Abstract
Oestrogen and family history are two of the most important risk factors for breast cancer. However, these risk factors cannot explain the differences in the incidence and recurrence of breast cancer between premenopausal and postmenopausal women. In this paper I propose that, in premenopausal women, an iron deficiency caused by menstruation stabilises hypoxia inducible factor-1α, which increases the formation of vascular endothelial growth factor. This mechanism results in premenopausal women being more susceptible to angiogenesis and, consequently, leads to a high recurrence of breast cancer. Conversely, increased concentrations of iron in post menopausal women, as a result of menstrual cessation, contribute to a high incidence of breast cancer via oxidative-stress pathways. Although the focus of this Personal View is on iron, this by no means negates the roles of other known risk factors in breast-cancer development. Characterisation of the role of iron in breast cancer could potentially benefit patients by decreasing recurrence and incidence and increasing overall survival.
Introduction
In developed countries, breast cancer is one of the leading causes of cancer-related death in women. Other than family history, oestrogen is the most significant and well-characterised risk factor for breast cancer. Oestrogen alone, however, cannot explain the differences in breast-cancer recurrence, aggressiveness, and incidence between premenopausal and postmenopausal women. In this paper, I propose that iron—a growth nutrient that has equal importance to oestrogen in female metabolism and development—and the changes in its concentration during menopausal transition, might have a key role in the development and recurrence of breast cancer in women.
Biology of the menopause
The menopause is a natural ageing process during which a woman loses her reproductive ability. In premenopausal women, complex interactions between hypothalamic-pituitary-ovarian (HPO) glands result in the pituitary gland stimulating the ovaries to mature and release an egg every month.1,2 When the egg is not fertilised, the endometrial matrix prepared for egg fertilisation is shed in the form of blood (figure 1). The average blood loss during menstruation is 35 mL per month with 10–80 mL considered healthy.3 Because of this blood loss, premenopausal women have a higher dietary requirement for iron to prevent iron deficiency than men or postmenopausal women. In general, iron concentrations are much lower in premenopausal women than in postmenopausal women.4,5 Ferritin, an iron-storage protein with a capacity of binding up to 4500 atoms of iron, and transferrin, an iron-transport protein with two binding sites for iron, are much less saturated in premenopausal women than in postmenopausal women.6,7 Thus, in young, premenopausal women, systemic oestrogen concentrations are high and iron concentrations are low.
Figure 1. Lining of the uterus during menstruation.
As women become older, their ovaries contain fewer eggs to release and menstruation becomes irregular. The dynamics of the HPO hormones change greatly from cyclic to static patterns and serum concentrations of oestrogen significantly diminish.8 Many women have physiological changes during and after the menopause. One of the first physiological changes is the cessation of menstruation, leading to increased concentrations of iron. Thus, the natural biological situation in postmenopausal women is the reverse of that in premenopausal women—ie, low systemic oestrogen concentrations and high iron concentrations. Although ovarian oestrogen is no longer an endocrine factor in postmenopausal women, oestrogen is produced locally in the breast tissue by aromatase cytochrome P450 (the product of CYP19A1).9 Despite the decline in serum oestrogen concentrations after the menopause, breast tissue 17β-oestradiol (E2) concentrations in pre menopausal and postmenopausal women do not significantly differ.10 Prostaglandin E2 increases intracellular cyclic AMP concentrations and stimulates oestrogen biosynthesis as a consequence of overexpression of cyclooxygenase type II (COX-2) in the breasts.11
Association between oestrogen and breast cancer
After family history, lifetime cumulative exposure to reproductive hormones, especially oestrogen, is the most important risk factor for breast cancer. Early age at menarche, nulliparity, late first full-time pregnancy, late menopause, and use of hormone replacement therapy (HRT), are all linked to increased breast-cancer risk.12,13 Oestrogen affects the growth, differentiation, and function of tissues of the female reproductive system—ie, the uterus, ovaries, and breasts.14 In general, oestrogen exerts its carcinogenicity by several mechanisms, including: its receptor-mediated hormonal activity; a cytochrome P450-mediated metabolic activation, which elicits direct genotoxic effects by increasing mutation rates; and the induction of aneuploidy.15 However, oestrogen alone cannot explain the age-related differences in breast-cancer incidence between premenopausal and postmenopausal women. For example, the incidence of breast cancer is higher in postmenopausal women than in premenopausal women (441 vs 75 per 100 000),16 but serum circulating oestrogen concentrations are lower in postmenopausal than in premenopausal women—eg, serum E2 is within the range of 0–110 pMol/L in postmenopausal women versus 275–1909 pMol/L in premenopausal women—and breast-tissue oestrogen concentrations are comparable. The way in which an overall low concentration of oestrogen contributes to a high breast-cancer incidence in postmenopausal women is not completely understood.
Young women diagnosed with breast cancer are known to have a higher risk of dying from the disease than older women because of the greater early recurrence and the increased aggressiveness of tumours in this age group.17–19 After surgery, recurrence in premenopausal women is twice as high as that in postmenopausal women,10,21 and node-positive premenopausal women with breast cancer have a substantially increased risk of recurrence compared with node-positive postmenopausal women with breast cancer.22 The BRCA1 mutation, which is linked to family history and was originally thought to contribute to poor outcome, has been shown to have no effect on breast-cancer mortality.23 Specific risk factors for this high breast-cancer recurrence in premenopausal patients have not been identified.
In view of these profound premenopausal and postmenopausal differences in iron concentrations, I propose that the change in iron status from iron deficiency to iron load during the menopausal transition contributes to the difference in breast-cancer incidence and recurrence between premenopausal and postmenopausal women. Thus, in addition to the other known risk factors of breast cancer (eg, genetic factors [ie, BRCA1 and BRAC2], HRT, lifestyle, pregnancy, and obesity), an imbalance in iron concentration should also be viewed as an important risk factor, which might account for some unexplained findings.
Iron deficiency and breast-cancer recurrence in premenopausal patients
Angiogenesis is necessary for any tumour to grow beyond a certain volume, and has an important role in tumour progression, malignancy, and recurrence.24,25 This process is regulated by several proangiogenic (eg, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor) and antiangiogenic (eg, thrombospondin-1) factors produced by both the tumour cells and the surrounding stroma.26 Cells buried in the centre of a tumour mass receive inadequate oxygen and nutrient supplies as a result of few blood vessels to transport them. This deprivation leads to a hypoxic cascade, in which hypoxia inducible factor-1α (HIF-1α) stabilises and upregulates genes stimulating angiogenesis, resulting in the formation of a new vasculature, which penetrates into the tumour core.27,28
Angiogenesis and metastasis are intrinsically connected and antiangiogenic strategies have been investigated for cancer treatment and the prevention of cancer recurrence and metastasis.29,30 Experimental data suggest that the establishment and growth of metastases are linked to soluble factors secreted from the primary tumour, followed by a spread of cancer cells through the newly formed blood vessels.29,30 VEGF is the most potent endothelial-cell mitogen and also a regulator of vascular permeability. Previous retrospective studies on the association of VEGF with relapse-free survival and overall survival have reported that patients with early-stage breast cancer who have tumours with increased concentrations of VEGF have a higher likelihood of recurrence or death than patients with low VEGF-producing tumours.31
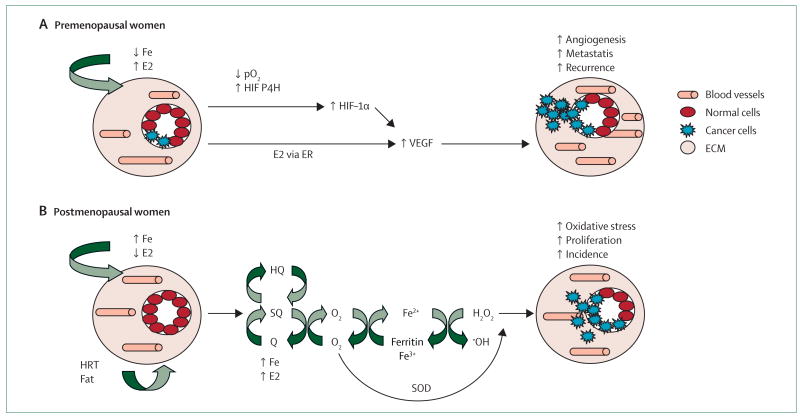
Iron deficiency is a relevant health issue in young women and affects 20% of non-pregnant women aged between 16 and 49 years in industrialised countries and over 40% of all women in developing countries.32–34 However, its relation with breast-cancer outcome has been overlooked. Figure 2 shows how a concomitant deficiency in iron with high oestrogen concentrations in young women can lead to increased VEGF formation, angiogenesis, metastasis, and consequently, high recurrence. This scheme is based on the fact that, by comparison with postmenopausal women, significantly higher concentrations of VEGF are evident in the healthy breast tissue of premenopausal women.35 Moreover, a higher percentage of VEGF-positive tumour cells have been shown to be present in premenopausal women than in postmenopausal women, which is apparently associated with oestrogen-receptor-negative tumours.36 Oestradiol can modulate VEGF concentrations in the breast by the classic oestrogen-receptor pathway.37,38 Indeed, metastatic-breast-cancer spread is more likely when resection of the primary tumour is done in the high oestrogen and low progesterone follicular phase of the menstrual cycle compared with the luteal phase of high progesterone and moderate oestrogen.39,40 However, iron deficiency could be an additional important factor in enhancing VEGF concentrations in premenopausal women. Iron deficiency is likely to increase HIF-1α stabilisation and angiogenesis by two different mechanisms: by lowering concentrations of HIF-degrading prolyl-4-hydroxylase because iron is a cofactor of this enzyme and by decreasing oxygen tension (increased hypoxia) in the body as a result of the presence of fewer red blood cells because of menstruation. HIF-1α prolyl-4-hydroxylase is an enzyme that hydroxylates HIF-1α and is iron dependent.41 Iron deficiency prevents hydroxylation of the proline and stabilises the HIF-1α protein, which subsequently leads to increased VEGF concentrations and angiogenesis.42 Iron deficiency can also result in hypoxic conditions in cancer tissue due to low haemoglobin concentrations in red blood cells. An association between low haemoglobin concentrations and increased serum VEGF concentrations has been reported in patients with cancer.43 Because many premenopausal women are likely to be iron deficient, lowered iron concentrations might contribute to angiogenesis, which could make premenopausal women with breast cancer more susceptible to breast-cancer recurrence than post menopausal women.
Figure 2. Proposed mechanisms for increased breast-cancer recurrence in premenopausal women (A) and for increased breast-cancer incidence in postmenopausal women (B).
Fe=iron. E2=17β-estradiol. pO2=partial pressure of oxygen. HIF-α1=hypoxia inducible factor-1α. P4H=prolyl-4-hydroxylase. VEGF=vascular endothelial growth factor. ER=oestrogen receptor. HQ= hydroquinone. SQ=semi-quinone. Q=quinine. SOD=superoxide dismutase. HRT=hormone replacement therapy. ECM=extracellular matrix.
Iron load and breast-cancer incidence in postmenopausal women
Many studies have suggested that a long lifetime exposure to oestrogen contributes to the development of breast cancer in postmenopausal women.44 However, although the incidence of breast cancer is higher in postmenopausal than in premenopausal women, serum circulating concentrations of oestrogen are lower in postmenopausal women and breast-tissue oestrogen concentrations are comparable. These findings strongly suggest that factors other than oestrogen contribute to the greater incidence of breast cancer in postmenopausal women.
Figure 2 shows that increased iron concentrations after the menopause could be an important aetiological factor in the development of breast cancer in this population. Iron is well known for catalysing Fenton/Haber-Weiss or autoxidation reactions, that lead to the formation of reactive oxygen species (ROS) and lipid peroxidation, as well as their products, which give rise to mutagenic aldehydyes, such as 4-hydroxynonenal.45,46 In conjunction with high local concentrations of oestrogen in the breast from either endogenous (eg, increased COX-2) or exogenous (eg, HRT use or intake of high-fat diet) sources, iron can catalyse the redox cycling of catechol oestrogen metabolites quinone and semi-quinone, which generate ROS shown to contribute to oestrogen-induced carcinogenesis.47 Redox cycling of the stilbene or steroid oestrogen metabolites (eg, oestrone-3,4-quinone) can form superoxide anions,48 which are capable of reducing ferritin-bound Fe3+ to Fe2+ and, thus, cause iron release from ferritin storage. In the presence of superoxide dismutase, the formed H2O2 from this reaction can be readily reduced by Fe2+ to hydroxyl radical (·OH), the most potent oxidant. DNA mutation is a crucial step in carcinogenesis and increased numbers of oxidative DNA lesions have been noted in various tumours, strongly implicating such damage in the aetiology of cancer.49 Furthermore, oestrogen and iron have been known to activate oxidant-mediated signalling through oxidative-stress pathways and promote cell proliferation. ROS produced within cells can act as secondary messengers in intracellular signalling cascades, which induce and maintain the oncogenic phenotype of cancer cells.49 Signal-transduction pathways that are known to be activated by ROS include the mitogen-activated protein kinase pathway, the AP-1 pathway, and the nuclear factor-κB pathway, which affect transcription of genes involved in cell growth and transformation.50 Therefore, an unhealthy diet or use of HRT can contribute to the redox cycling of these oxidant-generating processes, which make this postmenopausal population more susceptible to breast-cancer development.
Studying the association between iron imbalance and breast cancer
Generally, in-vivo rodent models of mammary cancer are appropriate vehicles for the study of human breast cancer.51 In these models, many confounding factors from either endogenous or exogenous sources can be controlled. In previous research, an ovariectomised mouse was used as a model of menopause,52 in which serum oestradiol baseline concentrations were substantially decreased compared with mice with ovaries, thus simulating one of the major postmenopausal settings. However, an important aspect that has not been investigated is the considerable difference in iron concentrations before and after menopause. Because appropriate animal models concurrently mimicking menopausal conditions of oestrogen and iron concentrations are absent, this iron-imbalance hypothesis is difficult to prove in a research laboratory.
Breast cancer develops in women as a result of a combination of external and endogenous factors, such as diet, socioeconomic status, and familial and genetic factors. Thus, identifying one specific factor or mechanism responsible for the disease is complicated in human research. However, if iron deficiency is, in fact, responsible for increased angiogenesis and for breast-cancer recurrence, supplementing young patients with breast cancer with iron before surgery should lead to a decrease in angiogenic response, such as a decline in VEGF formation and, in the long term, decreased relapse. Because iron supplementation is approved by the US Food and Drug Administration, such a study should carry no investigational risks to the patients. If an association between iron and breast-cancer recurrence is proven, a simple and inexpensive clinical solution to breast-cancer recurrence can be provided for premenopausal patients. Conversely, iron-chelation treatment could be proposed for postmenopausal patients.
Search strategy and selection criteria.
Data for this Personal View were identified by searches of Medline, Current Contents, PubMed, and references from relevant articles using the search terms “iron deficiency”, “anaemia”, “iron overload”, “breast cancer”, “menopause”, and “angiogenesis”. Only papers published in English were included. There were no date restrictions.
Conclusion
Iron imbalance is a unique physiological occurrence in women, which is likely to affect health before, during, and after the menopause. The possibility exists that iron deficiency contributes to the high recurrence of breast cancer in premenopausal women, whereas iron load might have a role in the incidence of breast cancer in postmenopausal women. Understanding the role of iron imbalance in breast cancer could lead to adjuvant therapeutic treatments, and potentially benefit patients by decreasing recurrence and incidence and increasing overall survival.
Acknowledgments
I would like to thank Krystyna Frenkel, Catherine Klein, Anne Jacquotte-Zeleniuch, and Julia Smith from NYU School of Medicine for their critical comments.
Footnotes
Conflicts of interest: The author declared no conflicts of interest.
References
- 1.Genazzani AR, Bernardi F, Pluchino N, et al. Endocrinology of menopausal transition and its brain implications. CNS Spectr. 2005;10:449–57. doi: 10.1017/s1092852900023142. [DOI] [PubMed] [Google Scholar]
- 2.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 3.International Women's Health Update. [May 27, 2008]; http://www.med.monash.edu.au/ob-gyn/research/menorr.html.
- 4.Milman N, Byg KE, Ovesen L, Kirchhoff M, Jürgensen KS. Iron status in Danish women, 1984–1994: a cohort comparison of changes in iron stores and the prevalence of iron deficiency and iron overload. Eur J Haematol. 2003;71:51–61. doi: 10.1034/j.1600-0609.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 5.Milman N, Clausen J, Byg KE. Iron status in 268 Danish women aged 18–30 years: influence of menstruation, contraceptive method, and iron supplementation. Ann Hematol. 1998;77:13–19. doi: 10.1007/s002770050405. [DOI] [PubMed] [Google Scholar]
- 6.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 7.Koziol JA, Ho NJ, Felitti VJ, Beutler E. Reference centiles for serum ferritin and percentage of transferrin saturation, with application to mutations of the HFE gene. Clin Chem. 2001;47:1804–10. [PubMed] [Google Scholar]
- 8.Bellino FL. Biology of menopause. New York: Springer; 2000. [Google Scholar]
- 9.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology. 2001;142:4589–94. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 10.Parl FF. Estrogens, estrogen receptor, and breast cancer. Oxford: IOS Press; 2000. [Google Scholar]
- 11.Brueggemeier RW, Richards JA, Petrel TA. Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol. 2003;86:501–07. doi: 10.1016/s0960-0760(03)00380-7. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among US women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Breast cancer facts and figures. [June 10, 2008]; http://www.cancer.org/downloads/STT/CAFF2003BrFPWSecured.pdf.
- 17.Bertheau P, Steinberg SM, Cowan K, Merino MJ. Breast cancer in young women: clinicopathologic correlation. Semin Diagn Pathol. 1999;16:248–56. [PubMed] [Google Scholar]
- 18.Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. doi: 10.1186/1471-2407-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai P, Cserni G, Van De Steene J, et al. Modeling the effect of age in T1-2 breast cancer using the SEER database. BMC Cancer. 2005;5:130. doi: 10.1186/1471-2407-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Zee KJ, Liberman L, Samli B, et al. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: the effect of age. Cancer. 1999;86:1757–67. doi: 10.1002/(sici)1097-0142(19991101)86:9<1757::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Retsky M, Demicheli R, Hrushesky WJM. Does surgery induce angiogenesis in breast cancer? Indirect evidence from relapse pattern and mammography paradox. Int J Surg. 2005;3:179–87. doi: 10.1016/j.ijsu.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Demicheli R, Bonadonna G, Hrushesky WJ, Retsky MW, Valagussa P. Menopausal status dependence of the timing of breast cancer recurrence after surgical removal of the primary tumour. Breast Cancer Res. 2004;6:R689–96. doi: 10.1186/bcr937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–23. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 24.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 25.Giordano FJ, Johnson RS. Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev. 2001;11:35–40. doi: 10.1016/s0959-437x(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 26.Nofech-Mozes S, Spayne J, Rakovitch E, Hanna W. Prognostic and predictive molecular markers in DCIS: a review. Adv Anat Pathol. 2005;12:256–64. doi: 10.1097/01.pap.0000184177.65919.5e. [DOI] [PubMed] [Google Scholar]
- 27.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–90. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 28.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 29.Benouchan M, Colombo BM. Anti-angiogenic strategies for cancer therapy (Review) Int J Oncol. 2005;27:563–71. [PubMed] [Google Scholar]
- 30.Kirsch M, Schackert G, Black PM. Metastasis and angiogenesis. Cancer Treat Res. 2004;117:285–304. doi: 10.1007/978-1-4419-8871-3_17. [DOI] [PubMed] [Google Scholar]
- 31.Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. 2000;5(suppl 1):37–44. doi: 10.1634/theoncologist.5-suppl_1-37. [DOI] [PubMed] [Google Scholar]
- 32.Hercberg S, Preziosi P, Galan P. Iron deficiency in Europe. Public Health Nutr. 2001;4:537–45. doi: 10.1079/phn2001139. [DOI] [PubMed] [Google Scholar]
- 33.Weiss G, Gordeuk VR. Benefits and risks of iron therapy for chronic anaemias. Eur J Clin Invest. 2005;35(suppl 3):36–45. doi: 10.1111/j.1365-2362.2005.01529.x. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 35.Dabrosin C. Positive correlation between estradiol and vascular endothelial growth factor but not fibroblast growth factor-2 in normal human breast tissue in vivo. Clin Cancer Res. 2005;11:8036–41. doi: 10.1158/1078-0432.CCR-05-0977. [DOI] [PubMed] [Google Scholar]
- 36.Fuckar D, Dekanic A, Stifter S, et al. VEGF expression is associated with negative estrogen receptor status in patients with breast cancer. Int J Surg Pathol. 2006;14:49–55. doi: 10.1177/106689690601400109. [DOI] [PubMed] [Google Scholar]
- 37.Garvin S, Nilsson UW, Huss FR, Kratz G, Dabrosin C. Estradiol increases VEGF in human breast studied by whole-tissue culture. Cell Tissue Res. 2006;325:245–51. doi: 10.1007/s00441-006-0159-7. [DOI] [PubMed] [Google Scholar]
- 38.Wood PA, Du-Quiton J, You S, Hrushesky WJ. Circadian clock coordinates cancer cell cycle progression, thymidylate synthase, and 5-fluorouracil therapeutic index. Mol Cancer Ther. 2006;5:2023–33. doi: 10.1158/1535-7163.MCT-06-0177. [DOI] [PubMed] [Google Scholar]
- 39.Hrushesky WJ, Bluming AZ, Gruber SA, Sothern RB. Menstrual influence on surgical cure of breast cancer. Lancet. 1989;2:949–52. doi: 10.1016/s0140-6736(89)90956-2. [DOI] [PubMed] [Google Scholar]
- 40.Wood PA, Bove K, You S, Chambers A, Hrushesky WJ. Cancer growth and spread are saltatory and phase-locked to the reproductive cycle through mediators of angiogenesis. Mol Cancer Ther. 2005;4:1065–75. doi: 10.1158/1535-7163.MCT-05-0028. [DOI] [PubMed] [Google Scholar]
- 41.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci. 2003;116:3041–49. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 42.Linden T, Wenger RH. Iron chelation, angiogenesis and tumor therapy. Int J Cancer. 2003;106:458–59. doi: 10.1002/ijc.11223. [DOI] [PubMed] [Google Scholar]
- 43.Dunst J, Pigorsch S, Hansgen G, Hintner I, Lautenschlager C, Becker A. Low hemoglobin is associated with increased serum levels of vascular endothelial growth factor (VEGF) in cancer patients. Does anemia stimulate angiogenesis? Strahlenther Onkol. 1999;175:93–96. doi: 10.1007/BF02742340. [DOI] [PubMed] [Google Scholar]
- 44.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 45.Frenkel K. Carcinogenesis: role of reactive oxygen and nitrogen species. In: Bertino J, editor. Encyclopedia of cancer. 2nd. New York: Academic Press; 2002. pp. 359–67. [Google Scholar]
- 46.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533:153–71. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 48.Wyllie S, Liehr JG. Release of iron from ferritin storage by redox cycling of stilbene and steroid estrogen metabolites: a mechanism of induction of free radical damage by estrogen. Arch Biochem Biophys. 1997;346:180–86. doi: 10.1006/abbi.1997.0306. [DOI] [PubMed] [Google Scholar]
- 49.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Sliva D. Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets. 2004;4:327–36. doi: 10.2174/1568009043332961. [DOI] [PubMed] [Google Scholar]
- 51.Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ Health Perspect. 1996;104:938–67. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raafat AM, Hofseth LJ, Haslam SZ. Proliferative effects of combination estrogen and progesterone replacement therapy on the normal postmenopausal mammary gland in a murine model. Am J Obstet Gynecol. 2001;184:340–49. doi: 10.1067/mob.2001.110447. [DOI] [PubMed] [Google Scholar]