Abstract
In mammals, the part of the nervous system responsible for most circadian behavior can be localized to a pair of structures in the hypothalamus known as the suprachiasmatic nucleus (SCN). Previous studies suggest that the basic mechanism responsible for the generation of these rhythms is intrinsic to individual cells. There is also evidence that the cells within the SCN are coupled to one another and that this coupling is important for the normal functioning of the circadian system. One mechanism that mediates coordinated electrical activity is direct electrical connections between cells formed by gap junctions. In the present study, we used a brain slice preparation to show that developing SCN cells are dye coupled. Dye coupling was observed in both the ventrolateral and dorsomedial subdivisions of the SCN and was blocked by application of a gap junction inhibitor, halothane. Dye coupling in the SCN appears to be regulated by activity-dependent mechanisms as both tetrodotoxin and the GABAA agonist muscimol inhibited the extent of coupling. Furthermore, acute hyperpolarization of the membrane potential of the original biocytin-filled neuron decreased the extent of coupling. SCN cells were extensively dye coupled during the day when the cells exhibit synchronous neural activity but were minimally dye coupled during the night when the cells are electrically silent. Immunocytochemical analysis provides evidence that a gap-junction—forming protein, connexin32, is expressed in the SCN of postnatal animals. Together the results are consistent with a model in which gap junctions provide a means to couple SCN neurons on a circadian basis.
Keywords: biocytin, circadian rhythms, dye coupling, gap junctions, Rattus rattus, SCN
The circadian timing system regulates many aspects of an organism’s behavior and physiology. In mammals, the part of the nervous system responsible for most circadian behavior can be localized to a pair of structures in the hypothalamus known as the suprachiasmatic nucleus (SCN). Importantly, when SCN cells are removed from the organism and maintained in a brain slice preparation, they continue to generate 24-h rhythms in electrical activity, secretion, and gene expression (reviewed by Gillette, 1997). Previous studies suggest that the basic mechanism responsible for the generation of these rhythms is intrinsic to individual cells in the SCN (Welsh et al., 1995) and perhaps in other cell populations (Balsalobre et al., 1998). The core molecular mechanism driving these cellular oscillations appears to be a negative feedback loop operating at the transcriptional/translational levels (e.g., Reppert, 1998; Sangoram et al., 1998; Wilsbacher and Takahashi, 1998). There is also evidence that the cells within the SCN are coupled to one another and that this coupling is important for the normal functioning of the circadian system. In the brain slice, SCN cells are thought to show only weakly coupled electrical activity (Bouskila and Dudek, 1993). But this coupling is sufficient to maintain the expression of rhythmic electrical activity in the SCN population. In contrast, in dissociated cultures of rat SCN, it appears that gap junctions couple astrocytes but not neurons (Welsh and Reppert, 1996). In these conditions, individual SCN neurons continue to express 24-h rhythms in electrical activity, but the cells drift out of phase with each other (Welsh et al., 1995) so that the population can no longer function as a time-keeping system. One mechanism that would coordinate electrical activity is the presence of direct electrical connections between cells mediated by gap junctions.
Gap junction channels form the basis of cell-to-cell electrotonic communication in the developing nervous system. These channels allow the passage of ions and other small molecules (up to 1 kD) between coupled cells and function to connect cells both electrically and metabolically. A gap junction channel is formed by two hemichannels, each composed of 6 connexin proteins. Connexins (Cx) are encoded by a multigene family of which Cx26, 32, 36, and 43 are the major isoforms expressed in the developing brain (e.g., Kumar and Gilula, 1996; Nadarajah et. al., 1997; Srinivas et al., 1999). Previous studies have found that Cx32 is typically expressed in neurons and oligodendrocytes, Cx43 is expressed in astrocytes, whereas Cx26 is restricted to nonneuronal cells in the ependyma and meninges. This distinction is not absolute as a previous study found that Cx43 was also expressed in a subpopulation of cortical neurons (Nadarajah et al., 1996). Nevertheless, Cx32 and perhaps Cx36 appear to be important for the formation of gap junctions in the central nervous system (CNS). Despite the recent progress made by the molecular description of this growing family of gap junction proteins, our understanding of the specific functions of these channels and their regulation is still limited.
The present study utilized whole-cell patch-clamp electrophysiology with biocytin-filled electrodes to examine if direct connections exist between developing SCN neurons in a brain slice preparation. The passage of biocytin from one cell to another will be taken as evidence for the presence of these direct connections presumably mediated by gap junctions. Next, experiments were run to determine if the permeability of these connections, that is, the extent of dye coupling, was modulated by activity-dependent mechanisms. The possibility of a circadian rhythm in electrical connections between SCN cells also was examined by making a day—night comparison between dye coupling in SCN slices of animals maintained in a light—dark (LD) cycle and in constant darkness. Finally, immunocytochemical analysis was used to characterize the possible expression of the Cx32 protein in the SCN of postnatal animals.
MATERIALS AND METHODS
Animals and Brain Slice Preparation
Brain slices were prepared using standard techniques from rats (Sprague-Dawley) between 10 and 14 days of age. The circadian oscillator based in the SCN is functional by this age (e.g., Reppert and Schwartz, 1984). Rats were killed by decapitation, and brains were dissected and placed in cold oxygenated artificial cerebral spinal fluid (ACSF) containing (in mM) NaCl, 130; NaHCO3, 26; KCl, 3; MgCl2, 5; NaH2PO4, 1.25; CaCl2, 1.0; glucose, 10 (pH 7.2–7.4; osmolality 290–300 mOsm). After cutting slices, transverse sections (350 μm) were placed in ACSF (25–27°C) for at least 1 h (in this solution CaCl2 was increased to 2 mM, MgCl2 was decreased to 2 mM, and 4 mM lactate was added). Slices were constantly oxygenated with 95% O2-–5% CO2. Slices were placed in a perfusion chamber (Warner Instruments, Hamden, CT) attached to the stage of the fixed-stage upright microscope. The slice was held down with thin nylon threads glued to a platinum wire and submerged in continuously flowing, oxygenated ACSF (25°C) at 2 mL/min. Solution exchanges within the slice were achieved by a rapid gravity feed delivery system. In our system, the effects of bath-applied drugs began within 15 s and were typically complete by 1–2 min.
IR Differential Interference Contrast Videomicroscopy
Slices were viewed with an upright compound microscope (Olympus BX50), using a water immersion lens (40×) and differential interference contrast (DIC) optics. They were illuminated with near IR light by placing an IR bandpass filter (750–1050 nm) in the light path. The image was detected with an IR-sensitive video camera (C2400; Hamamatsu, Bridgewater, NJ) and displayed on a video monitor. A camera controller allowed analog contrast enhancement and gain control. Cells were typically visualized from 30 to 100 μm below the surface of the slice. In the present study, IR videomicroscopy was used to visualize cells within the brain slice and to limit some of the uncertainty as to the cell type. This imaging technique allowed us to clearly see the SCN and to exclude cells from the surrounding hypothalamic regions. In addition, morphological criteria were used to target SCN neurons and to avoid taking measurements from cells that were clearly glia. Although size is hardly foolproof, electrophysiological recording were used to demonstrate that the cells expressed the electrical properties of neurons.
Whole-Cell Patch-Clamp Electrophysiology
Methods were similar to those described previously (e.g., Cepeda et al., 1998; Colwell et al., 1998). Briefly, electrodes were pulled on a multistage puller (P-97; Sutter, Novato, CA). Electrode resistance in the bath was typically 2–4 MΩ. The standard solution in the patch pipette contained (in mM): Cs-methanesulfonate, 125; EGTA, 9; Hepes, 8; MgATP, 8; NaCl, 4; KCl, 3; and MgCl2, 1. The pH was between 7.25 and 7.3, and the osmolality was between 280 and 290 mOsm. Whole-cell recordings were obtained with an Axon Instruments 200B amplifier and monitored on-line with pCLAMP (Axon Instruments, Foster City, CA). To minimize changes in offset potentials with changing ionic conditions, the ground path used an ACSF agar bridge. Cells were approached with slight positive pressure (23 cm H2O), and offset potentials were corrected. The pipette was lowered to the vicinity of the membrane, keeping a positive pressure. After forming a high-resistance seal (2–10 GΩ) by applying negative pressure, a second pulse of negative pressure was used to break the membrane. While entering the whole-cell mode, a repetitive 10-mV test pulse was delivered in a passive potential range (≈60 to -70 mV). Whole-cell capacitance and electrode resistance were neutralized and compensated (50–80%) using the test pulse. Data acquisition was then initiated. Series and input resistance was monitored throughout the experiment by checking the response to small pulses in a passive potential range.
Cell Identification
The morphology of cells that had been electrophysiologically analyzed were examined using intracellular marking with biocytin. The methods for tissue processing of biocytin-filled cells are well established (Kita and Armstrong, 1991) and commonly used by our research group (e.g., Colwell and Levine, 1995). Within 2 h of electrophysiological analysis, whole slices (350 μm) containing biocytin-filled cells were fixed by overnight immersion in paraformaldehyde (4%) in phosphate-buffered saline (PBS). The slices were then washed with Tris-buffered saline for 1 h and processed histochemically for biocytin staining. The slices were then incubated in peroxidase-conjugated streptavidin (1:250 dilution) for 2 h. The tissues were treated with diaminobenzidine tetrahydrochloride (DAB) and H2O2, and then mounted for examination.
Immunocytochemistry
Animals were anesthetized and then perfused transcardially with saline followed by 4% paraformaldehyde. Brains were immersed in fixative overnight and then sectioned (50 μm thick) on a cryostat. Sections were incubated with the primary antibody (diluted with 3% normal goat serum plus 0.1% Triton X-100 in PBS) for 48 h at 10°C. The tissue was then incubated for 1 h in biotinylated secondary antibody at room temperature, followed by incubation in an avidinbiotin-peroxidase complex at room temperature for 1 h. Sections were incubated in DAB (0.5%) for 5–20 min. All previous steps were followed by rinses in PBS (3 times for 5 min each). The tissue was mounted on slides, dehydrated, and cleared in alcohols and xylenes before coverslipping. Polyclonal antibodies for Cx32 and 43 raised in rabbit were purchased (Zymed, South San Francisco, CA). A dilution series was been performed for both antibodies in postnatal day 14 tissue, with optimal dilution found to be 1:500.
Lighting Conditions
In order to look for possible diurnal variation in dye coupling, animals were maintained on a daily LD cycle consisting of 12 h of light followed by 12 h of dark. It is already well established that cells in the SCN continue to show circadian oscillations when isolated from the animal in a brain slice preparation. Accordingly, care must be taken as to the time in the daily cycle when the data are collected. Most of the animals were killed 30 min before the time that the lights would have turned on in the LD cycle. The data from these animals were collected between zeitgeber time (ZT) 0 and 6 and pooled to form a “day” group. For comparison, some of the animals were killed immediately after the lights were turned off. The data from these animals were collected between ZT 12 and 18 and pooled to form a “night” group. In order to demonstrate that any diurnal rhythm is circadian, it is necessary to show that the rhythm continues in constant darkness. For these experiments, animals were placed in constant darkness for 2 days prior to the preparation of brain slices. The data were collected between circadian time (CT) 0–6 and CT 12–18 and were pooled to form “subjective day” and “subjective night” groups.
Statistical Analyses
Between-group differences were evaluated using Mann-Whitney rank sum tests. Between-group differences in the proportion of coupled cells were evaluated using a Z test. Values were considered significantly different if p < .05. All tests were performed using SigmaStat (SPSS, Chicago, IL). Values are shown as means ± S.E.M.
RESULTS
SCN Cells Are Dye Coupled
In the initial experiment, whole-cell recordings with biocytin-filled patch electrodes were performed in 19 neurons in the SCN of 14 rats between 10 and 14 days of age. All cells had resting membrane potentials more negative than −55 mV and had peak inward sodium currents of greater than 2000 pA. All cells were recorded for 20 min to allow the dye to diffuse into the cell and its processes. Unless otherwise noted, the cells were held at −70 mV. Of the 19 single cells filled during the day (ZT 2–8), histological examination revealed that in most cases (14/19) multiple cells were labeled with biocytin; that is, most cells were dye coupled (Fig. 1). In most cases in which dye coupling occurred, the original filled cell could be identified by reference to the IR DIC image and by its dark and extensive staining. Coupled cells were found in both the dorsomedial and ventrolateral subdivisions of the SCN. Although there was a tendency for the cells in the dorsomedial subdivision to be coupled to more cells (16 ± 3 vs. 9 ± 3), these differences were not significant. The clusters of coupled cells were restricted to one of the subdivisions or the other. For example, a cell filled in the dorsomedial subdivision was not coupled to cells in ventrolateral region. In each case, electrophysiological measurements were used to confirm that the original biocytin-filled cell was a neuron that generated action potentials. The coupled cells also appeared neuronal; however, it is difficult to rule out the involvement of other cell types. Bath application of the gap junction blocker halothane (0.1%) prevented the dye coupling (n = 6; Fig. 1). Of 9 additional cells filled in hypothalamic regions immediately outside the SCN, none exhibited dye coupling. Additional control experiments were performed in which biotin-filled electrodes were placed into slices for 20 min under slight positive pressure, and no cellular staining was observed (n = 8; Fig. 1).
Figure 1.
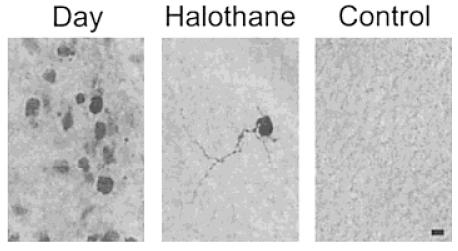
Multiple SCN cells are stained after intracellular injection of the tracer biocytin. In these experiments, single SCN neurons are voltage clamped with a whole-cell patch electrode that contains biocytin (2 mg/mL). After recording, the presence of biocytin within cells was visualized by HRP-streptavidin and reaction with DAB. Left: In most cases, multiple cells were found to stain for biocytin following this procedure. Middle: This dye coupling was prevented by the application of the gap junction blocker halothane (0.1%). Right: No staining was observed when biocytin-loaded electrodes were placed into the slice without patching a cell. Scale bar = 10 μm.
Dye Coupling between Cells in the SCN Appears to be Activity Dependent
In a number of preparations, gap junction permeability appears to be regulated by electrical activity and/or chemical synaptic transmission (e.g., Pereda et al., 1992; O’Donnell and Grace, 1993; Pereda and Faber, 1996). As a first step to determine whether this might occur in the SCN, tetrodotoxin (TTX; 1 μM) was applied to SCN slices during the day. TTX is a blocker of voltage-sensitive sodium channels that eliminates both action potentials and evoked synaptic activity in SCN neurons. This and other drug treatments were present in the bath for the entire duration of the patch recording. Of the 16 cells filled in the presence of TTX, in 11 cases only a single neuron was stained; that is, most of the cells were not coupled (Fig. 2). This effect of TTX on the proportion of cells that were dye coupled as well as the average number of cells that were coupled was significant ( p < .001). Because SCN neurons are mostly γ-aminobutyric acid (GABA)-containing cells that synapse onto other cells in the SCN, we also sought to determine the effect of agents that altered GABA-mediated synaptic communication. Although application of the GABAA receptor antagonist bicuculline (20 μM) did not alter dye coupling (n = 4), the GABAA receptor agonist muscimol decreased coupling during the day (Fig. 2). Of the 10 cells filled in presence of muscimol (10 μM), in 7 cases only a single neuron was stained; that is, most of the cells were not coupled. Similar to what was observed with TTX, the bath application of muscimol significantly decreased both the proportion of cells that exhibited dye coupling as well as the average number of cells in a coupled cluster (p < .01). Finally, holding the cell’s resting membrane potential at −80 mV for the entire duration of the patch recording altered the electrical activity of the SCN neuron originally filled with biocytin. Under these conditions, the neurons do not generate spontaneous action potentials, but still receive synaptic input from surrounding cells. Once again, this treatment was maintained and significantly reduced both the proportions of cells that exhibited dye coupling as well as the number of coupled cells (p < .05; Fig. 2). However, by our measures, the inhibition was less robust; for example, 6 of 14 cells still exhibited dye coupling and those 6 cells were coupled to an average of 10 other cells. Depolarizing the original filled neuron to −50 mV did not significantly increase the coupling frequency (n = 5). Together, these results suggest that dye coupling and, presumably, gap junction permeability is actively regulated in SCN neurons.
Figure 2.
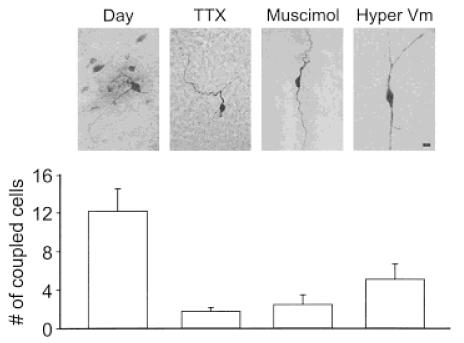
Dye coupling among SCN cells was activity-dependent. To determine if the transfer of dye between SCN cells may be actively regulated, TTX or muscimol was placed in the bath while the original neuron was being filled with biocytin. Both treatments inhibit the spontaneous electrical activity exhibited by these cells as well as chemical synaptic transmission. Both treatments inhibited dye coupling as indicated by images and corresponding cell counts. Similar results (labeled “Hyper Vm”) were obtained when the electrical activity of the SCN neuron originally filled with biocytin was inhibited by holding the cell’s resting membrane potential at −80 mV. Under these conditions, the neurons do not generate spontaneous action potentials but still receive synaptic input from surrounding cells. All three treatments produced a significant ( p < .05) decrease in the number of coupled cells. Scale bar = 10 μm.
Dye Coupling among Cells in the SCN Exhibits Circadian Variation
SCN neurons are known to undergo a daily rhythm of electrical activity with spontaneous activity high during the day and low during the night (Green and Gillette, 1982; Groos & Hendriks, 1982; Shibata et al., 1982). This rhythm provides another opportunity to test our hypothesis without pharmacological manipulation. If electrical activity regulates dye coupling in the SCN, then dye coupling should be more common during the day than during the night. Indeed, we found that few SCN neurons during the night appeared to be dye coupled (Fig. 3). Of the 8 cells filled during the night (ZT 13–18), in 6 cases only a single neuron was stained; that is, most cells were not coupled. This day/night difference in the proportion of cells that were dye coupled as well as the number of coupled cells was significant (p < .01). In order to demonstrate that any diurnal rhythm is circadian, it is necessary to show that the rhythm continues in constant darkness. For these experiments, animals were placed in constant darkness for 2 days prior to the preparation of brain slices. The data were collected between CT 0 – 6 and CT 12–18 and were pooled to form a “subjective day” and “subjective night” group. Again, there was a daily rhythm in dye coupling with significantly (p < .01) more cells coupled during the subjective day (12 ± 3; n = 8) than during the subjective night (4 ± 3; n = 7). Thus, there appears to be a circadian rhythm in coupling between cells in the SCN.
Figure 3.
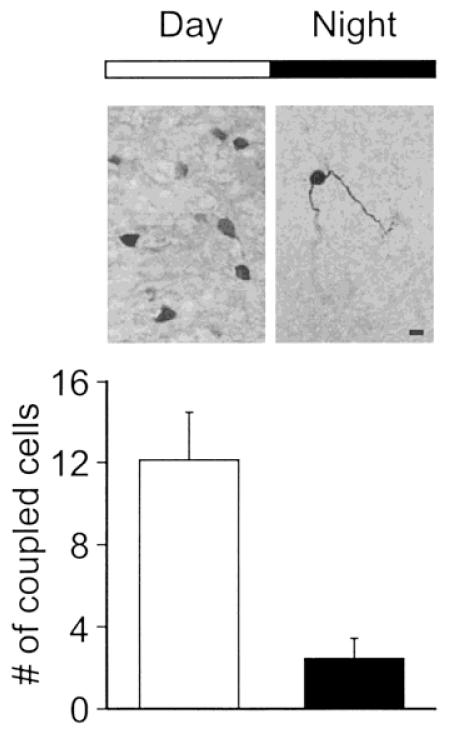
Dye coupling among SCN cells exhibited a circadian rhythm. SCN neurons are known to undergo a daily rhythm of electrical activity with spontaneous activity high during the day and low during the night. Although most SCN were dye coupled during the day (ZT 1–6), only a few SCN neurons during the night appeared to be dye coupled. The day/night difference in the number of coupled cells was significant ( p < .01).
Cx32 Is Expressed in SCN
Cx32 appears to be one of the main connexin isoforms responsible for the formation of gap junctions between neurons. Accordingly, a polyclonal antibody against Cx32 was used to stain SCN tissue from 14-day-old animals perfused during the day (ZT 2). Cx32 immunoreactivity was clearly present in the SCN and the surrounding region (Fig. 4). Sections from 5 of 6 animals were judged to exhibit labeled cell bodies, and all expressed more diffuse immunoreactivity. Within the SCN, no obvious differences in Cx32 immunoreactivity between the dorsomedial and ventrolateral subdivisions were apparent. Positive staining was also obtained with the antibody against Cx43 (data not shown), suggesting that the astrocytes may also be expressing gap junctions. None of the control sections incubated with preabsorbed primary antibody showed immunoreactivity. Next, Cx32 expression was compared between animals perfused during the day (ZT 2) with expression in animals perfused during the night (ZT 14). There were no consistent differences in the level of immunoreactivity expressed in the SCN in the day (n = 6) or night (n = 6). Finally, the developmental ontogeny of Cx32 expression was examined in the SCN. Labeled cells were observed at all ages examined (7, 14, 21, 28, and 60 days of age).
Figure 4.
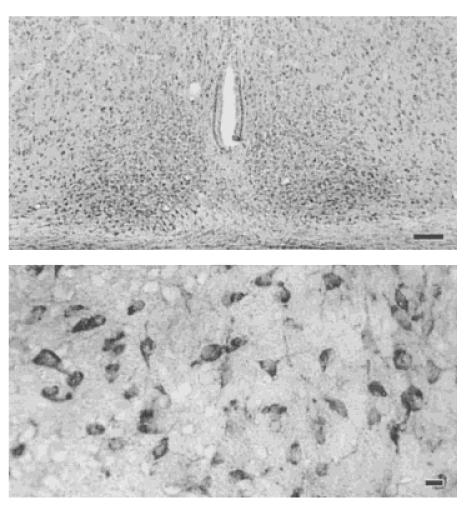
Connexin 32 protein is expressed in the SCN. Cx32 appears to be one of the main Cx isoforms responsible for the formation of gap junctions between neurons. Accordingly, a polyclonal antibody against Cx32 (Zymed, 1:500 dilution) was used to stain SCN tissue from 14-dayold animals perfused during the day (ZT 2). Top: Low magnification (100×) view of Cx32 expression in the SCN. Scale bar = 100 μm. Bottom: Higher magnification (400×) view of labeled cells within the SCN. Scale bar = 10 μm.
DISCUSSION
One of the most characteristic and functionally important features of neurons within the SCN is that they exhibit a daily rhythm of electrical activity. This rhythm continues when SCN neurons are maintained in a brain slice preparation (reviewed by Gillette, 1997) or even when maintained in culture conditions (Welsh and Reppert, 1996). This latter observation provides important evidence that the generation of the daily rhythm is an intrinsic property of single cells. However, these isolated cells quickly drift out of phase with one another and, as a population, would not form a functional timing system. So an important issue is to understand how these oscillatory cells communicate with each other. SCN neurons are GABAergic cells that mostly project within the nucleus. Undoubtedly, chemical synaptic mechanisms are important for communication within the SCN. However, it is important to note that in a low Ca2+ solution, SCN neurons exhibit loosely synchronized bursts of action potentials (Bouskila and Dudek, 1993). In addition, a circadian rhythm in glucose utilization is present in the SCN prior to synapse formation in the developing rat (Reppert and Schwartz, 1984; Shibata and Moore, 1988). The low Ca2+ prevents conventional chemical synaptic transmission and coupled with the developmental results, these studies raise the possibility that another mechanism underlying cell-to-cell communication is in operation within the SCN. We believe that one such mechanism may be the presence of gap junctions linking SCN cell populations. First, and perhaps most convincingly, SCN neurons exhibit dye coupling in brain slice preparations prepared from both immature (present study) and adult (Jiang et al., 1997) rats. Dye coupling is a phenomenon in which a labeled molecule or tracer injected into single cells spreads into surrounding cells. This is a diagnostic feature of gap junction mediated cell-to-cell communication. This dye coupling can be inhibited by halothane, a known gap junction blocker. Finally, immunocytochemical data indicate that SCN cells express one of the connexins (Cx32) that form gap junctions between neurons.
Evidence previously reported may be inconsistent with our suggestion that gap junctions serve to couple SCN neurons. First, a careful electron microscopic analysis of SCN cells in fixed tissue found gap junctions common between astrocytes but not neurons (Van den Pol, 1980). However, gap junctions that are small compared to section thickness and infrequent are difficult to identify with electron microscopy (EM) and may require a different set of techniques. For example, the use of a combination of confocal microscopy and “grid mapped” freeze-fracture demonstrated a high incidence of mixed chemical and electrical synapses on neurons within the spinal cord (Rash et al., 1996). These electrical connections were missed by conventional EM techniques. Second, in a primary cell culture preparation, astrocytes but not neurons were found to form gap junctions and express Cx43. One explanation may be that the preparation of these neurons in cell culture may have disrupted these direct cell-to-cell connections. Although it is possible that SCN cells are coupled via a low-resistance pathway not mediated by gap junctions, it seems more likely that gap junctions are present but at a low density. An appealing strategy for future work would be to combine freeze-fracture EM with immunocytochemical labeling of connexin proteins to confirm the presence of these channels between SCN neurons (see Fujimoto, 1997).
Data from the present study suggest that the extent of dye coupling, and presumably gap junction permeability, between SCN cells is activity dependent. The bath applications of two agents (TTX and muscimol) that block electrical activity of SCN neurons through distinct mechanisms both inhibited the extent of dye coupling. These treatments inhibit both electrical activity and synaptic transmission throughout the brain slice. In part to increase the specificity of the manipulation, the voltage clamp was used to specifically hyperpolarize the membrane potential of the originally filled neuron. These hyperpolarized cells still exhibited dye coupling, but the extent of the dye coupling was significantly reduced. Finally, we took advantage of the naturally occurring daily rhythm in the frequency of activity in SCN neurons in order to demonstrate that coupling varies as a function of time of day. This remarkable observation provides further support for the proposition that coupling varies as a function of the activity level of these neurons. The mechanisms underlying this regulation are unknown but must occur quickly on the time scale of minutes. These observations are consistent with a growing body of evidence that gap junction channels do not just passively allow the spread of signaling molecules but instead form an actively regulated communication system. In perhaps the best characterized examples, the chemical transmitter dopamine has been shown to modulate the degree of electrical and dye coupling between neurons in several regions of the nervous system (Piccolino et al., 1984; Lasater, 1987; Cepeda et al., 1989; Pereda et al., 1992; O’Donnell and Grace, 1993; McMahon and Brown, 1994). In addition, in Mauthner cells in the goldfish, activity-dependent enhancement of intercellular coupling has been demonstrated (Pereda and Faber, 1996). This enhancement of coupling was rapid and mediated by a Ca2+/calmodulin-dependent kinase II (Pereda et al., 1998). We have recently reported a daily rhythm in the resting Ca2+ concentration in SCN neurons that also peaks during the “day” when coupling is highest (Colwell, 2000). These regulatory mechanisms presumably serve physiological functions, and, in a few cases, it has been possible to link changes in gap junction permeability with changes in the physiological state of the organism. For example, in the retina, levels of light adaptation lead to changes in dye coupling among amacrine cells (Bloomfield et al., 1997). Furthermore, in the supraoptic nucleus of the hypothalamus, changes in the reproductive state of the rat are clearly linked to changes in gap junction permeability (Micevych et al., 1996; Hatton, 1997). In this context, SCN neurons may provide another example of “state-dependent” variations in gap junction permeability.
The gap junction—mediated communication between SCN cells may have a developmental function. Several groups have shown that dye coupling is developmentally regulated in the nervous system with extensive dye coupling for the first couple of weeks of postnatal development, which decreases as differentiation proceeds (e.g., LoTurco and Kriegstein, 1991; Cepeda et al., 1993; Conners et al., 1993; Peinado et al., 1993). The uncoupling of neurons in various regions in the nervous system overlaps with synapse formation in these areas (Katz and Shatz, 1996; Kandler and Katz, 1998). This inverted relationship between dye coupling and synapse formation leads to the suggestion that gap junctions help create a scaffolding within neuronal populations that guides the subsequent development of synaptic connections. This suggestion is supported by the observation that, in brain slices prepared from early postnatal rat neocortex, neuronal “domains” have been defined on the basis of spontaneous, restricted calcium waves that originate in a few neurons and propagate over a distance of 50–100 μm (Yuste et al., 1992). These domains appear to be organized as a result of coupling via gap junctions (Yuste et. al., 1995). In the present study, the data were collected from animals between 10 and 14 days of age, when circuit formation in the nervous system is still ongoing. In this regard, it is interesting to note that both the major subdivisions (ventrolateral or dorsomedial) within the SCN exhibit dye coupling; however, the coupling appears restricted within a subdivision. Many of the cells in the SCN express neuropeptides, and differences in peptide expression can serve as a basis to segregate SCN cells (e.g., Card et al., 1981; van den Pol and Tsujimoto, 1985). We did not investigate whether coupled cell clusters ultimately expressed the same neuropeptides or were otherwise linked. However, based on comparisons with other regions of the CNS, one possible function of these coupled cell clusters in the developing nervous system may be to link cells destined to form functional circuits in the adult.
There are also reasons to think that electrical coupling between neurons may also function in the adult SCN. A previous study in acute brain slices demonstrated that many neurons (at least 30%) in the adult rat SCN are dye coupled (Jiang et. al., 1997). Second, the Cx32 expression continues into adulthood (also see Micevych and Abelson, 1991). These observations raise the question of the functional significance of the coupling for the circadian timing system. Previous studies also provide some support for the hypothesis that gap junction—mediated communication may link SCN cells. Although SCN cells do not exhibit absolutely synchronized action potential generation, the population does show a pronounced daily rhythm with cells showing spontaneous activity during the day. A careful analysis of firing patterns suggests that the neurons were weakly coupled, such that the activity of one cell increases the probability that a neighbor will generate an action potential (Bouskila and Dudek, 1993). This weak coupling occurs in the presence of a low Ca2+ solution, suggesting a mechanism that does not involve chemical synaptic transmission, but is sufficient to maintain the expression of rhythmic electrical activity. In contrast, in dissociated cultures of rat SCN, it appears that gap junctions couple astrocytes but not neurons (Welsh and Reppert, 1996). In these conditions, SCN cells continue to express 24-h rhythms in electrical activity; however, the cells drift out of phase with each other (Welsh et al., 1995) so that the population can no longer function as a time-keeping system. This is in spite of the fact that the cultured cells appear to form functional synaptic connections. In addition, the administration of the gap junction blocker halothane disrupts the circadian rhythm in spontaneous neural activity recorded from a rat SCN brain slice (Prosser et al., 1994) as well as blocks light-induced phase shifts of the circadian system (Colwell et al., 1993). Comparatively, it is interesting to note that other structures that are known to function physiologically as circadian oscillators are also known to contain cells connected by gap junctions. In vertebrates this would include the retina and the pineal gland and, in non-vertebrates, the eyes of the marine mollusks Bulla and Aplysia. These observations are at least consistent with the hypothesis that electrical coupling via gap junctions may play a role in the coordination and synchronization of circadian pacemaker cells in the SCN.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: HL64582.
Contract grant sponsor: National Institutes of Health; contract grant number: MH59186.
Contract grant sponsor: National Institutes of Health; contract grant number: HD04612.
Contract grant sponsor: Whitehall Foundation; contract grant number: F98P15.
Footnotes
I am also grateful to Janelle Asai and Mary K. Lobo whose technical support was critical for this study as well as Donna Crandall for assistance with the figures. Finally, I would like to thank Drs. M. Levine and P. Micevych for their discussions of the experiments described in this article.
REFERENCES
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. Synchronous neuronal activity in the suprachiasmatic nucleus independent of chemical synaptic transmission. Proc Natl Acad Sci USA. 1993;90:3207–3210. doi: 10.1073/pnas.90.8.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Brecha N, Karten HJ, Moore RY. Immunocytochemical localization of vasoactive intestinal peptide-containing cells and processes in the suprachiasmatic nucleus of the rat. J Neurosci. 1981;1:1289–1303. doi: 10.1523/JNEUROSCI.01-11-01289.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole-cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Walsh JP, Hull DC, Howard SG, Buchwald NA, Levine MS. Dye coupling in the striatum of the cat. Modulation by dopamine-depleting lesions. Synapse. 1989;4:229–237. doi: 10.1002/syn.890040308. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Walsh JP, Peacock W, Buchwald NA, Levine MS. Dye-coupling in human neocortical tissue resected from children with intractable epilepsy. Cereb Cortex. 1993;3:95–107. doi: 10.1093/cercor/3.2.95. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Cepeda C, Crawford C, Levine MS. Postnatal development of NMDA evoked responses in the neostriatum. J Dev Neurobiol. 1998;20:154–163. doi: 10.1159/000017310. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Kaufman CM, Menaker M, Ralph M. Light-induced phase shifts and Fos expression in the hamster circadian system: the effects of anesthetics. J Biol Rhythms. 1993;8:179–188. doi: 10.1177/074873049300800301. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cyclic AMP-dependent mechanisms. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Benardo LS, Prince DA. Coupling between neurons of the developing rat neocortex. J Neurosci. 1993;3:773–782. doi: 10.1523/JNEUROSCI.03-04-00773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. SDS-digested freeze-fracture replica labeling electron microscopy to study the two-dimensional distribution of integral membrane proteins and phospholipids in biomembranes: practical procedure, interpretation and application. Histochem Cell Biol. 1997;107:87–96. doi: 10.1007/s004180050092. [DOI] [PubMed] [Google Scholar]
- Gillette MU. Cellular and biochemical mechanisms underlying circadian rhythms in vertebrates. Curr Opin Neurobiol. 1997;7:797–804. doi: 10.1016/s0959-4388(97)80138-9. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groos GA, Hendriks J. Circadian rhythm in electrical discharge of rat suprachiasmatic nucleus recorded in vitro. Neurosci Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Yang YQ, Allen CN. Tracer and electrical coupling of rat suprachiasmatic nucleus neurons. Neuroscience. 1997;77:1059–1066. doi: 10.1016/s0306-4522(96)00539-8. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Lasater EM. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1987;84:7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Kriegstein AR. Clusters of coupled neuroblasts in embryonic neocortex. Science. 1991;252:563–566. doi: 10.1126/science.1850552. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Brown DR. Modulation of gap junction channel gating at zebrafish retinal electrical synapses. J Neurophysiol. 1994;72:2257–2267. doi: 10.1152/jn.1994.72.5.2257. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Abelson L. Distribution of mRNAs coding for liver and heart gap junction proteins in the rat central nervous system. J Comp Neurol. 1991;305:96–118. doi: 10.1002/cne.903050110. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Popper P, Hatton GI. Connexin 32 mRNA levels in the rat supraoptic nucleus: up-regulation prior to parturition and during lactation. Mol Neuroendocrinol. 1996;63:39–45. doi: 10.1159/000126933. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Jones AM, Evans WH, Paravelas JG. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Thomaidou D, Evans WH, Paravelas JG. Gap junctions in the adult cerebral cortex: regional differences in their distribution and cellular expression of connexins. J Comp Neurol. 1996;376:326–342. doi: 10.1002/(SICI)1096-9861(19961209)376:2<326::AID-CNE13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A, Uste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Pereda AE, Bell TD, Chang BH, Czernik AJ, Nairn AC, Soderling TR, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc Natl Acad Sci USA. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Faber DS. Activity-dependent short-term enhancement of intercellular coupling. J Neurosci. 1996;16:983–992. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci USA. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M, Neyton J, Gerschenfeld H. Decrease in gap junctional conductance induced by dopamine and cyclic adenosine monophosphate in horizontal cells of the turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Edgar DM, Heller HC, Miller JD. A possible glial role in the mammalian circadian clock. Brain Res. 1994;643:296–301. doi: 10.1016/0006-8993(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Rash JE, Dillman RK, Bilhartz BL, Duffy HS, Whalen LR, Yasumura T. Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc Natl Acad Sci USA. 1996;93:4235–4239. doi: 10.1073/pnas.93.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM. A clockwork explosion! Neuron. 1998;21:1–4. doi: 10.1016/s0896-6273(00)80234-2. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. The suprachiasmatic nuclei of the fetal rat: characterization of a functional circadian clock using 14C-labeled deoxyglucose. J Neurosci. 1984;4:1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, Young MW, Weitz CJ, Takahashi JS. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Development of the fetal circadian rhythm after disruption of the maternal circadian system. Dev. Brain Res. 1988;41:313–317. doi: 10.1016/0165-3806(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247:154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. The hypothalamic suprachiasmatic nucleus of the rat: intrinsic anatomy. J Comp Neurol. 1980;91:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Reppert SM. Gap junctions couple astrocytes but not neurons in dissociated cultures of rat suprachiasmatic nucleus. Brain Res. 1996;706:30–36. doi: 10.1016/0006-8993(95)01172-2. [DOI] [PubMed] [Google Scholar]
- Wilsbacher LD, Takahashi JS. Circadian rhythms: molecular basis of the clock. Curr Opin Genet Dev. 1998;8:595–602. doi: 10.1016/s0959-437x(98)80017-8. [DOI] [PubMed] [Google Scholar]
- Yuste R, Nelson DA, Rubin WW, Katz LC. Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron. 1995;14:7–17. doi: 10.1016/0896-6273(95)90236-8. [DOI] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]


