacity, the lignin-compromised trees are not limited by water in support of transpiration and gas exchange, but rather are limited by a decrease in photosynthetic assimilation as a result of a significant decrease in sink strength. By inhibiting key steps in lignin biosynthesis and xylem cell wall formation, a normally strong sink in trees, the trees appear to respond by storing carbon in the leaves in the form of starch and soluble sugars. The accumulated carbohydrate is therefore not mobilized, as there is no sink to which it should be transported. Consequently, the sugar concentration in the leaves accumulates, resulting in a decrease in proteins (likely photosynthetic proteins) and a slowing of photosynthetic assimilation. As such, the obvious collapse and decrease in hydraulic conductivity directly related to lignin deposition in the cell wall does not preclude plant survival, or even plant growth at this early stage. Collapse and cavitation, and the supply of water in support of photosynthesis will undoubtedly become more important limitations for trees deficient in lignin as they grow and xylem tensions increase as a result of increased height and conduit length (Martinelli et al., 1998).
MATERIALS AND METHODS
Plant Material
Hybrid poplar (Populus alba × grandidentata) was transformed with an RNAi plasmid harboring a 370-bp sense and antisense fragment of a poplar C3′H sequence (GENBANK EU391631) as described previously (Coleman et al., 2008). Nine independent transgenic lines (ranging from no to 20-fold reduction in C3′H expression) and wild-type trees (12 trees of each) were grown in tissue culture and transferred to greenhouse conditions for 1.5 years. The initial growing season conditions included a 16-h photoperiod, 300 W m2, a minimum temperature of 21°C, and bidaily fertigated watering. After 4 months of growth, half of the plants were harvested for biomass measurements and biochemical analysis. The remaining plants were maintained in the greenhouse under greenhouse conditions and later utilized for gas exchange, water potential, δ13C, and elemental carbon and nitrogen analysis.
Biomass Measurements
Transgenic and wild-type trees were measured bimonthly for changes in height and diameter 10 cm above the root collar by caliper. After 4 months, plants were destructively harvested and all leaves, roots, and stems retained for biomass analysis. Leaf area was measured using an LI-3100 area meter (LI-COR Biosciences Inc.) and the leaves were then dried and weighed for determination of dry mass. Stem volume (V) was estimated using the formula for a cone:
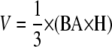 |
where BA is basal area and H is height. Stem and roots were oven dried at 105°C for 48 h and weighed to determine total DW. The number of leaves was recorded at harvest to determine changes in internode distance by dividing number of leaves by stem height. Leaf mass per area was calculated based on leaf area and total leaf oven DW.
Developmental stage was standardized by employing a plastichron index (PI), where PI = 0 was defined as the first leaf greater than 5 cm in length (petiole to leaf tip) and PI = 1 represented the leaf immediately below. Fully expanded leaves from the upper portion of the tree, representing PI 5, were flash frozen in liquid nitrogen and retained at −80°C for further analysis.
Soluble Sugars and Starch Analysis
Soluble sugars (Suc, Glc, and Fru) were extracted and quantified (Coleman et al., 2006). Lyophilized ground tissue was extracted in methanol:chloroform:water (12:5:3) at −20°C overnight (internal standard of 10 mg mL−1 Fuc). The sample was centrifuged and supernatant removed. The pellet was washed twice with methanol:chloroform:water and the supernatant fractions pooled. Water (5 mL) was added and the samples centrifuged to facilitate phase separation. The aqueous fraction was removed and dried by rotary evaporation and then resuspended in 3 mL of deionized water. Analysis was carried out using anion-exchange HPLC on a DX-600 HPLC system equipped with a PA1 column and an electrochemical detector (Dionex) and standard curves used for quantification.
Starch composition was quantified from extracted plant tissue, where the residual pellet was hydrolyzed in 4% sulfuric acid at 121°C for 3.5 min (internal standard of 5 mg mL−1 Fuc) and the liberated Glc quantified by HPLC (Coleman et al., 2006).
Gas Exchange and WUE
Net A, E, and gs were measured using a LI-6400 portable gas-exchange system (LI-COR Biosciences Inc.). Measurements were carried out on September 8, 2006 between 9 am and 12 pm. Chamber conditions were as follows: 25°C, 50% relative humidity, and 360 μmol mol−1 CO2. Three trees per line were used for these measurements and three independent leaves were evaluated on each tree. The leaves were allowed to equilibrate within the chamber for 15 min and then three individual measurements taken and averaged. Longer equilibration times did not alter results. Instantaneous WUE for the samples was calculated as A/E (Fisher and Turner, 1978).
The same leaves used for gas-exchange measurements were harvested for δ13C and elemental carbon and nitrogen analysis. Leaves were lyophilized and ground using mortar and pestle and analyzed for δ13C by the University of California at Davis Stable Isotope Facility using a ThermoFinnigan Isotope Ratio mass spectrometer. δ13C values were calculated from:
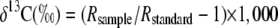 |
where Rsample and Rstandard are the ratios of 13C to 12C in the sample and the arbitrary standard (Vienna Peedee belemnite), respectively. Elemental carbon and nitrogen were analyzed as described (Verardo et al., 1990).
Water Potential and Relative Water Content
Shoot water potential (ψw) and leaf relative water content were measured on greenhouse-grown trees, between 9 am and 12 pm on a sunny day during active transpiration. Shoot ψw was measured with a pressure bomb (Plant Water Status Console model 3005, Soil Moisture Equipment Corp.) using the main stem of each tree (Tyree and Hammel, 1972).
Relative water content was measured by determining the FW, turgid weight (TW), and DW of leaf sections. Leaf sections were cut and weighed (FW). They were stored for 24 h in 10 mm KCN and then reweighed (TW). Samples were then dried for 48 h at 55°C and reweighed (DW). Relative water content was calculated as follows:
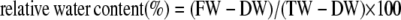 |
Hydraulic Conductivity and Susceptibility to Xylem Cavitation
Xylem conductivity and susceptibility to cavitation were measured as described (Wang et al., 2003) with minor modifications. Plants grown in the greenhouse were transferred intact to the laboratory. Samples were cut immediately prior to measurement and then recut and stripped of bark under water to prevent the entry of air. A 2-cm section was used for safranin staining (5% safranin dye) to determine if samples were experiencing unimpeded water flow, where full staining of the xylem was indicative of no blockage.
The proximal ends of the samples were then connected to tubing filled with degassed, deionized water pressurized to 10 kPa by gravity. Flux was measured by collecting flow through on a cotton swab at 1-min intervals and weighing the swab for the determination of initial hydraulic conductivity (kg cm−2). Measurements were carried out in triplicate on each sample. Samples were then placed in a double-ended pressure chamber (PMS Instrument) with both ends protruding and flux was measured again following pressurization. Pressurization occurred for 40 s and flux was measured over three 1-min intervals following the release of pressure. Following calibration, wild-type trees were analyzed with an applied pressure ranging from 1.5 to 3.5 MPa in 0.5 MPa increments. In contrast, transgenic trees required applied pressures ranging from 0.5 to 1.5 MPa in 0.5 MPa increments.
Susceptibility to cavitation is defined as the percent loss of hydraulic conductivity based on the change in flux at each pressure. Samples were compared based on their initial hydraulic conductivity and the water potential corresponding to 50% loss of hydraulic conductivity.
Electron Microscopy
Air-dried mature secondary xylem was dissected transversely with razor blades and wood samples were attached to SEM stubs using double-sided stick tape. Following gold coating, samples were viewed using a Hitachi S7600 at 3 kV and images captured digitally.
Statistical Analysis
All analysis was carried out using unpaired two-tailed t tests at 95% confidence.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number EU391631.
Acknowledgments
The authors would like to thank Virginie Pointeau and Tongli Wang for their assistance and instruction on instrumentation.
This work was supported by the Canadian Natural Sciences and Engineering Research Council (grant no. 238354 to S.D.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Shawn D. Mansfield (shawnman@interchange.ubc.ca).
References
- Abdulrazzak N, Pollet B, Ehlting J, Larsen K, Asnaghi C, Ronseau S, Proux C, Erhardt M, Seltzer V, Renou JP, et al (2006) A coumaroyl-ester-3-hydroxylase insertion mutant reveals the existence of nonredundant meta-hydroxylation pathways and essential roles for phenolic precursors in cell expansion and plant growth. Plant Physiol 140 30–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein N, Gödeke KH (1977) α-Amino-β-phenylpropionic acid-a potent inhibitor of L-phenylalanine ammonia lyase in vitro and in vivo. Plant Sci Lett 8 313–317 [Google Scholar]
- Anterola AM, Lewis NG (2002) Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry 61 221–294 [DOI] [PubMed] [Google Scholar]
- Araya T, Noguchi K, Terashima I (2006) Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant Cell Physiol 47 644–652 [DOI] [PubMed] [Google Scholar]
- Blount JW, Korth KL, Masoud SA, Rasmussen S, Lamb C, Dixon RA (2000) Altering expression of cinnamic acid 4-hydroxylase in transgenic plants provides evidence for a feedback loop at the point of entry into the phenylpropanoid pathway. Plant Physiol 122 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54 519–546 [DOI] [PubMed] [Google Scholar]
- Boyce CK, Zwieniecki MA, Cody GD, Jacobsen C, Wirick S, Knoll AH, Holbrook NM (2004) Evolution of xylem lignification and hydrogel transport regulation. Proc Natl Acad Sci USA 101 17555–17558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MM, Sederoff RR (1996) Variation in lignin content and composition. Plant Physiol 110 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MGR, Dewar RC (1994) Carbon allocation in trees: a review of concepts for modelling. Adv Ecol Res 25 60–103 [Google Scholar]
- Chabannes M, Barakate A (2001) Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J 28 257–270 [DOI] [PubMed] [Google Scholar]
- Coleman HD, Ellis DD, Gilbert M, Mansfield SD (2006) Up-regulation of sucrose synthase and UDP-glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotechnol J 4 87–101 [DOI] [PubMed] [Google Scholar]
- Coleman HD, Park JY, Nair R, Chapple C, Mansfield SD (2008) RNAi-mediated suppression of p-coumaroyl-CoA 3′hydroxylase in hybrid poplar impacts on lignin deposition and soluble secondary metabolism. Proc Natl Acad Sci USA 105 4501–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson LA (2002) Abnormal lignin distribution in wood from severely drought stressed Pinus radiata trees. IAWA J 23 161–178 [Google Scholar]
- Dwivedi UN, Campbell WH, Yu J, Datla RSS, Bugos RC, Chiang VL, Podila GK (1994) Manipulation of lignin biosynthesis in transgenic tobacco through expression of an antisense O-methyltransferase gene from Populus. Plant Mol Biol 26 61–71 [DOI] [PubMed] [Google Scholar]
- Elkind Y, Edwards R, Mavandad M, Hedrick SA, Ribak O, Dixon RA, Lamb CJ (1990) Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci USA 87 9057–9061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA, Turner NC (1978) Plant productivity in the arid and semi-arid zones. Annu Rev Plant Physiol 29 277–317 [Google Scholar]
- Franke R, Hemm MR, Denault JW, Ruegger MO, Humphreys JM, Chapple C (2002. a) Changes in secondary metabolism and deposition of an unusual lignin in the ref8 mutant of Arabidopsis. Plant J 30 47–59 [DOI] [PubMed] [Google Scholar]
- Franke R, Humphreys JM, Hemm MR, Denault JW, Ruegger MO, Cusumano JC, Chapple C (2002. b) The Arabidopsis REF8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. Plant J 30 33–45 [DOI] [PubMed] [Google Scholar]
- Franke R, McMicheal CM, Meyer K, Shirley AM, Cusumano JC, Chapple C (2000) Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. Plant J 22 223–234 [DOI] [PubMed] [Google Scholar]
- Gullo MAL, Salleo S, Piaceri EC, Rosso R (1995) Relations between vulnerability to xylem embolism and xylem conduit dimensions in young trees of Quercus cerris. Plant Cell Environ 18 661–669 [Google Scholar]
- Hancock JE, Loya WM, Giardina CP, Li L, Chiang VL, Pregitzer KS (2007) Plant growth, biomass partitioning and soil carbon formation in response to altered lignin biosynthesis in Populus tremuloides. New Phytol 173 732–742 [DOI] [PubMed] [Google Scholar]
- Hsiao TC, Xu LK (2000) Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. J Exp Bot 51 1595–1616 [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5 224–229 [DOI] [PubMed] [Google Scholar]
- Huntley SK, Ellis D, Gilbert M, Chapple C, Mansfield SD (2003) Significant increases in pulping efficiency in C4H-F5H transformed poplars: improved chemical savings and reduced environmental toxins. J Agric Food Chem 51 6178–6183 [DOI] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Ennos AR, Turner SR (2001) Cloning and characterization of irregular xylem4 (irx4) a severely lignin-deficient mutant of Arabidopsis. Plant J 26 205–216 [DOI] [PubMed] [Google Scholar]
- Kajita S, Hishiyama S, Tomimura Y, Katayama Y, Omori S (1997) Structural characterization of modified lignin in transgenic tobacco plants in which the activity of 4-coumarate:coenzyme A ligase is depressed. Plant Physiol 114 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195 313–323 [Google Scholar]
- Lee D, Meyer K, Chapple C, Douglas CJ (1997) Antisense suppression of 4-coumarate:coenzyme A ligase activity in Arabidopsis leads to altered lignin subunit composition. Plant Cell 9 1985–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhou Y, Cheng X, Sun J, Marita JM, Ralph J, Chiang VL (2003) Combinatorial modification of multiple lignin traits in trees through multigene cotransformation. Proc Natl Acad Sci USA 100 4939–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli LA, Almeida S, Brown IF, Moreira MZ, Victoria RL, Sternberg LSL, Ferreira CAC, Thomas WW (1998) Stable carbon isotope ratio of tree leaves, boles, and fine litter in a tropical forest in Rondonia, Brazil. Oecologia 114 170–179 [DOI] [PubMed] [Google Scholar]
- McCormick AJ, Cramer MD, Watt DA (2006) Sink strength regulates photosynthesis in sugarcane. New Phytol 171 759–770 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52 1383–1400 [DOI] [PubMed] [Google Scholar]
- Piquemal J, Lapierre C, Myton K, O'Connell A, Schuch W, Grima-Pettenati J, Boudet AM (1998) Down-regulation of cinnamoyl-CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J 13 71–83 [Google Scholar]
- Schoch G, Goepfert S, Morant M, Hehn A, Meyer D, Ullmann P, Werck-Reichhart D (2001) CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J Biol Chem 276 36566–36574 [DOI] [PubMed] [Google Scholar]
- Sewalt VJH, Hi W, Blount JW, Jung HG, Masoud SA, Howles PA, Lamb C, Dixon RA (1997) Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of l-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol 115 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS (2003) Evolution of water transport and xylem structure. Int J Plant Sci 164 S115–S127 [Google Scholar]
- Tyree MT, Hammel HT (1972) The measurement of the turgor pressure and the water relations of plants by the pressure bomb technique. J Exp Bot 23 267–282 [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem Structure and the Ascent of Sap, Ed 2. Springer, Heidelberg
- Verardo DJ, Froelich PN, McIntyre A (1990) Determination of organic carbon and nitrogen in marine sediments using the Carbo Erba NA-1500 Analyzer. Deep Sea Res 37 157–165 [Google Scholar]
- Wang T, Aitken SN, Kavanagh KL (2003) Selection for improved growth and wood quality in lodgepole pine: effects on phenology, hydraulic architecture and growth of seedlings. Trees (Berl) 17 269–277 [Google Scholar]