Abstract
Circadian changes in mood have been described earlier. A positive affect (PA) has been separated from a negative affect (NA), as independent components in opposite admittedly subjective directions, a circadian rhythm characterizing both aspects. Herein, the time structure (chronome) of human mood is re-examined and extended from the circadian to the circaseptan domain by a meta-analysis of data on 196 clinically healthy students who filled out the positive (PA) and negative (NA) affective scale (PANAS), consisting each of 10-item mood scales. Both PA and NA are found by cosinor to be characterized by a circaseptan, circasemiseptan, and circadian variation. The circaseptan and circasemiseptan amplitudes are found to be larger than the circadian amplitude for NA, whereas the circadian amplitude is largest for PA. Complementing differences in relative circaseptan-to-circadian prominence between PA and NA are differences in the timing of the circadian, circasemiseptan, and circaseptan components of PA and NA. An even broader spectrum of rhythms may include a circadecadal modulation. With this qualification, the information on the time structure of mood provides endpoints to be considered in any attempt to optimize psychological well-being by making sleeping, dietary, and/or other lifestyle adjustments.
Keywords: Circadian rhythm, Circaseptan rhythm, Human mood, Possitive and Negative affect, PANAS“
INTRODUCTION
Circadian (1) and infradian (2) rhythms have been documented for human mood, whether self-rated, e.g., along a 7-point scale (1) or otherwise (2). It was found consistently in clinically healthy medical students (3) as well as in patients (2, 4–8). In a 30-year long record of self-measurements carried out about 5 times a day each day with only few interruptions, components other than the circadian rhythm have been detected, notably an about 11.5-year variation (9). In manic-depressive disorder, a circadian rhythm in blood eosinophils may gauge a free-running adrenal cycle (2, 10), and is associated with overt infradian changes in mood as extreme as the alternating bipolar states.
These earlier studies did not differentiate between the reportedly independent positive and negative affect (11–15). In an about 3-month record on a clinically healthy man, a circadian variation was detected for both positive and negative affect, the independence of the two mood scales also being corroborated (16). Herein, 7-day records at mostly 3-hour intervals from 196 clinically healthy students previously analysed for the circadian variation (17) are re-analysed to map any circaseptan as well as circadian variation in positive and negative affect.
MATERIALS AND METHODS
As outlined elsewhere (17), the subjects were 196 college students in two studies who completed a mood rating form approximately 7 times a day for 1 week. Subjects filled out forms upon rising and retiring, and at scheduled 3-hour intervals in between. The PANAS questionnaire consists of two 10-item mood scales that are reportedly highly internally consistent, largely uncorrelated, and stable (11–15). Each item is rated on a 5-point scale. The positive affect (PA) was calculated as the sum of the scores for the 10-item scales for positive affect, and the negative affect (NA) was calculated as the sum of the scores for the 10-item scales for negative affect. The overall mood was calculated as the sum of the PA and NA scores.
Each data series was analysed by least squares spectrum, involving the least squares fit of cosine curves with fixed periods in the frequency range of one cycle per week to two cycles per day, by cosinor (18, 19). The results at a given trial period were summarized across all students by population-mean cosinor (18, 19). The circadian and circaseptan waveforms were further visualized by averaging the data from all students along the scales of an idealized day and week, respectively, after expressing the individual data as a percentage of the series’ mean value (to remove inter-individual variation in overall mood).
RESULTS
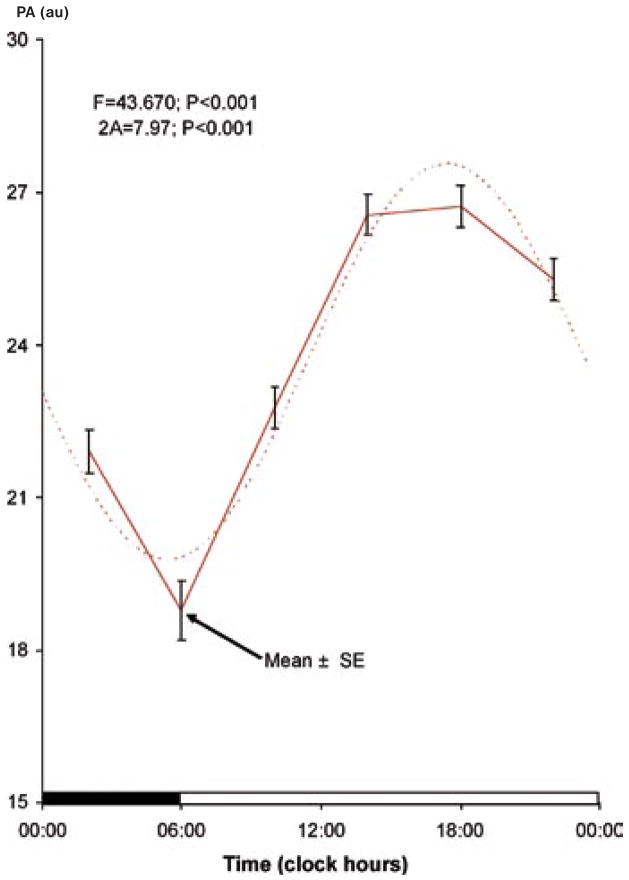
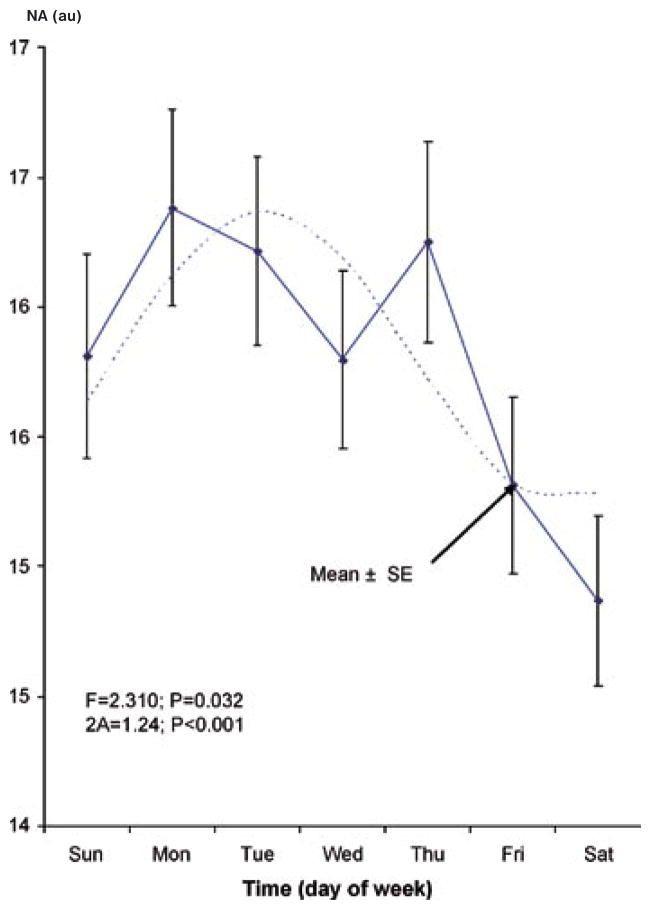
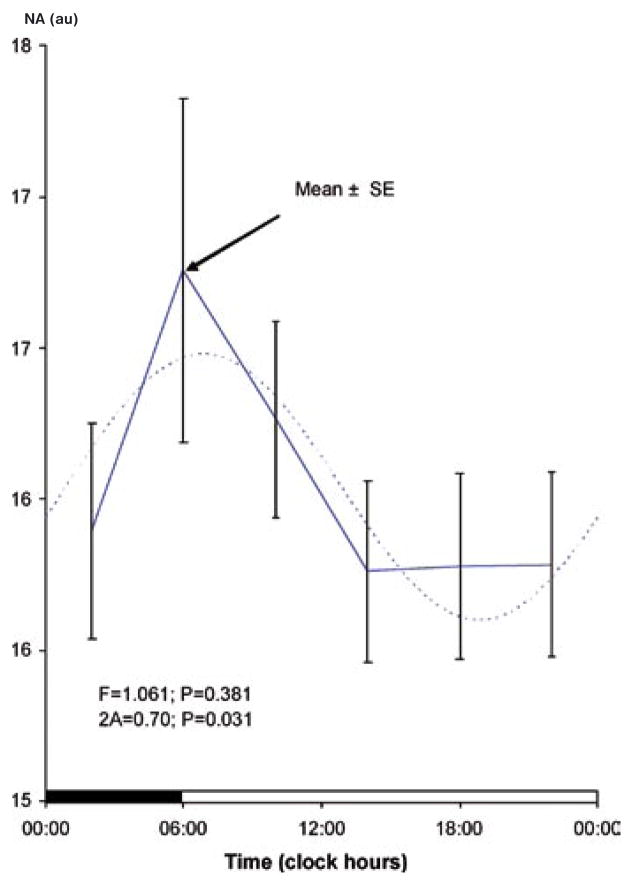
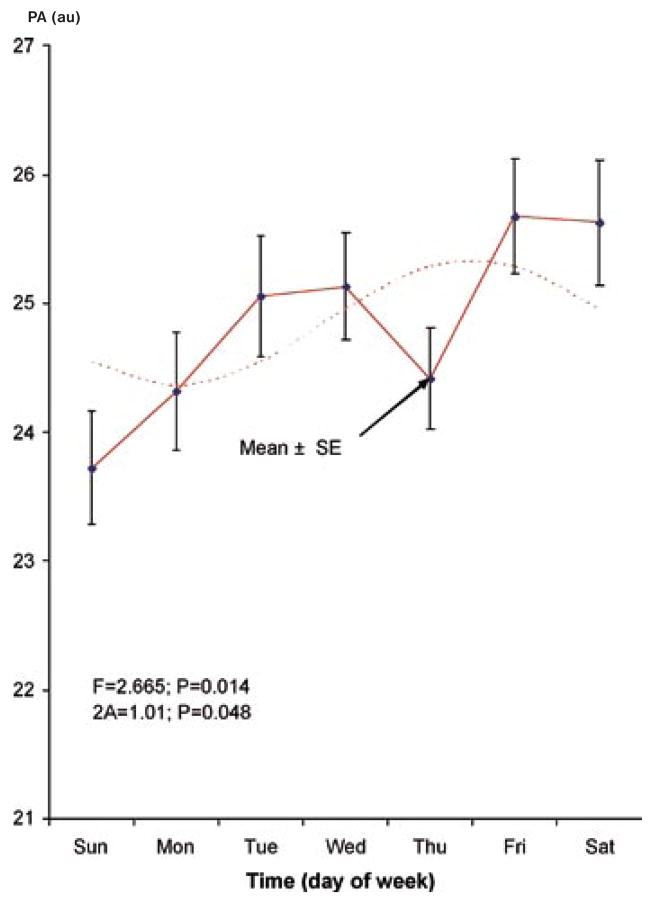
In addition to the previous demonstration of a circadian rhythm (17), a circaseptan variation is also found to be statistically significant, Table 1. The circadian and circaseptan acrophases differ between PA and NA, as evidenced by the non-overlap of the respective 95% confidence intervals for the acrophase (Table 1). The 84-hour (half-week) and 12-hour (half-day) components are also detected with statistical significance (except for the 12-hour component in NA), suggesting that the circadian and circaseptan waveforms depart from sinusoidality. As seen in Table 1, the weekly and half-weekly amplitudes are larger than the circadian amplitude for NA, whereas in the case of PA, the circadian variation is most prominent. The patterns in relative data (expressed as a percentage of each student’s average value) shown in Figs. 1–4 clearly illustrate the differences in relative circaseptan vs. circadian prominence and in the acrophase of each component between positive and negative affect.
Table 1.
Population-mean cosinor-derived point and interval estimates in positive affect (PA), negative affect (NA) and total affect of 196 clinically healthy subjects*
| Period (hours) | Variable | PR (%) | P | Amplitude (95% CI) | Acrophase (95% CI) |
|---|---|---|---|---|---|
| 168 | PA | 11.6 | 0.048 | 0.50 (0.11, 0.90) | −267° (−219, −317) |
| NA | 16.8 | <0.001 | 0.62(0.33, 0.91) | −134° (−102, −169) | |
| Total | 12.3 | 0.068 | 0.46 ( ) | −187° ( ) | |
| 84 | PA | 6.8 | <0.001 | 0.80 (0.54, 1.06) | −277° (−258, −297) |
| NA | 8.6 | <0.001 | 0.57 (0.34, 0.79) | −128° (−105, 155) | |
| Total | 8.0 | 0.058 | 0.42( ) | −235° ( ) | |
| 24 | PA | 18.6 | <0.001 | 3.99 (3.61, 4.37) | −249° (−244, 254) |
| NA | 7.2 | 0.031 | 0.35 (0.09, 0.61) | −81° (−49, −119) | |
| Total | 15.4 | <0.001 | 3.65 (3.23, 4.07) | −248° (−243, −253) | |
| 12 | PA | 5.7 | <0.001 | 1.03 (0.80, 1.27) | −148° (−136, −161) |
| NA | 4.3 | 0.365 | 0.10( ) | −229° ( ) | |
| Total | 5.6 | <0.001 | 1.05 (0.81, 1.30) | −154° (−140, −167) |
PR: Percentage Rhythm, average proportion of variance accounted for by cosine curve with given period fitted to individual data services; P: P-value from zero-aplitude test; MESORs (±SE) of PA, NA and Total are 23.73±0.35, 16.01±0.30 and 39.73±0.47
Fig. 1.
Circadian rhythm in positive affect
Fig. 4.
Circaseptan rhythm in negative affect
DISCUSSION AND CONCLUSION
The strength of the results herein stems from the relatively large population size. The greater prominence of the circaseptan as compared to the circadian variation in negative affect is in keeping with the results from a case report of a woman with bipolar disorder where the circaseptan component of overall mood was found to be amplified during a 2-week episode of depression (20). The results are also in keeping with those of a study of 14 healthy females showing a prominent circadian variation in positive affect but not in negative affect during a 27-hour constant routine (21).
The mapping of both circaseptan and circadian changes in mood may be helpful to optimize mood by making appropriate adjustments in sleeping habits, diet, and/or other lifestyle factors. For instance, vitamin D3 has been reported to enhance positive affect with some evidence of a reduction in negative affect (22). It has also been suggested that moderate changes in the timing of the sleep-wake cycle may have profound effects on subsequent mood (23). A study of 6 male graduate students over a 7-day span also led the authors to conclude that adrenaline accumulation correlated with physical fatigue, whereas cortisol was associated with alertness and ratios of adrenaline, noradrenaline, and dopamine were related to tenseness and irritability (24). Less daily illumination was also reportedly associated with poorer global functioning, longer but more disturbed sleep, and more depression (25).
Fig. 2.
Circadian rhythm in negative affect
Fig. 3.
Circaseptan rhythm in positive affect
Acknowledgments
US Public Health Service (GM-13981; FH), Dr hc hc Earl Bakken Fund (FH, GC), University of Minnesota Supercomputing Institute (FH, GC), MSM 0021622402 Ministry of Education, CZ.
References
- 1.Halberg F, Johnson EA, Nelson W, Runge W, Sothern R. Autorhythmometry procedures for physiologic self-measurements and their analysis. Physiol Tchr. 1972;1:1–11. [Google Scholar]
- 2.Bryson RW, Martin DF. Lancet. 1954;ii:365–367. doi: 10.1016/s0140-6736(54)92667-7. [DOI] [PubMed] [Google Scholar]
- 3.Günther R, Knapp E, Halberg F, Haus E. Cosinor mapping of physiologic and psychologic variables in 18 healthy men before and during balneotherapy. In: Scheving LE, Halberg F, Pauly JE, editors. Chronobiology, Proc Int Soc for the Study of Biological Rhythms, Little Rock, Ark. Stuttgart/Igaku Shoin Ltd, Tokyo: Georg Thieme Publishers; 1974. pp. 228–233. [Google Scholar]
- 4.Eckert E, Zimmermann RL, Sothern RB, Trapp G, Halberg F. Differing mood spectrum in identical twins with differing clinical stages of bipolar affective disease. Chronobiologia. 1979;6:93–94. [Google Scholar]
- 5.Halberg E, Cornélissen G, Bakken E, Halberg F. Chrononeuroimmunomodulation (chrono-NIM): lead-lag cross- correlations of mental state and tumor burden. Chronobiologia. 1994;21:144–145. [Google Scholar]
- 6.Halberg F, Luce G. Mental Health Program Reports #3, NIMH Publ. #1876. 1969. Techniques for assessing biological rhythms in medicine and psychiatry; pp. 111–204. investigator. [Google Scholar]
- 7.Madjirova NP, Halberg F, Dimitrov BD, Petrova N, Kitcheva L. Carbamazepine stability of the circadian rhythm of mood, vigor, temperature and pulse during combined antidepressive therapy in patients with affective disorders. In: Mikulecky M, editor. Chronobiology and its Roots in the Cosmos. High Tatras; Slovakia: Bratislava: Slovak Medical Society; September 2–6, 1997, 1997. pp. 285–300. [Google Scholar]
- 8.Simpson HW, Gjessing L, Fleck A, Kühl JFW, Halberg F. Phase analysis of the somatic and mental variables in Gjessing’s case 2484 or intermittent catatonia. In: Scheving LE, Halberg F, Pauly JE, editors. Chronobiology, Proc Int Soc for the Study of Biological Rhythms, Little Rock, Ark. Stuttgart/Igaku Shoin Ltd, Tokyo: Georg Thieme Publishers; 1974. pp. 535–539. [Google Scholar]
- 9.Halberg F, Cornélissen G, Otsuka K, et al. International BIOCOS Study Group. Cross-spectrally coherent ~10.5- and 21-year biological and physical cycles, magnetic storms and myocardial infarctions. Neuroendocrinol Lett. 2000;21:233–258. [PubMed] [Google Scholar]
- 10.Halberg F. Symposium on ‘Some current research methods and results with special reference to the central nervous system.’ Physiopathologic approach. Amer J ment Defic. 1960;65:156–171. [PubMed] [Google Scholar]
- 11.Tellegen A. Structures of mood and personality, and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Hills-dale, NJ: Erlbaum; 1985. pp. 681–706. [Google Scholar]
- 12.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Person Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 13.Tellegen A, Watson D, Clark LA. On the dimensional and hierarchical structure of affect. Psychol Sci. 1999;10:297–303. [Google Scholar]
- 14.Tellegen A, Watson D, Clark LA. Further support for a hierarchical model of affect: reply to Green and Salovey. Psychol Sci. 1999;10:307–309. [Google Scholar]
- 15.Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. J Person Soc Psychol. 1999;76:820–838. [Google Scholar]
- 16.Mitsutake G, Otsuka K, Cornélissen G, et al. Circadian and infradian rhythms in mood. Biomed Pharmacother. 2001;55(Suppl 1):94–100. doi: 10.1016/s0753-3322(01)90011-3. [DOI] [PubMed] [Google Scholar]
- 17.Clark LA, Watson D. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- 18.Halberg F. Chronobiology. Ann Rev Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- 19.Cornélissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. Vol. 1. Chichester: Wiley; 1998. pp. 642–649. [Google Scholar]
- 20.Rawson MJ, Cornélissen G, Holte J, et al. Circadian and circaseptan components of blood pressure and heart rate during depression. Scripta med. 2000;73:117–124. [Google Scholar]
- 21.Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiology International. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- 22.Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychop-harmacology. 1998;135:319–323. doi: 10.1007/s002130050517. [DOI] [PubMed] [Google Scholar]
- 23.Boivin DB, Czeisler CA, Dijk DJ, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 24.Fibiger W, Singer G, Miller AJ, Armstrong S, Datar M. Cortisol and catecholamines changes as functions of time-of-day and self-reported mood. Neuroscience Biobehavioral Reviews. 1984;8:523–530. doi: 10.1016/0149-7634(84)90009-5. [DOI] [PubMed] [Google Scholar]
- 25.Kripke DF, Girardin JL, Elliott JA, et al. Ethnicity, sleep, mood, and illumination in postmenopausal women. BMC Psychiatry. 2004;4:8. doi: 10.1186/1471-244X-4-8. http://www.biomedcentral.com/1471-244X/4/8. [DOI] [PMC free article] [PubMed]