Abstract
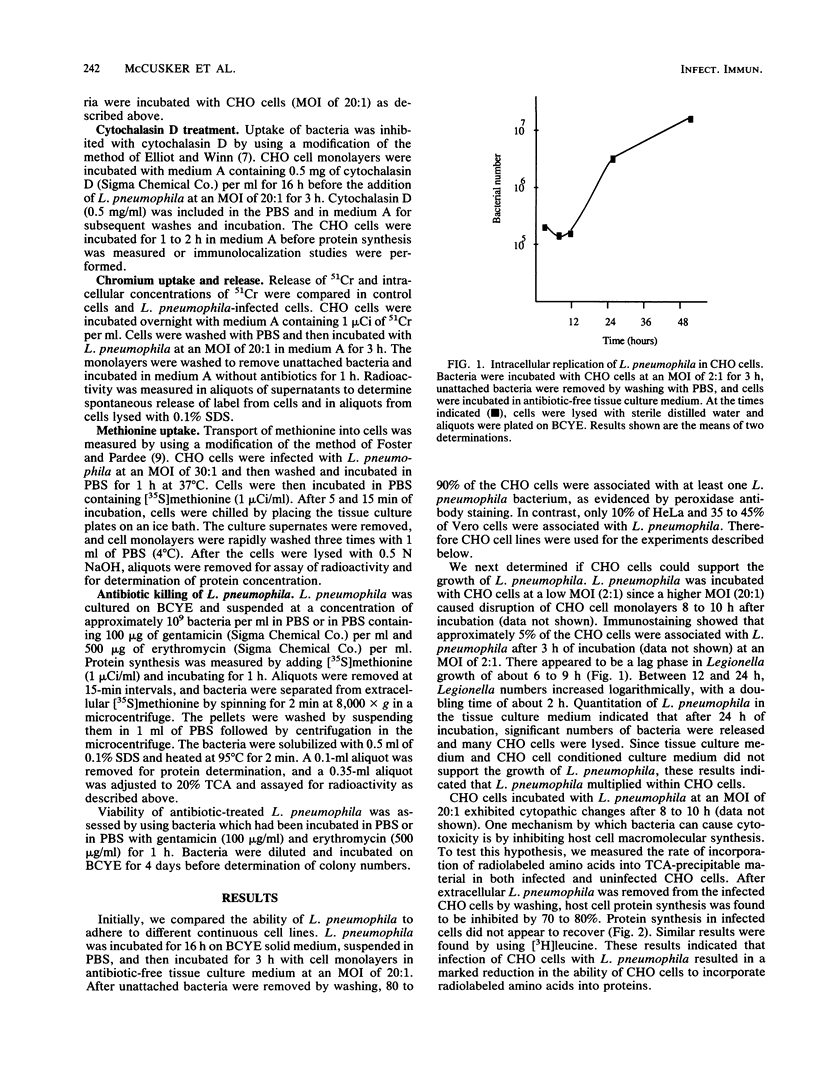
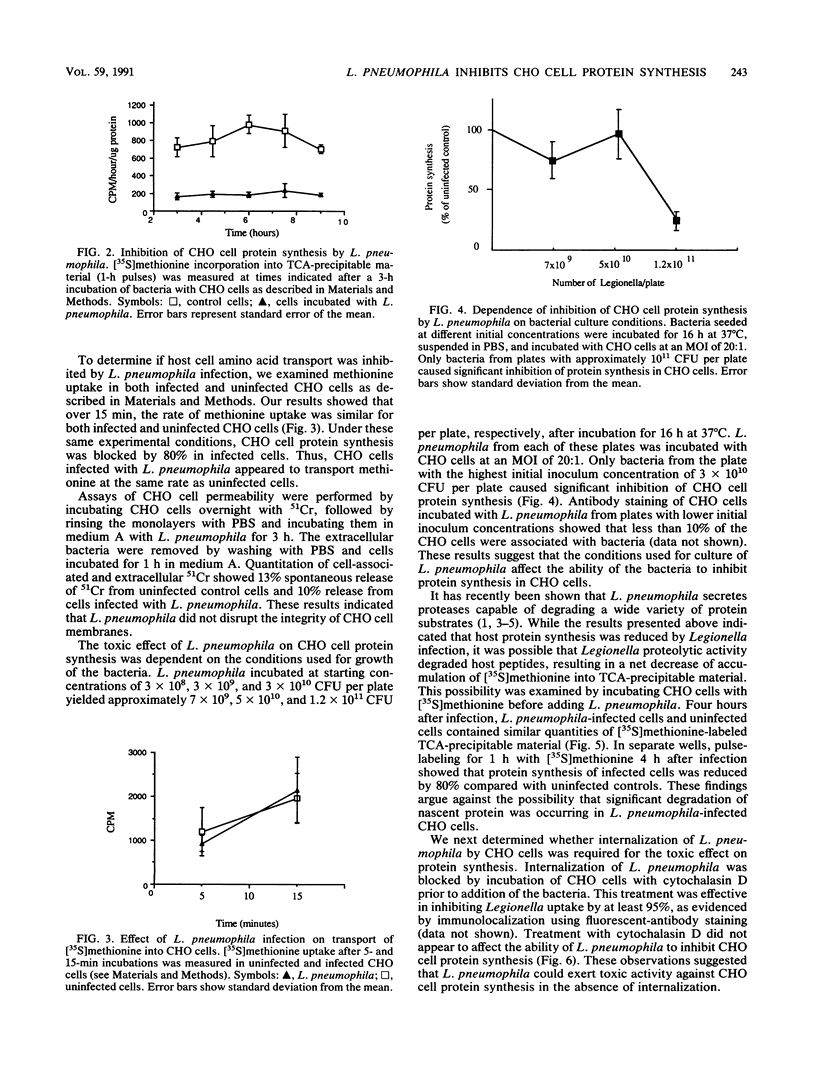
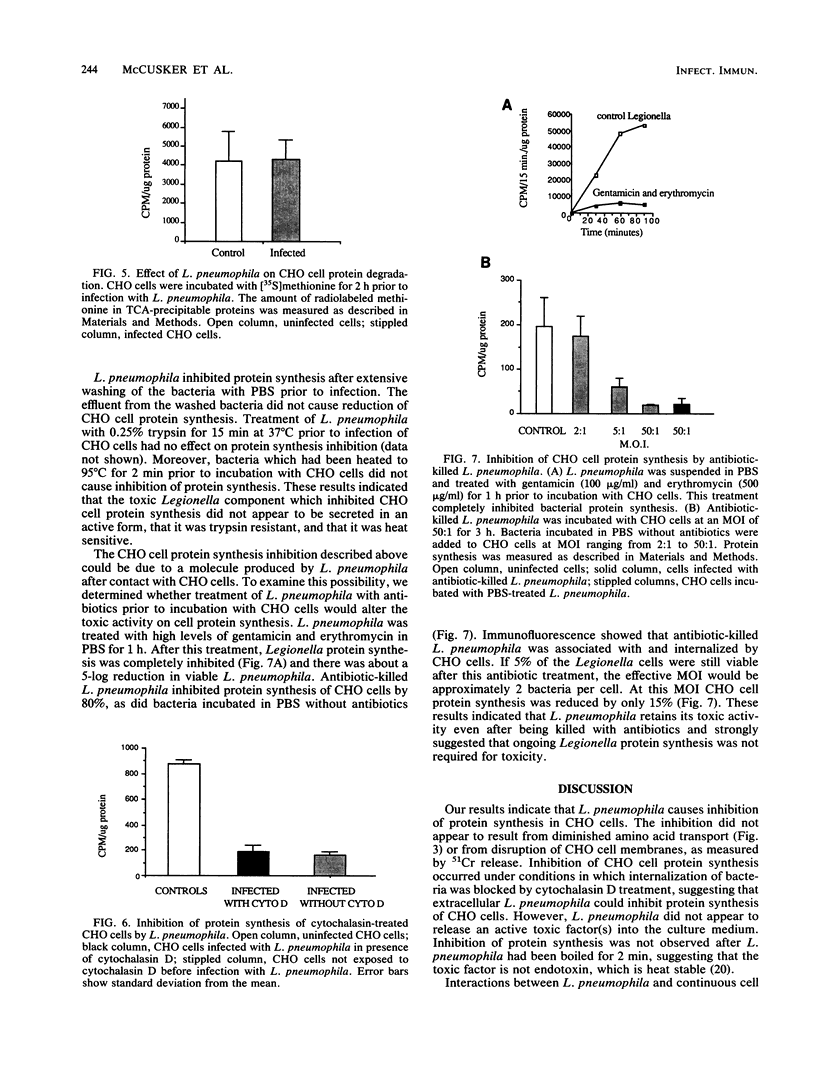
Legionella pneumophila is a gram-negative facultative intracellular parasite that causes Legionnaires disease. To explore the interactions between L. pneumophila and host cells, we have developed a continuous cell line model of infection. We show that about 80% of Chinese hamster ovary (CHO) cells were associated with L. pneumophila after incubation for 3 h at a multiplicity of infection of 20 bacteria per cell. Within 3 to 4 h of incubation with L. pneumophila, protein synthesis of CHO cells was markedly inhibited, as shown by the reduction of incorporation of radiolabeled amino acids into proteins. L. pneumophila did not inhibit transport of amino acids or cause degradation of newly synthesized proteins in CHO cells. Cytochalasin D blocked internalization of L. pneumophila by CHO cells, yet CHO cell protein synthesis was inhibited. These results indicated that L. pneumophila could inhibit host protein synthesis from the cell exterior. L. pneumophila that had been killed with antibiotics prior to incubation with CHO cells still inhibited protein synthesis, indicating that the inhibition of CHO cell protein synthesis occurred in the absence of de novo protein synthesis by L. pneumophila.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskerville A., Conlan J. W., Ashworth L. A., Dowsett A. B. Pulmonary damage caused by a protease from Legionella pneumophila. Br J Exp Pathol. 1986 Aug;67(4):527–536. [PMC free article] [PubMed] [Google Scholar]
- Brabender W., Hinthorn D. R., Asher M., Lindsey N. J., Liu C. Legionella pneumophila wound infection. JAMA. 1983 Dec 9;250(22):3091–3092. [PubMed] [Google Scholar]
- Conlan J. W., Williams A., Ashworth L. A. In vivo production of a tissue-destructive protease by Legionella pneumophila in the lungs of experimentally infected guinea-pigs. J Gen Microbiol. 1988 Jan;134(1):143–149. doi: 10.1099/00221287-134-1-143. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., Williams A., Ashworth L. A. Inactivation of human alpha-1-antitrypsin by a tissue-destructive protease of Legionella pneumophila. J Gen Microbiol. 1988 Feb;134(2):481–487. doi: 10.1099/00221287-134-2-481. [DOI] [PubMed] [Google Scholar]
- Dreyfus L. A., Iglewski B. H. Purification and characterization of an extracellular protease of Legionella pneumophila. Infect Immun. 1986 Mar;51(3):736–743. doi: 10.1128/iai.51.3.736-743.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. A., Johnson W. Virulence conversion of Legionella pneumophila serogroup 1 by passage in guinea pigs and embryonated eggs. Infect Immun. 1982 Mar;35(3):943–946. doi: 10.1128/iai.35.3.943-946.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Friedman R. L., Iglewski B. H., Miller R. D. Identification of a cytotoxin produced by Legionella pneumophila. Infect Immun. 1980 Jul;29(1):271–274. doi: 10.1128/iai.29.1.271-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Lochner J. E., Bigley R. H., Iglewski B. H. The effects of Legionella pneumophila toxin on oxidative processes and bacterial killing of human polymorphonuclear leukocytes. J Infect Dis. 1982 Sep;146(3):328–334. doi: 10.1093/infdis/146.3.328. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985 Aug;22(2):168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987 Nov 1;166(5):1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984 Jan;36(1):27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Hulkower K. I., Wnek A. P., McClane B. A. Evidence that alterations in small molecule permeability are involved in the Clostridium perfringens type A enterotoxin-induced inhibition of macromolecular synthesis in Vero cells. J Cell Physiol. 1989 Sep;140(3):498–504. doi: 10.1002/jcp.1041400314. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A., Horwitz M. A., Shuman H. A. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol. 1990 Apr 1;144(7):2738–2744. [PubMed] [Google Scholar]
- McClane B. A., Wnek A. P., Hulkower K. I., Hanna P. C. Divalent cation involvement in the action of Clostridium perfringens type A enterotoxin. Early events in enterotoxin action are divalent cation-independent. J Biol Chem. 1988 Feb 15;263(5):2423–2435. [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham L. J., Rodgers F. G. Adhesion, penetration and intracellular replication of Legionella pneumophila: an in vitro model of pathogenesis. J Gen Microbiol. 1985 Apr;131(4):697–706. doi: 10.1099/00221287-131-4-697. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Jiwa A. H., Engleberg N. C., Eisenstein B. I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988 Aug;5(2):87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Schwartzman J. D., Kasper L. H. Toxoplasma gondii: use of mutants to study the host-parasite relationship. Ciba Found Symp. 1983;99:74–91. doi: 10.1002/9780470720806.ch5. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Oliver C., Bateman J. L., Krag S. S., Galloway C. J., Mellman I. A single mutation in Chinese hamster ovary cells impairs both Golgi and endosomal functions. J Cell Biol. 1984 Oct;99(4 Pt 1):1296–1308. doi: 10.1083/jcb.99.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh M. J., Morse S. A., Miller R. D. Intermediary metabolism in Legionella pneumophila: utilization of amino acids and other compounds as energy sources. J Bacteriol. 1983 Jun;154(3):1104–1109. doi: 10.1128/jb.154.3.1104-1109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins L. S., Roessler B. J., Redd S. C., Markowitz L. E., Cohen M. L. Legionella prosthetic-valve endocarditis. N Engl J Med. 1988 Mar 3;318(9):530–535. doi: 10.1056/NEJM198803033180902. [DOI] [PubMed] [Google Scholar]
- Winn W. C., Jr Legionnaires disease: historical perspective. Clin Microbiol Rev. 1988 Jan;1(1):60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. C., Ewing E. P., Jr, Callaway C. S., Peacock W. L., Jr Intracellular multiplication of Legionella pneumophila in cultured human embryonic lung fibroblasts. Infect Immun. 1980 Jun;28(3):1014–1018. doi: 10.1128/iai.28.3.1014-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


