Summary
Male killing is caused by diverse microbial taxa in a wide range of arthropods [1–7]. This phenomenon poses important challenges to understanding the dynamics of sex ratios and host/pathogen interactions. However, the mechanisms of male killing are largely unknown. Evidence from one case in Drosophila suggests that bacteria can target components of the male-specific sex determination pathway [8]. Here, we investigated male killing by the bacterium Arsenophonus nasoniae in the haplo-diploid wasp Nasonia vitripennis, in which females develop as diploids from fertilized eggs and males develop parthenogenetically as haploids from unfertilized eggs. We found that Arsenophonus inhibits the formation of maternal centrosomes, organelles required specifically for early male embryonic development [9–11], resulting in unorganized mitotic spindles and developmental arrest well before the establishment of somatic sexual identity. Consistent with these results, rescue of Arsenophonus-induced male lethality was achieved by fertilization with sperm bearing the supernumerary chromosome paternal sex ratio (PSR) [12], which destroys the paternal genome but bypasses the need for maternal centrosomes by allowing transmission of the sperm-derived centrosome into the egg. These findings reveal a novel mechanism of male killing in Nasonia, demonstrating that bacteria have evolved different mechanisms for inducing male killing in the Arthropods.
Results and Discussion
Arsenophonus nasoniae is a γ-proteobacterium that induces male killing in the wasp Nasonia vitripennis, a parasite of several fly species [1,13,14]. Unlike other endocellular bacteria such as Wolbachia, which reside within the host germ cells and are transmitted to offspring through the developing egg [15,16], Arsenophonus is found only within the somatic tissue and interstitial fluid surrounding the germ cells [17]. The bacteria are injected into the parasitized fly puparium along with the wasp eggs during stinging [17]. Subsequently, they are ingested by feeding wasp larvae and invade through the gut to eventually re-infect the reproductive (and other) tissues [17]. Unfertilized eggs that normally develop as males fail to hatch into larvae [1,14], suggesting that Arsenophonus disrupts some unknown process of male embryonic development. The parthenogenetic development of male embryos found in haplo-diploid taxa presents various developmental challenges; for example, the fact that the centrosome is derived from the sperm in most diploid species has necessitated the evolution of de novo centrosome formation in unfertilized eggs of haplo-diploids [18,19]. Through a process that is poorly understood, several hundred free centrosomes form spontaneously in the egg cytoplasm following egg laying, two of which join the maternal pronucleus to initiate the first embryonic mitotic division [9,20]. These maternally derived centrosomes also form in fertilized female embryos. However, in fertilized embryos the sperm tail basal body nucleates a pair of centrosomes that is preferentially used to initiate the first mitotic division [9]. Thus, only male embryonic development requires maternally derived centrosomes. Our experiments demonstrate that Arsenophonus kills N. vitripennis males by inhibiting maternal centrosome formation.
To investigate the nature of male death, we first analyzed early embryos from Arsenophonus-infected N. vitripennis females. These females were from two common laboratory lines of Nasonia, LabII(INF) and AsymC(INF), which were transfected with Arsenophonus from a wild-caught line (see Experimental Procedures). These Arsenophonus-infected lines exhibited sex ratios biased toward female (Table 1a) and reduced male embryonic hatch rates (Table 1b) compared to control (uninfected) LabII and AsymC lines. To focus on male-specific effects, embryos were collected from unmated females, which produce all-male (unfertilized) broods. The relative male mortality from unmated females (Table 1b) is 72.1% and the relative male mortality from mated females based on the number of adult males from LabII and AsympC lines (Table 1a) is 73.3% and 71.9%, respectively. This indicates that male embryonic lethality due to Arsenophonus is similar in unfertilized eggs from mated and unmated females.
Table 1.
| Table 1a. Matings with control and Arsenophonus-infected females to wild type males | |||||||
|---|---|---|---|---|---|---|---|
| Class (Female) | Nm | Daughters | (SE) | Sons | (SE) | %Sons | P |
| AsymC | 25 | 32.8 | (2.7) | 19.2 | (2.0) | 36.9 | |
| AsymC(INF) | 17 | 27.9 | (4.2) | 5.4 | (0.9) | 16.2 | <0.0014 |
| LabII | 25 | 29.3 | (2.4) | 14.6 | (2.3) | 33.3 | |
| LabII(INF) | 11 | 18.5 | (3.5) | 3.9 | (1.7) | 17.4 | <0.035 |
| Table 1b. Number of eggs laid and number of surviving pupae for virgin Arsenophonus-infected and control virgin uninfected females. Virgin females produce only haploid male offspring. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Eggs | Pupae | |||||||
| Class | Nv | Ne | (SE) | Nv | Np | (SE) | %Survival | P |
| LabII | 47 | 26.06 | (1.44) | 45 | 22.91 | (1.26) | 87.9 | |
| LabII(INF) | 61 | 34.05 | (1.61) | 60 | 8.35 | (0.94) | 24.5 | <0.00001 |
Arsenophonus causes haploid male-specific lethality. (INF) denotes lines that are infected with Arsenophonus. LabII host lines, but not AsymC lines, are infected with Wolbachia. Nm is the number of mated females from each cross. SE is the standard error of the mean given for the number of daughters and the number of sons per mated female. The percent (%) sons for AsymC and LabII are below 50% due to reduced fertilization rates by mothers, but these values fall within the range of male progeny produced from Arsenophonus-uninfected females in previous studies. P values are shown for Mann-Whitney U tests comparing the % sons between each pair of laboratory line crosses.
Shown is the number of virgin females (Nv) provided with hosts, the average number of eggs laid per female (Ne) and the number of offspring surviving to the pupal stage (Np) from LabII (control) and LabII(INF) (Arsenophonus-infected) host lines. The standard error of the mean (SE) is shown for Ne and Np. %Survival is the ratio of eggs laid to surviving pupae. The P value is shown for bootstrap sampling with 10,000 replicates to test the difference in survival between the two classes. Note that the egg and pupal counts were made from different host sets, because opening the host to count eggs prevents their development to pupae.
In approximately half (N=54/107) of 0–5 hr unfertilized embryos collected from control LabII females, the rapidly dividing syncytial nuclei had reached the cortex (Figure 1b). The nuclei in these cortical embryos and the remaining pre-cortical embryos (N=53/107; Figure 1a) were evenly spaced and synchronous to within one-half a mitotic phase (Figure 1a’ and 1b’). In contrast, the nuclei in all 0–5hr unfertilized embryos (N=126) from LabII(INF) females were far fewer and had failed to reach the cortex (Figure 1c and 1d). These nuclei were mitotically asynchronous and unevenly spaced, with most containing hyper-condensed chromatin (Figure 1c’ and 1d’). Similar cellular defects were also observed in embryos from AsymC(INF) females (not shown). Embryos aged for longer periods of time did not develop further, suggesting that early embryogenesis is the lethal phase. These effects were likely caused by Arsenophonus since bacterial curing with antibiotics resulted in normal embryos (not shown but identical to LabII embryos in Figure 1a and 1b), restoration of normal all-male family sizes from unmated mothers (indicating development of unfertilized eggs), and sex ratios from mated mothers comparable to those from control mated mothers.
Figure 1.
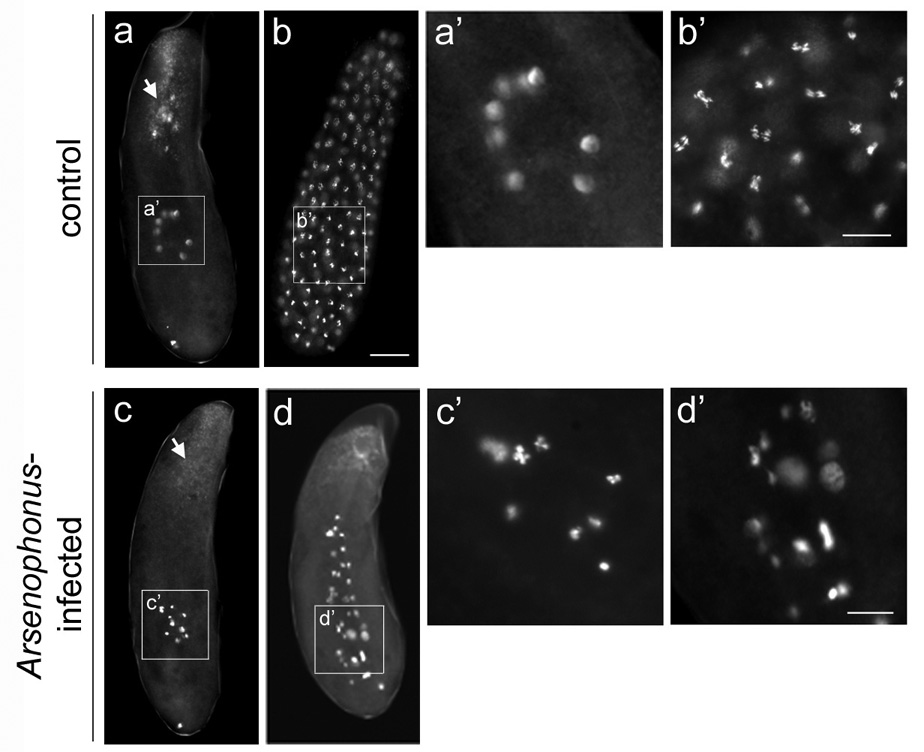
Early embryos from virgin Arsenophonus-infected females exhibit abnormal nuclear divisions. (a) A precortical embryo and (b) a cortical embryo, both from control LabII females. White arrow in (a) indicates endocellular Wolbachia bacteria at the posterior region of the embryo that accumulate into foci around maternal centrosomes. (a’) A higher magnification of eight interphase nuclei of the precortical embryo shown in (a). (b’) A higher magnification of anaphase nuclei of the precortical embryo shown in (b). (c and d) Defective pre-cortical embryos from virgin LabII(INF) females. White arrow in (c) indicates Wolbachia in the same region as in (a) but which fail to form foci due to the absence of maternal centrosomes (see text for explanation). (c’) and (d’) are higher magnifications of nuclei in (c) and (d), respectively. In all panels DNA is shown in greyscale. Scale bar equals 20 µm in (b) and 30 µm in (b’) and (d’).
Microtubule staining of unfertilized LabII(INF) embryos revealed severely unstructured spindles around hyper-condensed chromatin and, overall, minimal microtubule levels (Figure 2c–e) compared to unfertilized control embryos (Figure 2a–b’). There was no evidence of centrosomes, or microtubule-organizing centers (MTOCs), in embryos from infected females as indicated by the absence of astral microtubules or spindle microtubules focused into discrete poles (Figure 2d and 2e). The lack of centrosome activity likely explains the reduced nuclear divisions and migration to the cortex and is consistent with Arsenophonus-induced inhibition of maternal centrosome formation.
Figure 2.
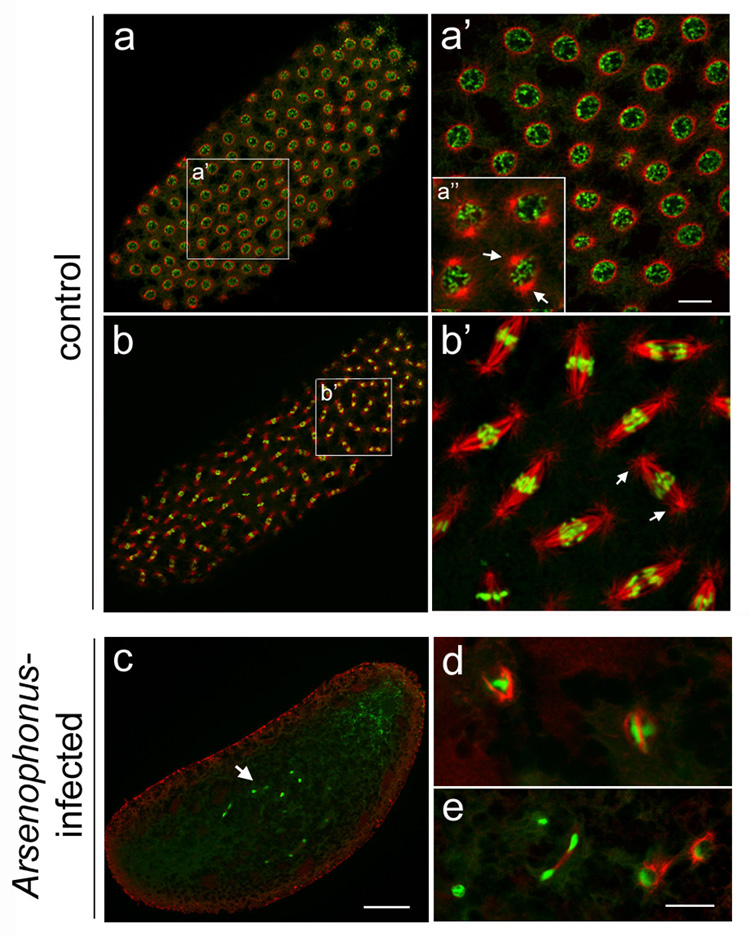
Early embryos from Arsenophonus-infected females lack microtubule-organizing centers (MTOCs). (a) An embryo from a control virgin LabII female whose nuclei are at prophase of the cell cycle. (a’) A higher magnification of nuclei from the embryo shown in (a). (a”) A higher magnfication of four nuclei from the embryo shown in (a), but at a focal plane near the plasma membrane. Each nucleus is associated with a pair of centrosomes that is highlighted by bright foci of microtubules (white arrows indicate a single pair of centrosomes around one nucleus). (b) An embryo from a LabII female whose nuclei are organized into spindles at anaphase. (b’) A higher magnification of nuclei in (b). White arrows indicate the centrosomes of a single spindle, highlighted by prominent microtubule foci at the ends of each spindle pole. (c) A pre-cortical embryo from a virgin Arsenophonus-infected LabII(INF) female. White arrow indicates defective nuclei. (d) and (e) Higher magnifications of two different clusters of nuclei in (c) but in different planes and with 2x higher gain order to visualize microtubules. In all panels, microtubules are shown in red and DNA in green. Scale bar equals 30 µm in (a’), 50 µm in (c), and 30 µm in (e).
To further test this hypothesis, we examined younger (0–2hr) embryos from control and Arsenophonus-infected females for the presence of maternal centrosomes. Previous work has shown that these organelles form during egg laying and persist until the third or fourth nuclear division (<1.5hr) [9]. Immuno-staining of α-Tubulin and other centrosome-associated components was hindered by our inability to remove the vitelline membrane at this early stage by using fixation conditions that preserve centrosome (microtubule) structure (see Experimental Procedures). This was in contrast to the easy removal of the vitelline membrane of embryos aged past 2 hours by using the same fixation conditions (see Figure 2), indicating substantial differences in membrane properties between these adjacent developmental periods. Lancing of early embryos allowed some penetration of anti-α–Tubulin antibodies and revealed the presence of maternal centrosomes in control embryos but not in Arsenophonus-infected embryos (Figure 3). This finding suggests that maternal centrosomes do not form in the presence of Arsenophonus. However, variability in antibody penetration among lanced embryos prevented accurate quantification of maternal centrosomes needed for robust comparison between experimental groups.
Figure 3.
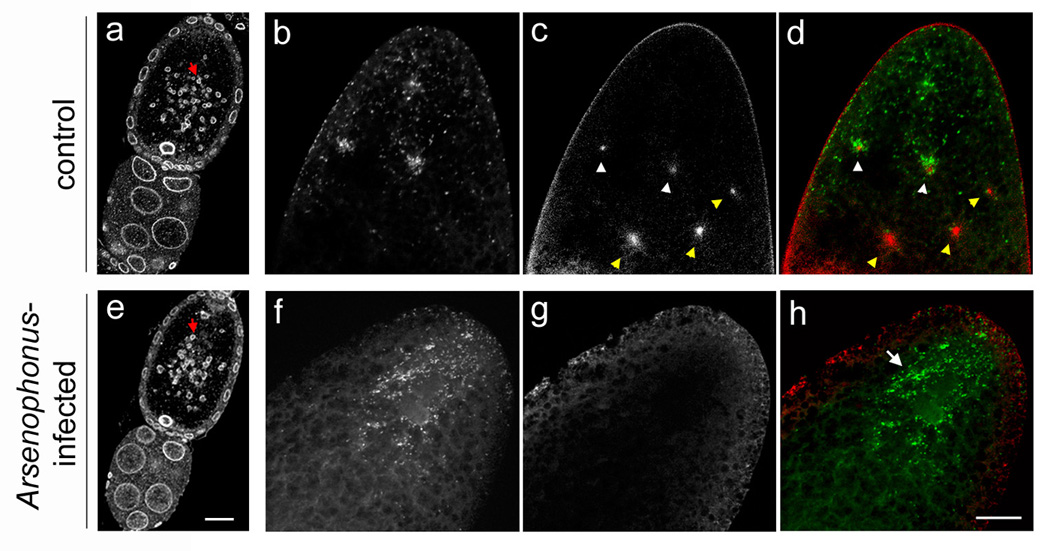
Wolbachia accumulate around maternal centrosomes at the posterior pole in control embryos but exhibit an abnormally diffuse pattern in embryos from Arsenophonus-infected females, in which maternal centrosomes fail to form. (a) and (e) Mid-stage egg chambers from a control LabII female and an Arsenophonus-infected LabII(INF) female, respectively. Lamin, which highlights accessory nuclei (AN) as well as nuclei of the oocyte, nurse cells, and follicle cells, is shown in greyscale. Red arrow points to AN within each oocyte (top). Nurse cells are on the bottom. (b to d) The posterior pole of a precortical embryo from a control LabII female showing the accumulation of Wolbachia bacteria (greyscale in b) around maternal centrosomes (greyscale in c). (d) A merge of panels (b) and (c). Wolbachia are green and centrosomes are red. White arrowheads in (c) and (d) indicate two maternal centrosomes near the posterior pole surrounded by Wolbachia, while yellow arrowheads indicate three maternal centrosomes that are not surrounded by Wolbachia because they are located further from the posterior pole where Wolbachia are not present. (f to h) The posterior pole of a pre-cortical embryo from an Arsenophonus-infected LabII(INF) female. Image colors are the same as in (c to d). White arrow in (h) points to diffusely scattered Wolbachia resulting from a lack of maternal centrosomes. Scale bar equals 30 µm in (e) and 20 µm in (f).
To circumvent this problem, we took advantage of the fact that the LabII and LabII(INF) lines are co-infected with Wolbachia. Wolbachia are known to closely associate with microtubules in the developing embryo [15,21,22] and have been shown to accumulate around the astral microtubules of maternal centrosomes [10]. Wolbachia do not play a role in the observed embryonic defects or skewed sex ratios in the LabII(INF) line because these phenotypes are also present in the AsymC(INF) line (Table 1a), which is not infected with Wolbachia. By using DNA dyes that penetrate the vitelline membrane to visualize Wolbachia [21–23], we observed these bacteria at the posterior pole of the early embryo, a pattern consistent with previous studies [24–26]. At the posterior region Wolbachia accumulated into easily recognizable foci around maternal centrosomes in unfertilized control LabII embryos (Figure 3b–d) but not in Arsenophonus-infected LabII(INF) embryos (Figure 3f–h; also compare Figure 1a to 1c). This result is consistent with our previous failure to detect maternal centrosomes by using α-Tubulin staining in unfertilized embryos from Arsenophonus-infected mothers. In control LabII embryos the Wolbachia foci began to disappear in embryos with 8 and 16 nuclei, when free maternal centrosomes are known to disintegrate [9,10], and were absent in more advanced embryos (not shown). Therefore, we were able to use Wolbachia foci as a proxy for scoring maternal centrosomes.
Wolbachia foci were detected in 77.8% (N=147/189) of unfertilized LabII embryos; most of the remaining embryos from this group had advanced past the 16-nucleus stage (N=31/189). In contrast, Wolbachia foci were present in only 3.6% (N=7/192) of unfertilized LabII(INF) embryos. In the remainder of these embryos (96.4%, N=185/192) Wolbachia were evenly distributed within the cytoplasm near the posterior pole (Figure 3f–h), which is an aberrant pattern compared to control embryos. Together these results strongly indicate that Arsenophonus inhibits maternal centrosome formation or activity.
Maternal centrosomes are formed from accessory nuclei (AN), vesicular organelles found in the eggs of hymenopteran insects (i.e., ants, bees and wasps) [20]. Derived from the nuclear envelop of the germinal vesicle during oogenesis, these Lamin-rich organelles sequester γ-Tubulin and possibly other centrosome components that are released into the egg cytoplasm following egg laying [20]. In a process that is poorly understood, this release triggers the immediate formation of maternal centrosomes. Using anti-Lamin antibodies to visualize AN [20], we found that these organelles were present and equally abundant in oocytes from control and Arsenophonus-infected females (Figure 3a and 3e). Therefore, Arsenophonus likely disrupts a process of centrosome formation that is downstream of AN formation in the oocyte.
To genetically test the hypothesized role of maternal centrosomes in Arsenophonus-induced male lethality, we took advantage of the “selfish” paternal sex ratio (PSR) supernumerary chromosome found in N. vitripennis [12]. PSR is transmitted through sperm to the fertilized egg but then causes complete loss of the paternal genome – except itself – during the first mitosis, allowing fertilized embryos to develop as haploid males from the maternal genome [12]. We reasoned that if Arsenophonus kills males by blocking maternal centrosome formation, then delivery of the paternally derived centrosome from PSR-bearing sperm should rescue male lethality. If, instead, downstream male-specific sex determination pathways are targeted by Arsenophonus, male rescue with PSR-bearing sperm should not occur. Crosses of LabII females to PSR males yielded all-male broods of similar size to those obtained from control (non-PSR) crosses (Table 2), consistent with conversion of fertilized eggs into haploid males due to PSR-mediated paternal genome loss. LabII(INF) females crossed with control males produced broods of reduced size and an average of 9.9% males, a 72% reduction in male progeny (Table 2). However, LabII(INF) females crossed with PSR males produced nearly all-male (90%) broods of similar size to those of LabII(INF) females crossed with control males (Table 2). These male progeny are infected with Arsenophonus and are healthy (data not shown), further indicating that Arsenophonus does not target males per se but rather prevents development of unfertilized eggs. Some female progeny were observed in crosses of LabII(INF) and PSR males but were produced from only one of 17 mothers. PCR testing of these daughters revealed that they did not carry the PSR chromosome although the father was positive for PSR. Therefore, the female production here is likely due to rare incomplete transmission of the PSR chromosome through sperm, which has been previously described [27]. These results show that PSR-bearing sperm rescue male killing of haploid embryos, presumably by providing a paternal centrosome.
Table 2.
Rescue of male killing by PSR.
| Cross | Nm | Daughters | (SE) | Sons | (SE) | Brood | (SE) | %Sons |
|---|---|---|---|---|---|---|---|---|
| LabII × LabII | 15 | 67.9 | (6.2) | 36.8 | (6.9) | 104.7 | (5.2)1,4 | 35.13 |
| LabII × LabII(PSR) | 15 | 0 | - | 103.7 | (8.3) | 103.7 | (8.3)1 | 100.0 |
| LabII(INF) × LabII | 18 | 55.3 | (5.9) | 6.1 | (1.3) | 61.4 | (5.6)2,4 | 9.93,5 |
| LabII(INF) × LabII(PSR) | 17 | 5.2 | (2.9) | 46.1 | (7.3) | 51.3 | (6.4)2 | 89.95 |
Arsenophonus-infected and -uninfected females were crossed to either wild type or PSR-carrying males. Indicated for each cross are the number of single mated mothers (Nm), the average number of daughters and sons surviving to adulthood and their respective standard errors (SE) of the mean, the average brood size and standard error (SE) of the mean, and the average percent (%) sons per brood. (PSR) denotes the presence of the PSR element in the paternal line, which converts fertilized eggs into haploids. These results show that fertilization of the egg with PSR-bearing sperm rescues Arsenophonus-induced lethality, because these offspring develop into haploid males.
The results of Mann-Whitney U tests for the indicated comparisons are as follows:
U=119.5, n1=15, n2=15, P=0.776
U=190.0, n1=18, n2=17, P=0.232
U=214.0, n1=17, n2=15, P<0.004
U=230.0, n1=17, n2=15, P<0.0005
U=284.5, n1=18, n2=17, P<8e-07
Taken together the results presented here indicate that Arsenophonus kills N. vitripennis males by targeting maternally derived centrosomes needed for early nuclear divisions in unfertilized embryos. Because Arsenophonus is not present within the developing eggs but only in the somatic tissue surrounding the germ line [17], the bacteria likely secretes an unknown toxin that diffuses across the vitelline and plasma membranes in utero or during egg laying in order to inhibit maternal centrosome formation. Without maternal centrosomes, unfertilized embryos undergo only a few rounds of abnormal nuclear divisions, ceasing to develop well before cellularization and formation of male somatic and germ line tissues. Thus, instead of killing males directly, Arsenophonus prevents the development of embryos from unfertilized eggs that are normally destined to become males. The few nuclear divisions that do occur in the absence of centrosomes are likely to result from unorganized spindles whose microtubules are nucleated from the chromosomes; this phenomenon is also known to occur in unfertilized (centrosome-less) eggs of the Dipteran Sciara coprophila [28]. Our data currently do not allow us to determine whether Arsenophonus (i) blocks the formation of maternal centrosomes from AN or (ii) inhibits the microtubule-nucleating properties of maternal centrosomes following their formation from AN. However, although maternally- and paternally-derived centrosomes form through distinct processes, both appear to be structurally and compositionally similar [10], suggesting that they utilize a common mechanism to nucleate microtubules. Thus, the fact that the sperm-derived centrosomes in diploid embryos and PSR-rescued haploid embryos nucleate microtubules normally in the presence of Arsenophonus (not shown) argues against the latter possibility.
It is particularly intriguing that Arsenophonus-induced male killing in Nasonia affects the pathway in which maternal centrosomes are formed from AN. Although AN are characteristic of hymenopteran eggs, these organelles also have been found in other diverse organisms, including non-hymenopteran insects, crustaceans, nematodes, amphibians and mammals, many of which are diploid and do not rely on maternal centrosomes for early development [29]. In addition to their role in maternal centrosome formation, AN have been implicated in the transport of small nuclear ribonucleoproteins (snRNPs) and other splicing-related molecules during early development [29]. Understanding the mechanisms by which Arsenophonus blocks AN function could therefore be relevant to understanding maternal (de novo) centrosome formation as well as other cellular processes.
Previous studies on the mechanisms of male killing have focused primarily on the intracellular bacterium, Spiroplasma poulsonii, in several Drosophila species [8,30–32]. S. poulsonii, a natural pathogen of D. willistoni, induces male death in this species during embryonic stages 12 and 13, well after cellularization, germ line formation and establishment of somatic sexual identity [30,32]. When transferred into D. melanogaster, S. poulsonii causes male death at similar developmental stages and genetic experiments revealed that a functional dosage compensation complex is required for this lethality [8]. Our data suggest that Arsenophonus does not operate through such downstream male-specific developmental pathways to kill males in Nasonia since PSR-rescued males develop normally in the presence of Arsenophonus.
Interestingly, S. poulsonii also induces an earlier embryonic lethal phase in D. willistoni, causing mitotic defects such as asynchronous nuclear divisions, abnormally elongated spindles, spindle fusion, and aneuploidy in the syncytial (pre-cleavage) embryo [30]; it is currently unknown whether only males or both sexes are affected at this stage. However, centrosome duplication and microtubule nucleation from centrosomes occur normally in all embryos [30], suggesting that Spiroplasma and Arsenophonus target different cellular components in their respective hosts. Nevertheless, the fact that both bacteria can disrupt mitotic progression during the early nuclear divisions suggests this may be a common ability of male-killing bacteria, a hypothesis that will be gradually tested as additional mechanistic studies of male killing are conducted in other systems. Such an ability is expected to be more effective at selectively killing male progeny in haplo-diploid hosts like Nasonia whose sex determination is based on fundamental mitotic differences between the sexes (i.e., the preferential use of maternal centrosomes in males). Our results, together with those mentioned above, suggest that male killing in other systems may be closely linked with the particular mode of sex determination of the host and will, perhaps, be manifested at the initial divergence of male/female development in a given host species.
Supplementary Material
Acknowledgements
We thank D. A. Barbash and three anonymous reviewers for helpful comments on this manuscript and P. A. Fisher for the anti-Lamin antibody. We also thank M. Clark and R. Edwards for assistance with the experiments and N. Clark and A. Greenberg for help with statistical bootstrapping. Financial support for this research was provided by the NIH (to P. M. F. and to J. H. W.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Werren JH, Skinner SW, Huger AM. Male-killing bacteria in a parasitic wasp. Science. 1986;231:990–992. doi: 10.1126/science.3945814. [DOI] [PubMed] [Google Scholar]
- 2.Williamson DL, Poulson DF. Sex ratio organisms (Spiroplasmas) of Drosophila. In: Whitcomb RF, Tully JG, editors. The Mycoplasmas. Vol. 175. New York: Academic Press; 1979. pp. 175–208. [Google Scholar]
- 3.Hurst GDD, Jiggins FM, Schulenberg JHGVD, Bertrand D, West SA, et al. Male-killing Wolbachia in two species of insect. Proc R Soc Lond Ser B Biol Sci. 1999;266:735–740. [Google Scholar]
- 4.Fialho RF, Stevens L. Male-killing Wolbachia in a flour beetle. Proc R Soc Lond Ser B Biol Sci. 2000;267:1469–1473. doi: 10.1098/rspb.2000.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeh DW, Zeh JA, Bonilla M. Wolbachia, sex ratio bias and apparent male killing in the harlequin beetle riding pseudoscorpion. Heredity. 2005;95:41–49. doi: 10.1038/sj.hdy.6800666. [DOI] [PubMed] [Google Scholar]
- 6.Hurst GDD, Jiggins FM, Majerus MEN. Inherited microorganisms that selectively kill male hosts: The hidden players of insect evolution? In: Bourtzis K, Miller TA, editors. Insect Symbiosis. Boca Raton, FL: CRC Press; 2003. pp. 177–198. [Google Scholar]
- 7.Nakanishi K, Hoshino M, Nakai M, Kunimi Y. Novel RNA sequences associated with late male killing in Homona magnanima. Proc Biol Sci. 2008;275:1249–1254. doi: 10.1098/rspb.2008.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veneti Z, Bentley JK, Koana T, Braig HR, Hurst GDD. A functional dosage compensation complex required for male killing in Drosophila. Science. 2005;307:1461–1463. doi: 10.1126/science.1107182. [DOI] [PubMed] [Google Scholar]
- 9.Tram U, Sullivan W. Reciprocal inheritance of centrosomes in the parthenogenetic hymenopteran Nasonia vitripennis. Curr Biol. 2000;10:1413–1419. doi: 10.1016/s0960-9822(00)00795-8. [DOI] [PubMed] [Google Scholar]
- 10.Riparbelli MG, Stouthamer R, Dallai R, Callaini G. Microtubule organization during the early development of the parthenogenetic egg of the hymenopteran Muscidifurax uniraptor. Dev Biol. 1998;195:89–99. doi: 10.1006/dbio.1997.8841. [DOI] [PubMed] [Google Scholar]
- 11.Riparbelli MG, Tagu D, Bonhomme J, Callaini G. Aster self-organization at meiosis: a conserved mechanism in insect parthenogenesis? Dev Biol. 2005;278:220–230. doi: 10.1016/j.ydbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Nur U, Werren JH, Eickbush DG, Burke WD, Eickbush TH. A selfish B chromosome that enhances its transmission by eliminating the paternal genome. Science. 1988;240:512–514. doi: 10.1126/science.3358129. [DOI] [PubMed] [Google Scholar]
- 13.Gherna RL, Werren JH, Weisburg W, Cote R, Woese CR, et al. Arsenophonus nasoniae, genus novel, species novel, causative agent of Son Killer trait in the parasitic wasp, Nasonia vitripennis. J Syst Bacter. 1991;41:563–565. [Google Scholar]
- 14.Skinner SW. Son-killer: a third extrachromosomal factor affecting the sex ratio in the parasitoid wasp, Nasonia (=Mormoniella) vitripennis. Genetics. 1985;109:745–759. doi: 10.1093/genetics/109.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tram U, Ferree PM, Sullivan W. Identification of Wolbachia-host interacting factors through cytological analysis. Microbes Infect. 2003;5:999–1011. doi: 10.1016/s1286-4579(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 16.Moran NA, Telang A. The evolution of bacteriocyte-associated endosymbionts in insects. BioScience. 1998;48:295–304. [Google Scholar]
- 17.Huger A, Skinner SW, Werren JH. Bacterial infections associated with the son-killer trait in the parasitoid wasp Nasonia (=Mormoniella) vitripennis (Hymenoptera: Pteromalidae) J Invert Pathol. 1985;46:272–280. doi: 10.1016/0022-2011(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 18.Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- 19.Callaini G, Giovanna M, Dallai R. Centrosome inheritance in insects: fertilization and parthenogenesis. Biol Cell. 1999;91:355–366. [PubMed] [Google Scholar]
- 20.Ferree PM, McDonald L, Fasulo B, Sullivan W. The origin of centrosomes in parthenogenetic hymenopteran insects. Curr Biol. 2006;16:801–807. doi: 10.1016/j.cub.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 21.Kose H, Karr TL. Organization of Wolbachia pipientis in the Drosophila fertilized egg and embryo revealed by an anti-Wolbachia monoclonal antibody. Mech Dev. 1995;5:275–288. doi: 10.1016/0925-4773(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 22.Callaini G, Dallai R, Riparbelli MG. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J Cell Sci. 1997;110:271–280. doi: 10.1242/jcs.110.2.271. [DOI] [PubMed] [Google Scholar]
- 23.Ferree PM, Frydman HM, Li JM, Cao J, Wieschaus E, et al. Wolbachia utilizes host microtubules and Dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 2005;1:e14. doi: 10.1371/journal.ppat.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breeuwer JAJ, Werren JH. Microorganism associated with chromosome destruction and reproductive isolation between two insect species. Nature. 1990;346:558–560. doi: 10.1038/346558a0. [DOI] [PubMed] [Google Scholar]
- 25.Zchori-Fein E, Roush RT, Rosen D. Distribution of parthenogenesis-inducing symbionts in ovaries and eggs of Aphytis (Hymenoptera: Apehlinidae) Curr Microbiol. 1998;36:1–8. doi: 10.1007/s002849900270. [DOI] [PubMed] [Google Scholar]
- 26.Serbus LR, Sullivan W. A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog. 2007;3:e190. doi: 10.1371/journal.ppat.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beukeboom LB, Werren JH. Transmission and expression of the parasitic Paternal Sex Ratio (PSR) chromosome. Heredity. 1993;70:437–443. [Google Scholar]
- 28.de Saint Phalle B, Sullivan W. Spindle assembly and mitosis without centrosomes in parthenogenetic Sciara embryos. J Cell Biol. 1998;141:1383–1391. doi: 10.1083/jcb.141.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilinski SM, Kloc M. Accessory nuclei revisited: the translocation of snRNPs from the germinal vesicle to the periphery of the future embryo. Chromosoma. 2002;111:62–68. doi: 10.1007/s00412-002-0186-4. [DOI] [PubMed] [Google Scholar]
- 30.Counce SJ, Poulson DF. Developmental effects of the sex ratio agent in embryos of Drosophila willistoni. J Exper Zool. 1962;151:17–31. doi: 10.1002/jez.1401510103. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi B, Poulson DF. Nature of “sex-ratio” agent in Drosophila. Science. 1961;12:1489–1490. doi: 10.1126/science.133.3463.1489. [DOI] [PubMed] [Google Scholar]
- 32.Bentley ZK, Veneti Z, Heraty J, Hurst GDD. The pathology of embryo death caused by the male-killing Spiroplasma bacterium in Drosophila nebulosa. BMC Biol. 2007;5:9–16. doi: 10.1186/1741-7007-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verheyen E, Cooley L. Looking at oogenesis. Methods Cell Biol. 1994;44:545–561. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


