Abstract
Background
Poorly controlled cardiovascular risk factors are common. Evaluating whether physicians respond appropriately to poor risk factor control in patients may better reflect quality of care than measuring proportions of patients whose conditions are controlled.
Objectives
To evaluate therapy modifications in response to poor control of hypertension, dyslipidemia, or diabetes in a large clinical population.
Design
Retrospective cohort study within an 18-month period in 2002 to 2003.
Setting
Kaiser Permanente of Northern California.
Patients
253 238 adult members with poor control of 1 or more of these conditions.
Measurements
The authors assessed the proportion of patients with poor control who experienced a change in pharmacotherapy within 6 months, and they defined “appropriate care” as a therapy modification or return to control without therapy modification within 6 months.
Results
A total of 64% of patients experienced modifications in therapy for poorly controlled systolic blood pressure, 71% for poorly controlled diastolic blood pressure, 56% for poorly controlled low-density lipoprotein cholesterol level, and 66% for poorly controlled hemoglobin A1c level. Most frequent modifications were increases in number of drug classes (from 70% to 84%) and increased dosage (from 15% to 40%). An additional 7% to 11% of those with poorly controlled blood pressure, but only 3% to 4% of those with elevated low-density lipoprotein cholesterol level or hemoglobin A1c level, returned to control without therapy modification. Patients with more than 1 of the 3 conditions, higher baseline values, and target organ damage were more likely to receive “appropriate care.”
Limitations
Patient preferences and suboptimal adherence to therapy were not measured and may explain some failures to act.
Conclusions
As an additional measure of the quality of care, measuring therapy modifications in response to poor control in a large population is feasible. Many patients with poorly controlled hypertension, dyslipidemia, or diabetes had their therapy modified and, thus, seemed to receive clinically “appropriate care” with this new quality measure.
Despite strong evidence that pharmacotherapeutic interventions contribute to preventing cardiovascular events in high-risk individuals (1-5), risk factor control remains suboptimal (6-9) and medications to reduce risk factors seem to be underused (10, 11). Underuse of evidence-based medications in chronic disease care may be partly due to “clinical inertia” (12) by physicians and health care systems. As such, it represents a possible avenue for quality improvement in health care (13, 14).
The Health Plan Employer Data and Information Set (HEDIS) has developed measures to assess health plan and individual physician performance (15) that are based directly on the proportions of high-risk patients whose low-density lipoprotein (LDL) cholesterol levels, hemoglobin A1c levels, and blood pressure are in control. Although such measures may identify important deficiencies in quality (16) and are directly related to the health status of patients, they may be confounded by population differences in case mix and therefore cannot distinguish between a patient who is receiving poor care and a patient who is being appropriately treated but who either does not adhere to treatment recommendations or has particularly severe disease that is less responsive to treatment (17). Ideally, quality measures should reflect whether physicians and systems deliver appropriate or optimal care. Provider responses to poorly controlled risk factor levels, such as intensification of either behavioral therapy or pharmacotherapy, have recently been proposed as “tightly linked” process indicators of quality. Solid evidence suggests that these processes of care are directly linked to improved patient outcomes (18, 19). Such “tightly linked” process measures might be more clinically relevant than the levels of either testing or control (18). Higher testing rates have not been shown to be associated with better clinical control (7, 19). To better understand the potential utility and the feasibility of measuring therapy modifications in response to poor risk factor control as an additional measure of quality, we examined the frequency and predictors of therapy modifications in response to poorly controlled hyper-tension, dyslipidemia, and diabetes in a large managed care population.
Context
What actions do health care providers take for patients with poorly controlled cardiovascular risk factors?
Content
This large retrospective cohort study from Kaiser Permanente of Northern California found that, within a 6-month period, most patients received therapy modifications for the following poorly controlled risk factors: systolic blood pressure (64%), diastolic blood pressure (71%), low-density lipoprotein cholesterol level (56%), and hemoglobin A1c level (66%). The most frequent modifications were adding drugs and increasing dosages.
Cautions
We do not know how often patient preferences or suboptimal adherence influenced treatment decisions. The study did not directly link therapy modifications to clinical outcomes.
—The Editors
Methods
Study Participants
Description of the Study Population
We performed a retrospective cohort study within an 18-month period from 1 July 2002 to 31 December 2003 of all members of Kaiser Permanente Medical Care Program of Northern California who were older than 20 years of age. Kaiser Permanente Medical Care Program is a fully integrated health care delivery system that provides comprehensive medical care to more than 3 million members. Members comprise approximately 30% of the northern California population and are demographically representative of the population (20).
Primary care physicians, who worked with specialty care, health education, and nutrition education staff located in close proximity, followed most patients. Many primary care teams included care managers and either nurses or pharmacists, to whom patients with poor control could be directly referred. All services were covered benefits.
Diagnostic Criteria
We searched clinical databases, including inpatient and ambulatory diagnoses, ambulatory blood pressure measurements, laboratory results, and prescriptions for the 18-month period before 31 December 2003, to identify all patients categorized as having hypertension, dyslipidemia, diabetes mellitus, or a combination of these conditions and to assess modifications in therapy in response to poor control. Criteria used to identify each condition (Appendix Table 1, available at www.annals.org) have been described previously (21). Previous studies have documented the accuracy of the clinical databases used in our study for several diagnoses, including diabetes, coronary artery disease (CAD), and stroke (22, 23). Diabetes diagnoses, myocardial infarction, and stroke are confirmed at chart review in 98%, 99%, and 75% of cases, respectively.
Study Cohort
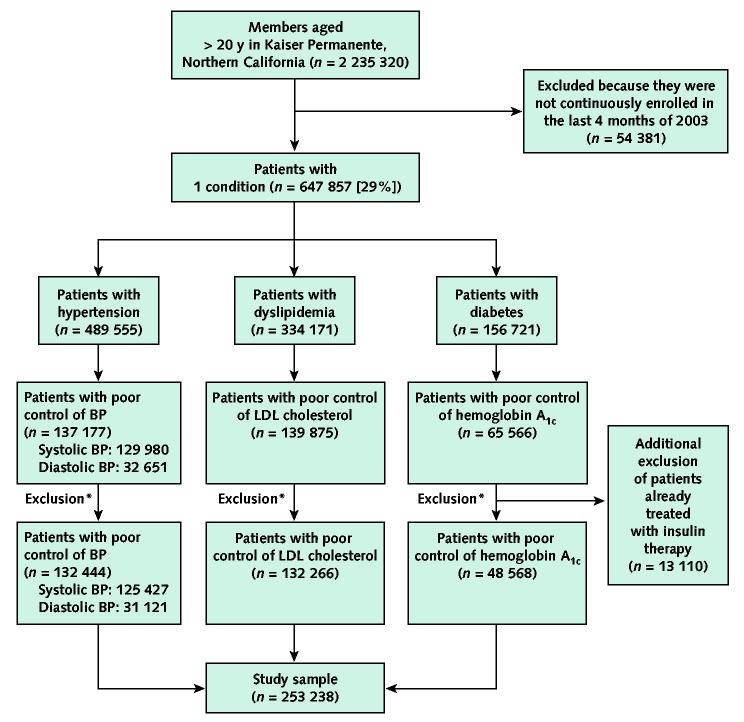
To assess therapy modifications in response to poorly controlled hypertension, dyslipidemia, or diabetes mellitus, we identified patients who met criteria for poor control of 1 condition or more at some point during the study period. Appendix Table 2 (available at www.annals.org) details our definitions of poor control. We did not include patients with moderately elevated risk factors because initial lifestyle modification is often appropriate for these participants (4, 5), so pharmacotherapeutic interventions may not be warranted. To allow a complete 6-month follow-up, we extended observation into 2004 for those whose poor control was first identified in the second half of 2003. We excluded members who were not continuously enrolled 4 months before and 6 months after the date of the defining measure of poor control because we did not have adequate time for observation (Figure). We also excluded patients with diabetes who were already treated with insulin therapy, because day-to-day adjustments in insulin dosage cannot be reliably identified from automated data sources.
Figure.
Flow of participants in the study.
Levels of control and poor control for each condition are defined in Appendix Table 2 (available at www.annals.org). Poor control of blood pressure (BP) is defined as poor control of systolic blood pressure, diastolic blood pressure, or both. LDL = low-density lipoprotein. *Exclusion of patients who were not continuously enrolled 4 months before and 6 months after the date with abnormal value.
Measurements
Therapy Modifications in Response to Poor Control
On the basis of previous definitions of “tightly linked” quality measures (17), we defined a potential therapy modification as an increase in the number of drug classes, increased dosage of 1 medication or more, or a switch to another medication in a different therapeutic class. We did not treat switches to medications in the same therapeutic class as a therapy modification, unless the daily dose of the new agent represented an increase in bioequivalent dose compared with the previous agent, because a switch could be in response to side effects rather than an intensification of therapy. Other outcomes included a return to control without therapy modification, which was based on the last risk factor measurement recorded during the 6-month period; a return to “near control” (Appendix Table 2, available at www.annals.org); and no therapy modification with either no return to control or no further measurement. This last category included patients with no follow-up visit during the interval. Recognizing that physicians may sometimes opt for nonpharmacologic recommendations for an elevated risk factor value, we created an outcome called “appropriate care,” defined as either a therapy modification or return to control without therapy modification within 6 months. We also examined responses within 3 months.
Pharmacologic Management
We defined pharmacologic therapy from prescriptions filled during the 4 months before and the 6 months after the first abnormal risk factor measure. We grouped drugs by drug codes into therapeutic classes, with 7 classes for hypertension (thiazide diuretics, other diuretics, β-blockers, calcium-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, and other antihypertensive agents), 5 classes for dyslipidemia (statins, fibric acid derivatives, niacin, bile acid resins, and other lipid-lowering agents), and 5 classes for diabetes mellitus (insulin, sulfonylureas, metformin, thiazolidinediones, and other diabetes-related medications). We recorded daily dosages and defined increased dosage as any increase in daily dose in milligrams. When drugs from more than 1 medication class were taken, we considered a dosage increase for any class.
For patients who did not have therapy modifications, we assessed the proportion of patients who may have already been receiving maximal therapy. We defined maximal therapy as receiving 3 medications for hypertension or diabetes (including the proportion who were receiving high doses of all 3 medications) or receiving a “maximal” dose of statin therapy (80 mg of simvastatin or ≥40 mg of atorvastatin). Combination lipid-lowering therapy was very uncommon.
Covariates
We examined the relationship between several patient factors and receipt of “appropriate care” in response to poor control, notably whether physicians responded differently in relation to patients' level of cardiovascular risk as reflected by the presence of several conditions or target organ disease. We defined 3 mutually exclusive categories of cardiovascular risk: history of CAD, other target organ damage, and no history of either condition. We defined previous CAD as any diagnosis of myocardial infarction, angina pectoris, atherosclerotic heart disease, or coronary revascularization recorded during the previous 5 years. We defined target organ damage on the basis of the definitions of the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) (24): previous diagnoses of hypertensive heart disease, congestive heart failure, cerebrovascular or peripheral arterial disease, and nephropathy (defined as renal failure or insufficiency during the previous 5 years or by a most recent serum creatinine level greater than 124 μmol/L [>1.4 mg/dL] for women or greater than 133 μmol/L [>1.5 mg/dL] for men). Race and ethnicity data were available for 76% of patients from responses to previous member surveys and previous hospital discharges.
Statistical Analysis
For each condition, we determined the proportions of poorly controlled patients who experienced each possible response described earlier. All 95% CIs were smaller than ± 1.0% and are therefore not presented. We assessed independent patient factors associated with “appropriate care” at 6 months (vs. “inappropriate care”) by using multivariable logistic regression. To account for the clustered study design (patients clustered within 1570 physicians within 17 medical centers), we used hierarchical logistic regression models (SAS GLIMMIX macro, SAS Institute, Inc., Cary, North Carolina), with medical center as a fixed factor and physician as a random factor (25). Because logistic regression odds ratios become poor estimates of relative risk when outcomes are common (26), we used predicted adjusted proportions of patients receiving “appropriate care” and 95% CIs.
To examine variability in “appropriate care” among medical centers and physicians, we calculated the interquartile ranges for the adjusted proportions from the regression models. Because we treated medical center as a fixed effect, we could directly calculate adjusted proportions. For physicians, we estimated the adjusted proportions by using the assumed normal distribution with mean zero. To assess whether the inclusion of patients who returned to control without therapy modification in the “appropriate care” category affected our findings, we performed a sensitivity analysis that excluded those patients from the multivariable models. Because of missing data on race or ethnicity (Table 1), we used complete case analysis for the multivariate models, which is less subject to bias than treating observations with missing race or ethnicity as a separate category (28). We conducted all analyses using SAS, version 8 (SAS Institute, Inc., Cary, North Carolina).
Table 1.
Baseline Characteristics of 253 238 Patients with Poorly Controlled Hypertension, Dyslipidemia, and Diabetes Mellitus
| Characteristic | Patients with Poorly Controlled Hypertension |
Patients with Poorly Controlled Dyslipidemia (n = 132 266) | Patients with Poorly Controlled Diabetes Mellitus (n = 48 568) | |
|---|---|---|---|---|
| Systolic Blood Pressure (n = 125 427) | Diastolic Blood Pressure (n = 31 121) | |||
| Demographic | ||||
| Mean age, y | 67.3 | 57.7 | 61.7 | 58.1 |
| Age, % | ||||
| 20–44 y | 4.8 | 16.3 | 8.0 | 13.5 |
| 45–64 y | 34.6 | 53.3 | 50.3 | 56.2 |
| ≥65 y | 60.6 | 30.4 | 41.7 | 30.4 |
| Women, % | 58.1 | 48.0 | 49.4 | 44.3 |
| Race or ethnicity, % | ||||
| White | 53.6 | 41.5 | 47.5 | 34.4 |
| African American | 10.5 | 14.4 | 7.8 | 9.6 |
| Asian | 8.0 | 7.7 | 9.2 | 12.3 |
| Latino | 6.3 | 5.1 | 6.0 | 10.7 |
| Mixed | 3.0 | 3.0 | 2.5 | 3.6 |
| Native American | 0.6 | 0.6 | 0.5 | 0.7 |
| Pacific Islander | 0.1 | 0.1 | 0.1 | 0.2 |
| Missing data | 17.8 | 27.6 | 26.5 | 28.5 |
| Comorbid conditions, % | ||||
| Coronary artery disease | 27.7 | 19.2 | 20.7 | 16.6 |
| Other target organ damage | 53.0 | 46.9 | 28.2 | 37.0 |
| Cerebrovascular disease | 19.7 | 14.7 | 10.3 | 9.0 |
| Peripheral arterial disease | 13.0 | 7.8 | 6.8 | 8.6 |
| Nephropathy | 29.3 | 27.5 | 15.3 | 25.9 |
| Other disease* | 16.7 | 14.1 | 7.9 | 8.9 |
| Co-occurrence of the 3 conditions | ||||
| Hypertension only | 32.1 | 38.8 | – | – |
| Dyslipidemia only | – | – | 22.9 | – |
| Diabetes mellitus only | – | – | – | 13.3 |
| Hypertension and dyslipidemia | 26.8 | 24.0 | 41.1 | – |
| Hypertension and diabetes mellitus | 10.4 | 11.3 | – | 16.7 |
| Dyslipidemia and diabetes mellitus | – | – | 9.3 | 17.4 |
| All 3 conditions | 30.7 | 25.9 | 26.7 | 52.6 |
| Baseline medications, % | ||||
| Medications for each condition | ||||
| 0 medication | 20.2 | 25.9 | 72.6 | 34.6 |
| 1 medication | 29.4 | 30.1 | 26.0 | 35.9 |
| 2 medications | 27.4 | 24.5 | 1.4 | 25.9 |
| 3 medications | 16.4 | 13.7 | 0.0 | 3.6 |
| ≥4 medications | 6.5 | 5.8 | 0.0 | 0.1 |
| Highest level of dosage if receiving medications for each condition† | ||||
| Low | 34.1 | 34.7 | 21.4 | 36.7 |
| Medium | 31.3 | 31.2 | 56.6 | 21.1 |
| High | 34.6 | 34.1 | 22.1 | 42.2 |
| Medications for others of the 3 conditions | ||||
| 0 medication | 46.6 | 55.4 | 34.1 | 27.1 |
| 1 medication | 30.3 | 26.4 | 21.4 | 23.6 |
| 2 medications | 13.7 | 11.0 | 21.2 | 19.2 |
| 3 medications | 7.2 | 5.6 | 12.9 | 15.6 |
| ≥4 medications | 2.3 | 1.6 | 10.3 | 14.5 |
Other disease included hypertensive heart disease and heart failure.
In this table, daily dosages were categorized as low, medium, or high on the basis of inspection of dosage distributions for all users of each medication and on the basis of recommendations of the Physicians' Desk Reference (27). When drugs from more than 1 medication class were taken, the highest dosage of any class was considered.
Role of the Funding Source
The Pharmaceuticals Outcomes Research Group of Pfizer Inc. funded the data collection and analysis. Investigators at Kaiser Permanente performed the analyses. By contract, the final author (J.V. Selby) retains the right to publish the paper without approval from Pfizer Inc. Kaiser Permanente's Institutional Review Board approved the study.
Results
Population Characteristics and Degree of Control
Among 647 857 patients identified as having hypertension, dyslipidemia, diabetes mellitus, or a combination of these conditions, 28% of those with hypertension, 42% of those with diabetes, and 42% of those with dyslipidemia met criteria for poor control (Figure). By using the tighter definitions of “in control” (Appendix Table 2, available at www.annals.org), we found that 55%, 55%, and 49% of patients, respectively, were not in control at the end of 2003.
Table 1 details the baseline demographic characteristics and comorbid conditions. Relatively few patients received several medications at baseline and relatively small proportions of patients were receiving maximal doses of any medication. Most of the 1570 physicians were internists (64%) or family physicians (16%); other physicians' specialties were uncommon (each < 3%). The mean number of patients per physician for each condition ranged from 18 patients (range, 1 patient to 85 patients) for diastolic blood pressure to 86 patients (range, 1 patient to 236 patients) for LDL cholesterol level.
Therapy Modifications during 3-Month and 6-Month Periods
Among patients with poor control, 54% experienced therapy modifications for poorly controlled systolic blood pressure within 3 months, 63% for poorly controlled diastolic blood pressure, 47% for poorly controlled LDL cholesterol level, and 57% for poorly controlled hemoglobin A1c level (Table 2). At 3 months, the most frequent modifications were increases in the number of drug classes (from 62% to 84%) and increased dosage (from 14% to 37%). With 3 further months of observation, the proportion of patients experiencing therapy modifications increased to 64% for poorly controlled systolic blood pressure, 71% for diastolic blood pressure, 56% for LDL cholesterol level, and 66% for hemoglobin A1c level.
Table 2.
Proportion of Patients with Poorly Controlled Hypertension, Dyslipidemia, or Diabetes Mellitus Who Had Subsequent Therapy Modifications within a 3-Month and 6-Month Period*
| Characteristic |
Patients with Poorly Controlled Hypertension (Systolic BP) (n = 125 427), % |
Patients with Poorly Controlled Hypertension (Diastolic BP) (n = 31 121), % |
Patients with Poorly Controlled Dyslipidemia (LDL Cholesterol) (n = 132 266), % |
Patients with Poorly Controlled Diabetes Mellitus (Hemoglobin A1c) (n = 48 568), % |
||||
|---|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 3 Months | 6 Months | 3 Months | 6 Months | 3 Months | 6 Months | |
| “Appropriate care” | 60.9 | 71.1 | 74.3 | 82.4 | 48.5 | 58.6 | 58.1 | 69.7 |
| Any therapy modification | 54.3 | 64.3 | 62.7 | 71.1 | 47.0 | 55.8 | 57.0 | 66.2 |
| Increase in the number of drug classes | 61.8 | 70.3 | 65.5 | 73.3 | 84.2 | 83.5 | 70.1 | 73.7 |
| Increased dosage of ≥1 medication | 36.6 | 39.9 | 33.7 | 37.1 | 13.9 | 15.3 | 29.7 | 34.0 |
| Switch to another medication in a different class† | 15.1 | 8.2 | 16.0 | 9.5 | 2.1 | 1.6 | 7.9 | 3.7 |
| Several therapy modifications | 13.8 | 18.8 | 15.6 | 20.3 | 0.4 | 0.9 | 7.7 | 11.6 |
| Return to control without therapy modification‡ | 6.6 | 6.8 | 11.6 | 11.3 | 1.5 | 2.8 | 1.1 | 3.5 |
| “Inappropriate care” | 39.1 | 28.8 | 25.7 | 17.6 | 51.5 | 41.4 | 41.9 | 30.3 |
| No therapy modification and return to near control‡ | 7.8 | 7.7 | 4.1 | 3.6 | 3.2 | 6.2 | 3.0 | 5.7 |
| No therapy modification and no return to control‡ | 12.7 | 11.5 | 6.0 | 5.3 | 4.5 | 8.6 | 5.3 | 7.3 |
| No further measurement and no therapy modification§ | 18.6 | 9.6 | 15.6 | 8.7 | 43.8 | 26.6 | 33.6 | 17.3 |
Confidence intervals for all proportions in this table are narrower than ±1.0%. BP = blood pressure; LDL = low-density lipoprotein.
A switch to another medication in a different class was counted only when a new class of medication for the condition was added, but the total number of drug classes remained the same.
Levels of control and near control for each condition are defined in Appendix Table 2 (available at www.annals.org).
This category included those with no new visit or no new measurements.
Most patients (≥80%) had 1 therapy modification within 6 months, compared with those who had several modifications. For all conditions, most patients (>70%) had a therapy modification in the first 2 months after the elevated level for all conditions, and only 2% to 6% of modifications occurred during the sixth month. Furthermore, 7% to 11% of those with poorly controlled blood pressure, but only 3% to 4% of those with elevated LDL cholesterol level or hemoglobin A1c level, returned to control without therapy modification. Thus, 71% to 82% of all patients with poorly controlled blood pressure had “appropriate care,” defined as either a therapy modification or a return to control. For elevated levels of LDL cholesterol and hemoglobin A1c, 59% and 70% of patients had “appropriate care,” respectively.
Approximately 50% of patients with “inappropriate care” were not measured further in the observation period. Among those with “inappropriate care,” 24% of those with poorly controlled systolic blood pressure, 18% of those with poorly controlled diastolic blood pressure, and 5% of those with poorly controlled hemoglobin A1c level were already receiving 3 or more medications at baseline, but only 0.6% to 1.5% of these patients were receiving high doses of the 3 medications. Only 4% were already receiving a maximal dose of statins.
Factors Associated with “Appropriate Care”
Higher baseline values, co-occurrence of 1 or more of the 3 conditions, and previous CAD or target organ damage were independently associated with “appropriate care” in response to poor control at 6 months (Table 3). The main exceptions were that patients with diabetes were less likely to have “appropriate care” in response to elevated systolic blood pressure and were not more likely to have “appropriate care” in response to elevated hemoglobin A1c levels in the presence of CAD or target organ damage. The effects of demographic characteristics varied by condition. Women were more likely to have “appropriate care” in response to all poorly controlled risk factors except for systolic blood pressure. Older age was associated with “appropriate care” in response to poorly controlled diastolic blood pressure and LDL cholesterol levels, and younger age was associated with “appropriate care” in response to poorly controlled hemoglobin A1c level and systolic blood pressure.
Table 3.
Multivariable Analyses of Factors Associated with “Appropriate Care” in Response to Poorly Controlled Hypertension, Dyslipidemia, and Diabetes Mellitus*
| Characteristic |
Adjusted Proportions (95% CI), % |
|||
|---|---|---|---|---|
| Poorly Controlled Hypertension |
Poorly Controlled Dyslipidemia (n = 132 266) | Poorly Controlled Diabetes Mellitus (n = 48 568) | ||
| High Systolic BP (n = 125 427) | High Diastolic BP (n = 31 121) | |||
| Age | ||||
| 20–44 y | 71.2 (69.5–72.8)† | 78.4 (76.7–80.1)† | 54.4 (52.9–56.0)‡ | 73.0 (71.2–74.7)‡ |
| 45–54 y | 71.5 (70.5–72.5)† | 80.0 (78.9–81.1)† | 57.9 (56.9–58.9)‡ | 68.0 (66.8–69.2)‡ |
| 55–64 y | 69.9 (69.1–70.6)† | 81.7 (80.7–82.7)† | 58.6 (57.8–59.4)‡ | 67.2 (66.1–68.2)‡ |
| ≥65 y | 70.1 (69.5–70.6)† | 85.5 (84.7–86.3)† | 58.5 (57.8–59.2)‡ | 68.0 (67.0–69.1)‡ |
| Sex | ||||
| Women | 69.3 (68.7–69.8) | 83.7 (82.9–84.4) | 58.8 (58.1–59.5) | 69.6 (68.7–70.5) |
| Men | 71.6 (71.0–72.2)§ | 80.7 (79.9–81.5)§ | 57.6 (56.9–58.3)§ | 66.9 (66.0–67.8)§ |
| Race or ethnicity | ||||
| White | 69.3 (68.8–69.8) | 81.8 (81.0–82.6) | 57.7 (57.1–58.4) | 70.5 (69.6–71.4) |
| African American | 72.6 (71.6–73.5)§ | 82.2 (80.9–83.4) | 57.0 (55.8–58.2) | 65.9 (64.2–67.5)§ |
| Asian | 72.3 (71.3–73.3)§ | 83.9 (82.3–85.4)∥ | 60.8 (59.8–61.9)§ | 66.0 (64.6–67.4)§ |
| Latino | 71.6 (70.4–72.7)§ | 83.0 (81.1–84.8) | 59.4 (58.1–60.7)∥ | 66.3 (64.8–67.7)§ |
| Mixed | 70.1 (68.5–71.7) | 82.3 (79.7–84.6) | 58.7 (56.8–60.5) | 66.2 (63.7–68.6)§ |
| Native American | 70.8 (67.3–74.1) | 83.1 (77.3–87.6) | 57.7 (53.6–61.6) | 63.5 (57.8–68.9)¶ |
| Pacific Islander | 73.2 (61.8–82.2) | 87.6 (63.2–96.7) | 63.9 (52.4–74.0) | 66.2 (55.7–75.4) |
| Co-occurrence of other conditions | ||||
| None | 68.6 (67.9–69.3) | 79.6 (78.6–80.6) | 51.3 (50.4–52.3) | 59.9 (58.0–61.7) |
| Hypertension and dyslipidemia | 71.6 (70.9–72.3)§ | 83.4 (82.3–84.4)§ | 54.3 (53.6–55.0)§ | – |
| Hypertension and diabetes mellitus | 66.0 (64.9–67.0)§ | 81.2 (79.6–82.8) | – | 66.3 (64.9–67.7)§ |
| Dyslipidemia and diabetes mellitus | – | – | 66.9 (65.7–68.1)§ | 66.6 (65.1–68.0)§ |
| All 3 conditions | 72.1 (71.4–72.7)§ | 85.1 (84.2–86.0)§ | 66.5 (65.7–67.3)§ | 70.7 (69.9–71.5)§ |
| Risk status | ||||
| No target organ disease | 70.0 (69.3–70.7) | 80.4 (79.4–81.4) | 54.2 (53.5–54.9) | 69.1 (68.2–70.0) |
| Target organ disease (not CAD) | 70.1 (69.5–70.8) | 83.2 (82.3–84.0)§ | 60.5 (59.6–61.4)§ | 67.5 (66.4–68.6)∥ |
| CAD | 70.5 (69.8–71.2) | 83.7 (82.5–84.7)§ | 65.6 (64.8–66.4)§ | 66.7 (65.4–68.0)¶ |
| Baseline level of each condition | ||||
| Systolic BP | ||||
| 140–159 mm Hg | 65.3 (64.7–65.9)† | – | – | – |
| 160–179 mm Hg | 75.5 (74.9–76.1)† | – | – | – |
| ≥180 mm Hg | 81.5 (80.7–82.3)† | – | – | – |
| Diastolic BP | ||||
| 90–99 mm Hg | – | 79.5 (78.7–80.3)† | – | – |
| 100–109 mm Hg | – | 85.0 (84.1–85.8)† | – | – |
| ≥110 mm Hg | – | 89.2 (87.8–90.4)† | – | – |
| LDL cholesterol level | ||||
| 3.4–4.1 mmol/L (130–159 mg/dL) | – | – | 50.4 (49.7–51.2)† | – |
| 4.2–4.9 mmol/L (160–189 mg/dL) | – | – | 59.9 (59.1–60.6)† | – |
| ≥5.0 mmol/L (≥190 mg/dL) | – | – | 72.6 (71.8–73.4)† | – |
| Hemoglobin A1c | ||||
| 8.0%–8.9% | – | – | – | 61.8 (60.8–62.7)† |
| 9.0%–9.9% | – | – | – | 69.8 (68.6–71.0)† |
| ≥10% | – | – | – | 78.1 (77.2–79.0)† |
Hierarchical logistic regression with adjustment for all the covariates in this table. These multivariable models assessed patient factors associated with “appropriate care” (vs. “inappropriate care”) at 6 months. “Appropriate care” was defined as therapy modifications or return to control without therapy modification. BP = blood pressure; CAD = coronary artery disease; LDL = low-density lipoprotein.
P for trend < 0.001.
P for trend < 0.05.
P < 0.001 compared with reference group.
P < 0.05 compared with reference group.
P < 0.01 compared with reference group.
In general, nonwhite patients (African-American, Asian, or Latino patients) were less likely than white patients to have responses for hemoglobin A1c control but were slightly more likely to have “appropriate care” in response to poor blood pressure control. Latino and Asian patients, but not African-American patients, were more likely than white patients to have “appropriate care” in response to poorly controlled LDL cholesterol levels. For other race or ethnicity groups, most results were not statistically significant and had wide CIs.
Excluding the patients who returned to control without any therapy modification from the multivariable models yielded similar results for all factors. We also evaluated the number of medications that patients were taking for any other of the 3 conditions to see whether medication burden may be a barrier to treatment modification. The number of these medications did not decrease the likelihood of a therapy change for each condition. Its inclusion in the multivariate models diminished the strength of cooccurrence of other conditions with the outcomes. The variation in “appropriate care” was slightly larger at the physician level than at the medical center level for all outcomes: from 66.8% to 75.1% (interquartile ranges) at the physician level versus from 70.3% to 73.2% at the medical center level for systolic blood pressure; 79.5% to 85.0% versus 81.1% to 84.5%, respectively, for diastolic blood pressure; 52.6% to 64.5% versus 55.9% to 61.1%, respectively, for dyslipidemia; and 64.7% to 74.2% versus 67.4% to 70.6%, respectively, for diabetes.
Discussion
In this large clinical population, many patients with poorly controlled hypertension, dyslipidemia, or diabetes were receiving relatively few medications and relatively low doses when poor control was first noted. For each condition, most patients received a therapy modification within the following 6 months. Moreover, physicians were more likely to respond to poor risk factor control when cardiovascular risk was higher. These data demonstrate that measuring therapy modifications as possible markers of quality in a large patient population is feasible. These “tightly linked” process measures (18, 19) might represent an additional opportunity for improving quality of care. They met an important criterion for utility as a quality measure in that variation among physicians was substantial in each measure.
The frequency of therapy modifications in response to poorly controlled cardiovascular risk factors was higher in our study than that in 2 previous studies using similar measures. In a Veterans Affairs study in 1998 to 1999, 39% of 307 patients with diabetes and LDL cholesterol levels of 3.4 mmol/L or greater (≥130 mg/dL) began or increased lipid-lowering medication therapy within 6 months and 6% returned to control without a therapy modification (17). In a study in 30 U.S. academic medical centers in 2000 to 2001, 14% of patients with diabetes and untreated blood pressure greater than 150/100 mm Hg had therapy initiated, 15% of those patients with untreated LDL cholesterol levels of 3.4 mmol/L or greater (≥130 mg/dL) had therapy initiated, and 46% of patients with hemoglobin A1c levels of 8% or greater had modifications in hypoglycemic therapy, but these changes were only assessed at 1 visit (19). These studies were limited to patients with diabetes and had examined an earlier study period.
We observed that therapy modifications in response to poor control were more likely in the presence of increased risk, as measured by co-occurrence of another of the 3 conditions, higher baseline risk factor values, and presence of previous CAD or target organ damage. Recent guidelines for control of both blood pressure and LDL cholesterol level emphasize tighter control in patients who are at greatest cardiovascular risk (4, 5), which is likely to have a greater effect on preventing cardiovascular disease compared with treating low-risk patients.
For 18% to 41% of patients who were in poor control for 1 of these risk factors, we observed no “appropriate care” within 6 months. These apparent failures to respond have several potential explanations in addition to poor quality of care. Very small proportions of these patients were already receiving maximal medical therapy. For these patients, physicians may have been reluctant to further intensify therapy because of concerns over side effects. Patients' lack of adherence to pharmacotherapy may undoubtedly explain some failures. In our pharmacy data system, changes in pharmacotherapy are only observed if the patient fills the new prescription. Missed appointments and missed laboratory tests would also lead to apparent failures to respond. A previous study in this population demonstrated that the rate of missed outpatient appointments was highly predictive of glycemic control among patients with diabetes (29). Finally, physician choices to recommend lifestyle modification may also explain some failures to respond. We could not identify such recommendations in our data. However, we counted return to control within 6 months, which may have been due to successful lifestyle modification, as “appropriate care.”
Nevertheless, in view of the relatively low levels of treatment intensity at baseline, most observed failures to change therapy are probably due to poor quality of care or clinical inertia (12). Proposed explanations for clinical inertia include physicians' overestimation of their adherence to guidelines (12) or acceptance of elevated risk factor levels in their patients (30), lack of training on achieving therapeutic goals, possible lack of motivation in clinicians to treat asymptomatic chronic conditions (12), pharmacotherapy pill burden (31), acute symptoms that supersede risk factor management, and time limitations (32). Quality improvement strategies that incorporate more clinically specific measures of “appropriate care,” such as those examined in our study, into performance feedback or physician reminder systems (12) may be more effective in overcoming these barriers and improving risk factor levels.
The rates of nonresponse differed between conditions, which might provide information on the reasons for non-response. Similar to previous studies (33, 34), therapy modifications were more likely in response to poor control of diastolic than systolic blood pressure, possibly reflecting a continued lack of awareness among physicians of the greater importance of systolic blood pressure in predicting cardiovascular risk (34, 35). The frequency of therapy modifications was higher for poorly controlled blood pressure than for diabetes and particularly for dyslipidemia, possibly because more drug classes are available for hypertension. For diabetes, concerns about hypoglycemia and patient or physician reluctance to initiate insulin therapy may be specific additional barriers to the implementation of more intensive control (36, 37). Moreover, contraindications for therapies may further restrict therapeutic options. As patients got older, physicians were less likely to intensify treatment for poorly controlled systolic blood pressure and hemoglobin A1c level, possibly reflecting a greater concern about treatment side effects, pill burden, or adherence to therapy.
An intriguing finding is the variation in response by patient race or ethnicity across the 3 conditions. These differences were small but persisted after adjustment for factors associated with higher cardiovascular risk and demographic characteristics. Our data suggest that a lower likelihood of therapy modifications in response to elevated hemoglobin A1c levels may explain the poorer glycemic control that have been widely found in nonwhite patients with diabetes in the United States (22, 38). Higher therapy modification rates in nonwhite patients for poorly controlled hypertension and LDL cholesterol levels have not been noted previously and should be further explored.
Our study has several limitations and remaining questions. Our data do not permit identification of many causes of nonresponse to poor control that were discussed earlier. Thus, optimal rates of therapy intensification are unknown and are certainly less than 100%. Although our analyses do not directly demonstrate that therapy modifications led to better control, their validity is indirectly supported by the several randomized, controlled trials that have demonstrated the efficacy of these pharmacotherapeutic interventions (1-5). Several previous studies have also shown that clinical inertia was associated with poor risk factor control (12, 39) and intensification of therapy with better control (17, 39-41). A final validation would examine whether higher rates of treatment intensification or “appropriate care” are associated with better levels of control across populations or over time. This was beyond the scope of our study. Our measure for diabetes is not currently applicable to patients who are already receiving insulin because day-to-day adjustments in insulin dosage are not available in automated data sources. Finally, therapy changes for hypertension might have been modestly misclassified if these drugs were prescribed primarily for reasons other than hypertension. However, these drugs were introduced in the 6 months after an elevated risk factor value, and the timing of the changes (>70% during the first 2 months) suggests that most changes were related to the elevated risk factor value. Even if drugs were prescribed primarily for a condition other than hypertension, the medication may help to lower blood pressure. We also could not assess whether the total number of medications was inversely related to the likelihood of a change in therapy because our data set did not include noncardiovascular medications, but we found that the total number of medications taken for any other of the 3 conditions did not decrease the likelihood of a change in therapy.
We believe that our study is the first to demonstrate the feasibility of assessing “tightly linked” measures in a large patient population, which should generalize to other health care organizations with electronic clinical databases (18). Such measures have been proposed to have several advantages over simpler process measures, such as rates of risk factor testing, and also over levels of control. Intensification of therapy, but not higher testing rates for risk factors (7, 19), has been shown to lead to better control (17, 39-41). For example, a study in 30 U.S. academic medical centers found a very high proportion of testing rates (88% to 97%) for cardiovascular risk factors in patients with diabetes, but a low proportion of patients in control (33% to 46%) and a low rate of therapy modifications in those with elevated risk factor levels (14% to 46%) (19). Unlike measurements of risk factor control, measures of treatment intensification are directly actionable by physicians or by systems (18). These process-of-care measures do not penalize physicians or systems that care for sicker or less adherent patients (42). Hofer and colleagues (43) showed that high outlier physicians could dramatically improve profiles on the basis of levels of control alone, simply by avoiding caring for some patients with elevated risk factor levels.
However, the measure we examined considers only patients with poorly controlled risk factors and could not, therefore, be the only quality indicator for a condition. An assessment of overall quality must consider both the proportions of patients with the condition who are in control and the clinician behavior in response to those who are not. Future studies should assess whether reporting performance that is partly based on this new measure would be more clinically relevant and acceptable to physicians than simply reporting levels of testing or control and would improve risk factor control.
In summary, we have demonstrated that therapy modifications in response to poor risk factor control can be measured as an additional marker of quality in a large patient population. Most patients with poor control of hypertension, dyslipidemia, and diabetes experienced a therapy modification within 6 months. Because appropriate physician response to poor control is a critical intermediate step between risk factor assessment and clinical outcomes (18), inclusion of such responses, in conjunction with standard assessments of proportions of patients who are already in control, may provide a more accurate index of the quality of clinical care than relying solely on measures of proportions of patients who are in control. It may lead to improved control of risk factors.
Acknowledgments
The authors thank Jon P. Edwards and Fiona Nitsche for editorial support.
Appendix Table 1
Diagnostic Criteria for Diabetes Mellitus, Hypertension, and Dyslipidemia*
| Condition | Diagnostic Criteria |
|---|---|
| Diabetes mellitus | At least 1 prescription of insulin or an oral hypoglycemic agent; at least 2 outpatient diagnoses of diabetes mellitus; 1 outpatient diagnosis of diabetes mellitus plus at least 1 hemoglobin A1c measurement ≥ 7%; or at least 1 hospital discharge with a primary diabetes mellitus–related diagnosis (ICD-9 code 250.X) |
| Hypertension | At least 1 prescription for an antihypertensive medication plus an outpatient diagnosis of hypertension; at least 2 outpatient diagnoses of hypertension; at least 1 prescription for an antihypertensive medication plus at least 1 elevated outpatient blood pressure reading (≥140 mm Hg [systolic] or ≥90 mm Hg [diastolic]); or at least 1 outpatient diagnosis of hypertension plus at least 1 blood pressure reading of ≥140 mm Hg (systolic) or ≥ 90 mm Hg (diastolic) |
| Dyslipidemia | At least 1 prescription for an antilipemic agent or outpatient diagnosis of hyperlipidemia or hypercholesterolemia with a previous LDL cholesterol value ≥ risk-appropriate cut-point value, as defined in Appendix Table 2 |
ICD-9 = International Classification of Diseases, Ninth Revision; LDL = low-density lipoprotein.
Appendix Table 2
Levels of Control for Hypertension, Dyslipidemia, and Diabetes Mellitus*
| Condition | Measure | In Control | Near Control | Poor Control |
|---|---|---|---|---|
| Hypertension | ||||
| No diabetes mellitus and no target organ disease | 2 consecutive SBP | <140 mm Hg | 140–159 mm Hg | ≥160 mm Hg |
| 2 consecutive DBP | <90 mm Hg | 90–99 mm Hg | ≥100 mm Hg | |
| With diabetes mellitus or target organ disease | 2 consecutive SBP | <130 mm Hg | 130–139 mm Hg | ≥140 mm Hg |
| 2 consecutive DBP | <85 mm Hg | 85–89 mm Hg | ≥90 mm Hg | |
| Dyslipidemia | ||||
| Low risk | ||||
| No diabetes mellitus or CAD, <2 CAD risk factors | LDL cholesterol level | <4.2 mmol/L (<160 mg/dL) | 4.2–4.9 mmol/L (160–189 mg/dL) | ≥5.0 mmol/L (≥190 mg/dL) |
| Moderate risk | ||||
| 2 CAD risk factors but 10-y Framingham risk score < 20% | LDL cholesterol level | <3.4 mmol/L (<130 mg/dL) | 3.4–4.1 mmol/L (130–159 mg/dL) | ≥4.2 mmol/L (≥160 mg/dL) |
| High risk | ||||
| ≥ 2 CAD risk factors and 10-y Framingham risk score ≥ 20% | LDL cholesterol level | <2.6 mmol/L (<100 mg/dL) | 2.6–3.3 mmol/L (100–129 mg/dL) | ≥3.4 mmol/L (≥130 mg/dL) |
| With diabetes mellitus or previous CAD | LDL cholesterol level | <2.6 mmol/L (<100 mg/dL) | 2.6–3.3 mmol/L (100–129 mg/dL) | ≥3.4 mmol/L (≥130 mg/dL) |
| Diabetes mellitus | Hemoglobin A1c | <7.0% | 7.0%–7.9% | ≥8.0% |
CAD = coronary artery disease; DBP = diastolic blood pressure; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Footnotes
Grant Support: Research funding for the collection and analysis of these data was provided by the U.S. Outcomes Research Group of Pfizer Inc. Dr. Rodondi was supported by a grant from the Swiss National Foundation (PBLAB-102353). Dr. Kerr was supported by the Veterans Affairs Health Services Research and Development Service Diabetes Mellitus Quality Enhancement Research Initiative (DIB #98-001) and the Michigan Diabetes Research and Training Center (National Institues of Health/National Institute of Diabetes & Digestive & Kidney Diseases #5 P50 DK 20572).
Potential Financial Conflicts of Interest: Employment: S. Tang (Pfizer Inc.), D. Pettitt (Pfizer Inc.); Expert testimony: J.V. Selby (Kaiser Permanente); Stock ownership or options (other than mutual funds): S. Tang (Pfizer Inc.), D. Pettitt (Pfizer Inc.); Grants received: N. Rodondi (Pfizer Inc., Swiss National Foundation), A.J. Karter (Pfizer Inc.), J.V. Selby (Pfizer Inc.); Grants pending: J.V. Selby (Pfizer Inc.).
References
- 1.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [PMID: 12114036] [DOI] [PubMed] [Google Scholar]
- 2.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [PMID: 12479763] [DOI] [PubMed] [Google Scholar]
- 3.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [PMID: 12556541] [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [PMID: 12748199] [DOI] [PubMed] [Google Scholar]
- 5.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [PMID: 11368702] [DOI] [PubMed] [Google Scholar]
- 6.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [PMID: 12851274] [DOI] [PubMed] [Google Scholar]
- 7.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [PMID: 11955024] [DOI] [PubMed] [Google Scholar]
- 8.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [PMID: 14734596] [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003;107:2185–9. doi: 10.1161/01.CIR.0000066320.27195.B4. [PMID: 12719276] [DOI] [PubMed] [Google Scholar]
- 10.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–86. doi: 10.1056/NEJMoa010273. [PMID: 11519501] [DOI] [PubMed] [Google Scholar]
- 11.Stafford RS, Blumenthal D, Pasternak RC. Variations in cholesterol management practices of U.S. physicians. J Am Coll Cardiol. 1997;29:139–46. doi: 10.1016/s0735-1097(96)00441-x. [PMID: 8996306] [DOI] [PubMed] [Google Scholar]
- 12.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, et al. Clinical inertia. Ann Intern Med. 2001;135:825–34. doi: 10.7326/0003-4819-135-9-200111060-00012. [PMID: 11694107] [DOI] [PubMed] [Google Scholar]
- 13.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000–5. doi: 10.1001/jama.280.11.1000. [PMID: 9749483] [DOI] [PubMed] [Google Scholar]
- 14.Lenfant C. Conquering cardiovascular disease: progress and promise [Editorial] JAMA. 1999;282:2068–70. doi: 10.1001/jama.282.21.2068. [PMID: 10591390] [DOI] [PubMed] [Google Scholar]
- 15.The Health Plan Employer Data and Information Set (HEDIS) National Committee for Quality Insurance; Washington, DC: Feb 7, 2006. www.ncqa.org/programs/hedis Accessed at. [Google Scholar]
- 16.Bell RA, Camacho F, Goonan K, Duren-Winfield V, Anderson RT, Konen JC, et al. Quality of diabetes care among low-income patients in North Carolina. Am J Prev Med. 2001;21:124–31. doi: 10.1016/s0749-3797(01)00328-2. [PMID: 11457632] [DOI] [PubMed] [Google Scholar]
- 17.Kerr EA, Smith DM, Hogan MM, Hofer TP, Krein SL, Bermann M, et al. Building a better quality measure: are some patients with ‘poor quality’ actually getting good care? Med Care. 2003;41:1173–82. doi: 10.1097/01.MLR.0000088453.57269.29. [PMID: 14515113] [DOI] [PubMed] [Google Scholar]
- 18.Kerr EA, Krein SL, Vijan S, Hofer TP, Hayward RA. Avoiding pitfalls in chronic disease quality measurement: a case for the next generation of technical quality measures. Am J Manag Care. 2001;7:1033–43. [PMID: 11725807] [PubMed] [Google Scholar]
- 19.Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337–442. doi: 10.2337/diacare.28.2.337. [PMID: 15677789] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [PMID: 1566949] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby JV, Peng T, Karter AJ, Alexander M, Sidney S, Lian J, et al. High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care. 2004;10:163–70. [PMID: 15005509] [PubMed] [Google Scholar]
- 22.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [PMID: 12020332] [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [PMID: 10610643] [DOI] [PubMed] [Google Scholar]
- 24.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [PMID: 9385294] [DOI] [PubMed] [Google Scholar]
- 25.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. Springer-Verlag; New York: 2005. [Google Scholar]
- 26.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [PMID: 9832001] [DOI] [PubMed] [Google Scholar]
- 27.Physicians' Desk Reference. 54th ed. Medical Economics Company; Montvale, NJ: 2001. [Google Scholar]
- 28.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–64. doi: 10.1093/oxfordjournals.aje.a117592. [PMID: 7503045] [DOI] [PubMed] [Google Scholar]
- 29.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Ferrara A, Liu JY, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care. 2004;42:110–5. doi: 10.1097/01.mlr.0000109023.64650.73. [PMID: 14734947] [DOI] [PubMed] [Google Scholar]
- 30.Oliveria SA, Lapuerta P, McCarthy BD, L'Italien GJ, Berlowitz DR, Asch SM. Physician-related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–20. doi: 10.1001/archinte.162.4.413. [PMID: 11863473] [DOI] [PubMed] [Google Scholar]
- 31.Asai Y, Heller R, Kajii E. Hypertension control and medication increase in primary care. J Hum Hypertens. 2002;16:313–8. doi: 10.1038/sj.jhh.1001385. [PMID: 12082491] [DOI] [PubMed] [Google Scholar]
- 32.Yarnall KS, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–41. doi: 10.2105/ajph.93.4.635. [PMID: 12660210] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyte JL, Lapuerta P, L'Italien GJ, Franklin SS. The challenge of controlling systolic blood pressure: data from the National Health and Nutrition Examination Survey (NHANES III), 1988–1994. J Clin Hypertens (Greenwich) 2001;3:211–6. doi: 10.1111/j.1524-6175.2001.00461.x. [PMID: 11498651] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med. 2002;162:577–81. doi: 10.1001/archinte.162.5.577. [PMID: 11871926] [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [PMID: 10678282] [DOI] [PubMed] [Google Scholar]
- 36.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–12. doi: 10.2337/diacare.26.6.1902. [PMID: 12766131] [DOI] [PubMed] [Google Scholar]
- 37.Davis S, Alonso MD. Hypoglycemia as a barrier to glycemic control. J Diabetes Complications. 2004;18:60–8. doi: 10.1016/S1056-8727(03)00058-8. [PMID: 15019602] [DOI] [PubMed] [Google Scholar]
- 38.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–8. doi: 10.2337/diacare.22.3.403. [PMID: 10097918] [DOI] [PubMed] [Google Scholar]
- 39.Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–63. doi: 10.1056/NEJM199812313392701. [PMID: 9869666] [DOI] [PubMed] [Google Scholar]
- 40.Cook CB, Ziemer DC, El-Kebbi IM, Gallina DL, Dunbar VG, Ernst KL, et al. Diabetes in urban African-Americans. XVI. Overcoming clinical inertia improves glycemic control in patients with type 2 diabetes. Diabetes Care. 1999;22:1494–500. doi: 10.2337/diacare.22.9.1494. [PMID: 10480515] [DOI] [PubMed] [Google Scholar]
- 41.Asch SM, McGlynn EA, Hiatt L, Adams J, Hicks J, DeCristofaro A, et al. Quality of care for hypertension in the United States. BMC Cardiovasc Disord. 2005;5:1. doi: 10.1186/1471-2261-5-1. [PMID: 15638933] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krein SL, Hofer TP, Kerr EA, Hayward RA. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Serv Res. 2002;37:1159–80. doi: 10.1111/1475-6773.01102. [PMID: 12479491] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA. 1999;281:2098–105. doi: 10.1001/jama.281.22.2098. [PMID: 10367820] [DOI] [PubMed] [Google Scholar]