Abstract
In osteoarthritis (OA), low-grade joint inflammation promotes altered chondrocyte differentiation and cartilage catabolism. S100/calgranulins share conserved calcium-binding EF-hand domains, associate noncovalently as homodimers and heterodimers, and are secreted and bind receptor for advanced glycation end products (RAGE). Chondrocyte RAGE expression and S100A11 release are stimulated by IL-1β in vitro and increase in OA cartilage in situ. Exogenous S100A11 stimulates chondrocyte hypertrophic differentiation. Moreover, S100A11 is covalently cross-linked by transamidation catalyzed by transglutaminase 2 (TG2), itself an inflammation-regulated and redox stress-inducible mediator of chondrocyte hypertrophic differentiation. In this study, we researched mouse femoral head articular cartilage explants and knee chondrocytes, and a soluble recombinant double point mutant (K3R/Q102N) of S100A11 TG2 transamidation substrate sites. Both TG2 and RAGE knockout cartilage explants retained IL-1β responsiveness. The K3R/Q102N mutant of S100A11 retained the capacity to bind to RAGE and chondrocytes but lost the capacity to signal via the p38 MAPK pathway or induce chondrocyte hypertrophy and glycosaminoglycans release. S100A11 failed to induce hypertrophy, glycosaminoglycan release, and appearance of the aggrecanase neoepitope NITEGE in both RAGE and TG2 knockout cartilages. We conclude that transamidation by TG2 transforms S100A11 into a covalently bonded homodimer that acquires the capacity to signal through the p38 MAPK pathway, accelerate chondrocyte hypertrophy and matrix catabolism, and thereby couple inflammation with chondrocyte activation to potentially promote OA progression.
Maintenance of the structural and functional integrity of articular cartilage requires a balance between anabolic and catabolic activities of chondrocytes, the sole cells in hyaline articular cartilage (1). In osteoarthritis (OA),3 low-grade inflammation, associated with increased chondrocyte IL-1β expression, develops within cartilage and variably in synovium (2, 3). IL-1β promotes chondrocyte de-differentiation, and stimulates oxidative stress and matrix catabolism modulated partly by induction of inducible NO synthase (3), matrix metalloproteinase (MMP)-13, and ADAMTS-5 (a disintegrin and metalloproteinase with thrombospondin motifs) (1, 4). Certain other conventional inflammatory cytokines up-regulated in OA cartilage in situ (TNF-α (5) and the chemokines CXCL1 and CXCL8 (6)) induce chondrocyte maturation to hypertrophy (7, 8), a state intimately linked in growth plate physiology to a shift in collagen synthesis from type II to type X, as well as other changes in extracellular matrix organization and an increased capacity to calcify (9, 10). Significantly, evidence of chondrocyte hypertrophic differentiation typically appears within OA cartilage chondrocyte populations in the course of OA (11, 12).
Inhibition of IL-1βonly partially inhibits experimental OA (13–15), and thus the respective roles of other inflammatory mediators in OA remain to be defined. S100/calgranulins are a family of over 20 proteins of ~10–12 kDa that share conserved calcium-binding EF-hand domains and associate noncovalently as homodimers and heterodimers (16). Inside cells, the major functions of S100/calgranulins appear to be mediated by their capacity to shuttle calcium between subcellular compartments (16). When released by cells, some S100/calgranulins can act as unconventional inflammatory cytokines (e.g., phagocyte-derived S100A8 and S100A9) (17, 18). S100/calgranulins S100B, S100A4, and S100A11 can activate cultured chondrocytes (19 –21). S100/calgranulins signal in part by binding the receptor for advanced glycation end products (RAGE), a member of the Ig superfamily (22). RAGE, a patterning receptor for at least four classes of ligands, does not scavenge ligands by internalization, but instead can promote activation of NF-κB, JAK-STAT, and MAPK signaling dependent on the RAGE cytosolic tail domain (23–25). IL-1β increases RAGE expression in cultured chondrocytes. RAGE expression and S100A11 surrounding chondrocytes are elevated in OA cartilage in situ (19, 21). In cultured chondrocytes, IL-1β, TNF-α, and CXCL8 induce S100A11 release. Moreover, hypertrophic chondrocyte differentiation induced by exogenous S100A11, CXCL8, and TNF-α is dependent on RAGE signaling transduced in part by the p38 MAPK pathway (19).
Clustering of RAGE by multimeric S100/calgranulins appears to enhance RAGE-dependent signal transduction (26–28). Significantly, transglutaminase (TG)2 stimulates covalent S100A11 homodimerization by catalyzing formation of N-ε(γ-glutamyl)lysyl isopeptide bonds (29, 30). Moreover, cultured articular chondrocytes express TG2 (31), and TG catalytic activity and TG-catalyzed isopeptide bond formation are substantially increased in human knee OA cartilage chondrocytes (32, 33). Furthermore, TG2 is the principal catalytically active TG isoenzyme in OA cartilage extracellular matrix in situ (34). Significantly IL-1β, TNF-α, CXCL1, and CXCL8 induce TG activity in cultured chondrocytes (8, 33), and TG2 release is essential for chemokine-induced chondrocyte hypertrophy (8, 34, 35). Hence, S100A11, RAGE, and TG2 are expressed by chondrocytes, are modulated by inflammatory cytokines, and each can promote chondrocyte hypertrophic differentiation. In this study, we observe that transamidation by TG2 transforms S100A11 into an unconventional inflammatory cytokine that, by acquiring the capacity to signal through RAGE, modulates chondrocyte differentiation and promotes matrix catabolism.
Materials and Methods
Mice
All mouse procedures were humanely performed and were reviewed and approved by the Institutional Animal Care and Use Committee. We established breeding colonies of RAGE null mice from Dr. A. M. Schmidt (Columbia University, New York, NY) and TG2 null mice from Dr. B. Graham (Victor Chang Research Institute, Darlinghurst, New South Wales, Australia), each on a C57BL/6 background. Congenic wild-type mice were used as controls. C57BL/6 wild-type controls were purchased from The Jackson Laboratory.
Reagents
Unless otherwise indicated, all chemical reagents were obtained from Sigma-Aldrich. Goat polyclonal Abs to RAGE and the rabbit polyclonal Abs to type I collagen, type II collagen, and aggrecan were from Chemicon International.
Site-directed mutagenesis and preparation of recombinant proteins
The S100A11 mutant K3R/Q102N was made using normal human S100A11 cDNA (19) as the template, and using the QuikChange Site-Directed Mutagenesis kit (Stratagene) with the following primers: K3R (forward) GCTCCAACATGGCAAGAATCTCCAAGC, (reverse) CTG TAGGGCTGGAGATTCTTGCCATG; and Q102N (forward) CAAG GCTGTCCCTTCCAACAAGCGGA and (reverse) CCTCAGGTCCGCT TGTTGGAAGGGAC. Purification of S100A11 polypeptides after cDNA transfection into HEK-293 cells was performed as previously described, with SDS-PAGE or Western blotting done to confirm purity (19). LPS was undetectable (<0.025 endotoxin U/ml) by the Limulus amebocyte lysate assay in all recombinant S100A11 preparations. Preparation of the TG2 mutants K173L and C277G was previously described (36). For copper chloride copper chloride oxidation of S100A11 to generate S-S bonded oligomers (37), 1 µg of S100A11 mutant K3R/Q102N was incubated in 6 µM CuCl2 in 20 mM Tris-HCl for 1 h, after which 90 µM copper-chelating diethylenetriamine-N,N,N′,N′,N′-pentaacetic acid was added and the reaction products studied by SDS-PAGE under nonreducing or reducing conditions.
Studies of mouse femoral head cartilage explants
We adapted the methods of Glasson et al. (4) to study femoral head cartilage caps from mice at 2 mo of age. In brief, isolated femoral head caps were placed in a 96-well plate containing 0.05 ml of DMEM/high glucose supplemented with 10% FCS, 1% L-glutamine, 100 U/ml penicillin, and 50 µg/ml streptomycin at 37°C in 5% CO2 for 18 h before the described treatments. Glycosaminoglycans (GAG) release into conditioned medium at 48 h was measured spectrophotometrically in triplicate at 525 nm using 200× 1,9-dimethyl-methylene blue in wells containing 5 µl of medium and 100 µl of 0.4 M glycine, 0.4 M NaCl, 0.095 M HCl (pH 3.0), with chondrotin sulfate used as the standard (38). Nitrite levels in conditioned medium were assayed by the Griess reaction to measure NO generation, with sodium nitrite used as the standard (8). We assayed TG transamidation catalytic activity by 5-biotinamidopentylamine binding to dimethylcasein (8), with 1 U of TG designated as 1 µM substrate catalyzed per hour.
For immunohistochemistry, 9-µm frozen sections were fixed using 100% ethanol for 10 min and allowed to dry for 30 min. Sections were then incubated with 1% Triton X-100 for 5 min or with 0.1 U chondroitinase and 0.1 U keratanase for 1 h at 37°C. Sections were washed two times with PBS containing 0.05% Tween 20 and blocked with 1% BSA/1% casein for 30 min at 37°C, then incubated for 1 h at 37°C with primary Ab in the same blocking buffer. After three washes, primary Ab was detected via the avidin-biotin conjugate method using the Histostain-Plus reagent (Invitrogen), and with Fast Green (0.001%) added for 5 min followed by two washes in water. Whole serum containing rabbit polyclonal Abs to type X collagen used for immunohistochemistry were from Cosmobio at a 1/100 dilution. Rabbit polyclonal Abs to the aggrecanase neoepitope NITEGE were from GeneTex and used at 10 µg/ml.
Murine chondrocyte isolation and characterization
To isolate immature mouse knee articular chondrocytes, we adapted a recently described method (39). In brief, patellar groove and femoral condyle cartilage dissected out from 7- to 8-day-old mice were digested using 3 mg/ml collagenase D (Roche) in DMEM/high glucose containing 10% serum for 45 min, then transferred into 2 mg/ml type II collagenase for an additional digestion of 3 h. The supernatant was filtered through a 70-µm cell strainer and the cells were centrifuged at 500 × g for 5 min, washed in PBS and plated in monolayer culture in DMEM/high glucose supplemented with 10% FCS, 1% l-glutamine, 100 U/ml penicillin, and 50 µg/ml streptomycin at 37°C in 5% CO2. All experiments were performed after 3 days. Chondrocyte differentiation was validated via RT-PCR for the following: type II collagen (forward) GCCCGTCAGGAAGTACC, (reverse) ACCAGCATCTCCTTTCTGT; and aggrecan (forward) TTCCATCTG GAGGAGAGGG, (reverse) ATCTACTCCTGAAGCAGATGTC (using type I collagen (forward) CCCTGGTATGACTGGCTT, (reverse) GAC CACGAATCCCTTCCT as a negative control). For immunocytochemical analysis, chondrocytes were plated on glass cover slips and after 3 days the cells were fixed in 4% paraformaldehyde, and then blocked with 1% BSA/1% casein for 1 h. Primary Abs (diluted to 10 µg/ml) were added for 1 h, and after three washes the primary Ab was detected via the avidinbiotin conjugate method using the Histostain-Plus reagent applied according to the manufacturer’s instructions.
Studies of cultured mouse chondrocytes and human chondrocytic CH-8 cells
Murine chondrocytes were carried in monolayer culture in DMEM/high glucose supplemented with 10% FCS, 1% l-glutamine, 100 U/ml penicillin, and 50 µg/ml streptomycin at 37°C with 5% CO2. Where first passage chondrocytes were stimulated with agonists such as S100A11, serum supplementation in the medium was decreased from 10% to 1%. CH-8 cells, a line of immortalized normal human knee articular chondrocytes (36), were studied between passage 5 and passage 15 under the same culture conditions described, under which maintenance of type II collagen and aggrecan expression were confirmed by RT-PCR as described (36). For transfection studies, aliquots of 0.5 × 106 CH-8 cells were grown in 60-mm culture dishes for 18 h in DMEM/high glucose containing 10% serum. For RNA knockdown, transfection of human short hairpin TG2 (shTG2) and the control scrambled sequence of TG2 (scrTG2) were performed using Lipofectamine Plus, according to the manufacturer’s protocol (Invitrogen). The shTG2 was prepared using the Ambion Web-based small interfering RNA design program to identify 21-mer regions within TG2 effective for targeting. Five sequences were originally tested to find an optimal sequence. The 21-mers were then used to generate 55-bp oligo-nucleotides, which included two 16-bp regions specific to human TG2 complementary to each other to form the hairpin, a loop sequence separating the complementary domains and a dinucleotide overhang that can hybridize with the RNA target (part of the original 21-mer). Both 55-bp complementary oligonucleotides were annealed and then ligated into the pSilencer 4.1-CMV Neo Vector (Ambion). The optimal sequences used in the experiments were for shTG2, 5′-GATCCGAGCGAGATGATCTG GATTCAAGAGAGTTCCAGATCATCTCGCTCTTA-3′ and the control scrambled sequence of TG2 (scrTG2) 5′-GATCCGAGAGCTTAAGAG GTAATGGATCGACACGTTCAAGAGACTCTTA-3′.
SDS-PAGE and Western blotting
Western blotting of samples from cell cultures analyzed aliquots of 30 µg of protein obtained from whole cell lysates or precipitated from conditioned medium using 15% TCA, followed by separation via 10% SDSPAGE. Polyclonal Abs in rabbit serum diluted 1/3000 for type X collagen were from Calbiochem. Rabbit polyclonal Abs recognizing the human S100A11-specific peptide CHDSFLKAVPSQKRT (diluted to 1 µg/ml) were generated by Zymed Laboratories and previously characterized as a mouse polyclonal Ab (19). Rabbit polyclonal Abs to phosphorylated p38 and total p38 were from BioSource International (diluted to 1 µg/ml). Polyclonal Abs specific for TG2 in goat serum were diluted 1/2000 (Upstate Biotechnology). HRP-conjugated goat anti-rabbit IgG, anti-mouse IgG, or rabbit anti-goat IgG used at 0.1 µg/ml were obtained from Pierce, and immunoreactive products were detected using ECL.
Assays of S100A11 binding to cells
For assays of binding of S100A11 to cells, aliquots of 1 × 104 CH-8 cells were plated in a 96-well plate for 18 h, after which the cells were incubated with a 1/500 dilution of goat anti-RAGE Abs in serum or normal goat serum for 30 min at 4°C. After two washes in growth medium, 1 µg/ml S100A11 or S100A11 mutant K3R/Q102N was added for 1 h at 4°C. The polyclonal anti-S100A11 Abs were added at 1 µg/ml for 1 h at 22°C after three washes, and after three further washes, biotin-labeled anti-rabbit IgG was added at 1 µg/ml for 1 h at 22°C. After three subsequent washes, streptavidin-alkaline phosphatase (Invitrogen) was added for 1 h at 22°C, and after three further washes, binding of S100A11 was detected using the Alkaline Phosphatase Yellow (pNPP) Liquid Substrate (Sigma-Aldrich). Cell binding of S100A11 was normalized to protein concentration per sample.
Statistical analyses
Where indicated, error bars represent SD. Statistical analyses were performed using the Student t test. Mean was tested using paired two-sample testing.
Results
Differentiation and function of TG2 and RAGE knockout cartilage explants and chondrocytes
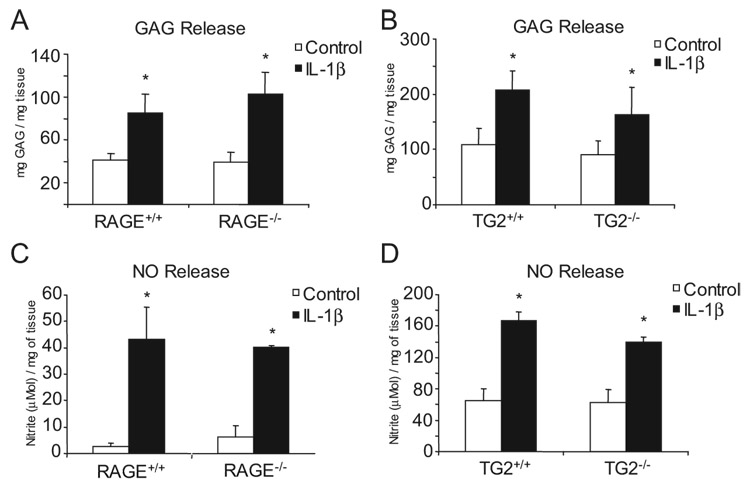
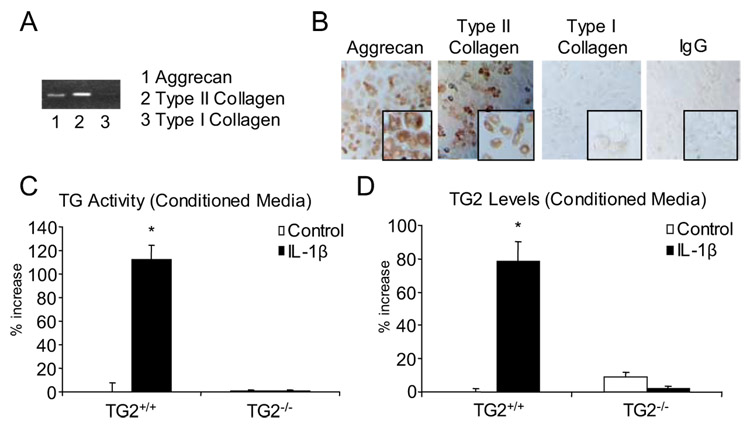
We first isolated TG2 and RAGE knockout acetabular cartilage explants, which we observed to retain responsiveness to IL-1β comparable to that for wild-type cartilage explants, as assessed by IL-1β-induced release of both GAG and NO (Fig. 1). In addition, we isolated immature mouse knee articular chondrocytes, and verified, using RT-PCR and immunocytochemistry, expression of the chondrocyte-specific matrix proteins collagen type II and aggrecan, with only trace expression of collagen I (Fig. 2, A and B). In response to IL-1β, the wild-type chondrocytes but not TG2−/− chondrocytes increased the level of TG catalytic activity in the conditioned medium in association with secretion of TG2 (Fig. 2C, and D).
FIGURE 1.
Both RAGE and TG2 knockout mouse cartilage explants retain responsiveness to IL-1β. Mouse femoral head cartilage was isolated as described in Materials and Methods. IL-1β (10 ng/ml) was added and GAG release was measured at 48 h (A and B) and NO generation at 24 h (C and D) assayed using the Griess reaction. Data are representative of three separate experiments. *, p < 0.05 compared with unstimulated cells.
FIGURE 2.
Characterization of extracellular matrix molecule expression and TG2 release in mouse articular chondrocytes. Articular chondrocytes from 7- to 8-day-old mice were isolated as described in the Materials and Methods, and after 3 days in culture, RT-PCR analysis (A) and immunocytochemistry (visualized at magnification ×40 (inset magnification at ×100)) (B) were performed for collagen type II, collagen type I, and for aggrecan. Results are representative of five different donors. C, Mouse chondrocytes were stimulated with 10 ng/ml IL-1β for 2 days, and released TG catalytic activity was measured in the conditioned medium. Results shown are the percentage of increase above the TG activity in unstimulated cells. D, TG2 was measured by ELISA in the conditioned medium, with results representing the percentage of increase above TG2 released by unstimulated cells. Data pooled from four experiments performed in triplicate. *, p < 0.05.
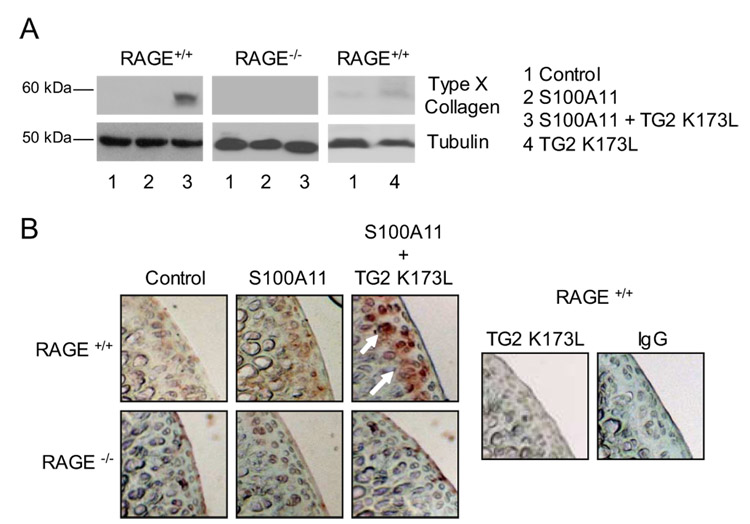
Next, we used exogenous recombinant human TG2 K173L, a catalytically active TG2 mutant that lacks the capacity to bind GTP, and which we confirmed (36) to be insufficient by itself to induce chondrocyte hypertrophy. Exogenous S100A11 induces the stereotypic chondrocyte hypertrophy marker type X collagen at 4 days in cultured normal human chondrocytes (19). In this experiment, addition of the TG2 mutant K173L to murine knee chondrocytes rendered exogenous S100A11 able to induce type X collagen at 2 days in culture, a point at which S100A11 had not yet induced type X collagen (Fig. 3A).
FIGURE 3.
Exogenous TG2 accelerates S100A11-induced type X collagen expression in cultured chondrocytes and cartilage in organ culture. A, After 3 days in culture that immediately followed isolation, mouse chondrocytes were treated with 100 ng/ml S100A11 with or without 100 ng/ml TG2 K173L. SDS-PAGE and Western blotting on cell lysates were performed for type X collagen after 2 days in culture. As a control, the effects on expression of type X collagen of the catalytically active but GTP-binding site functional mutant TG2 K173L were examined. B, Mouse femoral head cartilage was treated as in A. Frozen sections were examined by immunohistochemistry for type X collagen as described in Materials and Methods. Data representative of five different mouse donors.
Previous studies of RAGE function in chondrocytes have not studied complete deficiency of RAGE by assessment of knockout animals (19, 21). Here, we first observed that S100A11 failed to induce type X collagen in RAGE−/− chondrocytes (Fig. 3A). Second, we studied induction of type X collagen by immunohistochemistry of mouse hip cartilage explants, bearing in mind the limitations that normal mouse knee articular cartilage does demonstrate some type X collagen expression in situ, and that subchondral bone in such specimens normally bears hypertrophic chondrocytes (40). We observed that RAGE knockout blunted the capacity of S100A11 to induce type X collagen in the articular cartilage zone of explants (Fig. 3B). Under these conditions, the capacity of S100A11 to induce type X collagen in the articular cartilage zone of the explants was markedly increased by concurrent addition of TG2 K173L (Fig. 3B).
Effects of TG2 transamidation on the capacity of S100A11 to activate chondrocytes
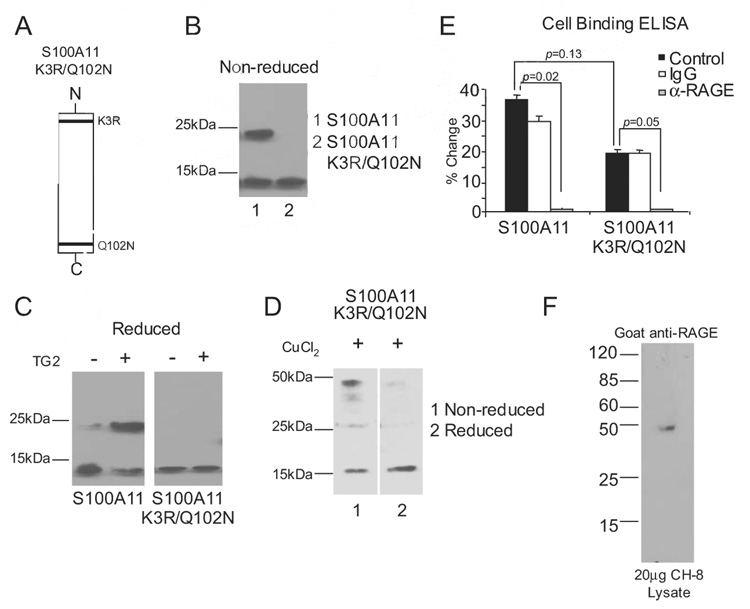
We conducted charge-conservative point mutations of the described transamidation substrate sites of human S100A11 (K3 and Q102 to R3 and N102, respectively) (Fig. 4A). Isolated recombinant human S100A11 K3R/Q102N, examined under nonreducing conditions, was monomeric, whereas wild-type S100A11 was found to be in monomeric and dimeric forms (Fig. 4B). Upon incubation with 100 ng/ml TG2, and examination by SDS-PAGE and Western blotting under reducing conditions, we observed that TG2 stimulated covalent dimerization of wild-type S100A11 but not S100A11 K3R/Q102N (Fig. 4C). Because the S100A11 K3R/Q102N mutant did not naturally assemble into multimers and because other S100/calgranulins can oligomerize via formation of disulfide bonds (37), we tested whether S100A11 K3R/Q102N was able to form disulfide-bonded multimers. We observed that copper oxidation of S100A11 K3R/Q102N induced assembly into dimers and tetramers sensitive to reduction of the disulfide bonds with 2-ME (Fig. 4D).
FIGURE 4.
Characterization of the S100A11 K3R/Q102N mutant. A, Graphic representation of the TG2 transamidation substrate sites of K3R and Q102N in the S100A11 protein. B, Both TG2 transamidation sites in recombinant human S100A11 were altered by site-directed mutagenesis to generate S100A11 K3R/Q102N. After purification, S100A11 and S100A11 K3R/Q102N proteins were isolated. S100A11 was separated by SDS-PAGE under nonreducing conditions and Western blotting performed. The wild-type S100A11 was detected as both monomeric (~12 kDa) and dimeric (~24 kDa), but the TG double transamidation site mutant was only detected in monomeric form. C, A total of 1 µg of S100A11 and S100A11 K3R/Q102N were incubated with 100 ng/ml TG2 for 2 h at 37°C, and SDS-PAGE under reducing conditions and Western blotting for S100A11 were performed. Data are representative of four different experiments. D, A total of 1 µg of S100A11 K3R/Q102N was incubated with 6 µM CuCl2 for 1 h at 37°C. SDS-PAGE under nonreducing and reducing conditions and Western blotting were performed for S100A11. Data are representative of three different experiments. E, Cell-associated RAGE binding ELISA was performed as described in Materials and Methods. Results are presented as the percentage of change compared with wells not receiving S100A11 or S100A11 K3R/Q102N, with data pooled from four experiments. F, A total of 20 µg of human chondrocytic CH-8 cell lysates was separated by SDS-PAGE under reducing conditions, and Western blotting was performed for RAGE using goat polyclonal Abs.
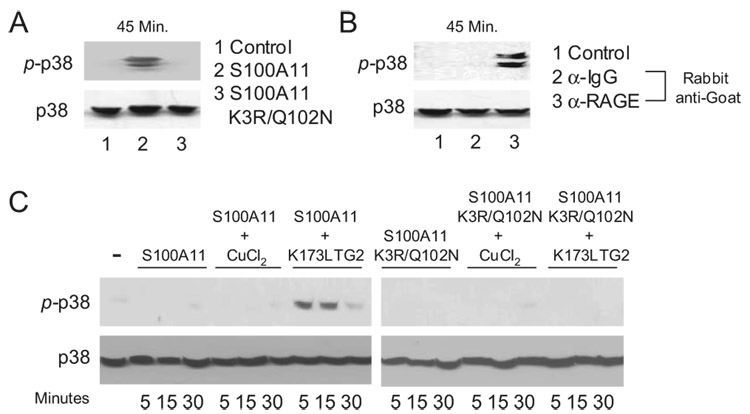
Using an ELISA system to assess S100A11 binding to human chondrocytic CH-8 cells, we observed that both wild-type S100A11 and K3R/Q102N mutant S100A11 were able to bind the chondrocytic cells in a RAGE-dependent manner (Fig. 4E). The cells were assayed using a goat polyclonal blocking Ab that recognized RAGE with high selectivity in the CH-8 human chondrocytic cells (Fig. 4F). Because we previously established critical linkage between MKK3 signaling and its kinase substrate p38 MAPK in the hypertrophic differentiation response to S100A11 (19), we next assessed the capacity of the S100A11 K3R/Q102N mutant to signal (Fig. 5). For these signaling studies, we used the CH-8 cells because we observed CH-8 cells to express significantly less extracellular basal transamidation-specific activity than normal human articular chondrocytes (data not shown). We demonstrated that Ab-induced cross-linking of RAGE induced p38 phosphorylation, but that the S100A11 K3R/Q102N mutation eliminated the capacity of S100A11 to induce detectable p38 phosphorylation in chondrocytic CH-8 cells (Fig. 5, A and B). We noted that S100A11 induced detectable p38 phosphorylation at 45 min but not at earlier tested time points in CH-8 cells (Fig. 5, A and C), whereas wild-type S100A11 pretreated with the catalytically active K173L TG2 mutant induced p38 phosphorylation by 5 min in CH-8 cells (Fig. 5C). In contrast, both the copper-oxidized wild-type S100A11, and the K3R/Q102N S100A11 induced to multimerize (via disulfide bond formation) in response to copper oxidation (Fig. 4D), failed to induce the rapid p38 phosphorylation response in CH-8 cells (Fig. 5C).
FIGURE 5.
Decreased signaling capacity of the S100A11 K3R/Q102N mutant in human chondrocytic CH-8 cells. A, CH-8 cells were treated with 100 ng/ml S100A11 or S100A11 K3R/Q102N for 45 min. SDS-PAGE and Western blotting of cell lysates was performed to assess p38 phosphorylation. Total p38 was visualized as a loading control. Data are representative of five different experiments. B, CH-8 cells were pretreated with anti-RAGE or control IgG. After several washes, 10 µg/ml rabbit anti-goat Ab was added for 45 min. SDS-PAGE and Western blotting of cell lysates were performed for p38 phosphorylation. Data are representative of four experiments. C, S100A11 and S100A11 K3R/Q102N were incubated with 100 ng/ml TG2 for 2 h at 37°C or 6 µM CuCl2 for 1 h at 37°C, and then CH-8 cells were stimulated with 100 ng/ml S100A11, 100 ng/ml S100A11 K3R/Q102N, 100 ng/ml TG2 pretreated calgranulins or 100 ng/ml CuCl2 treated calgranulins for the times indicated. SDS-PAGE was Western blotting on cell lysates were performed for p38 phosphorylation and total p38. Data are representative of three different experiments.
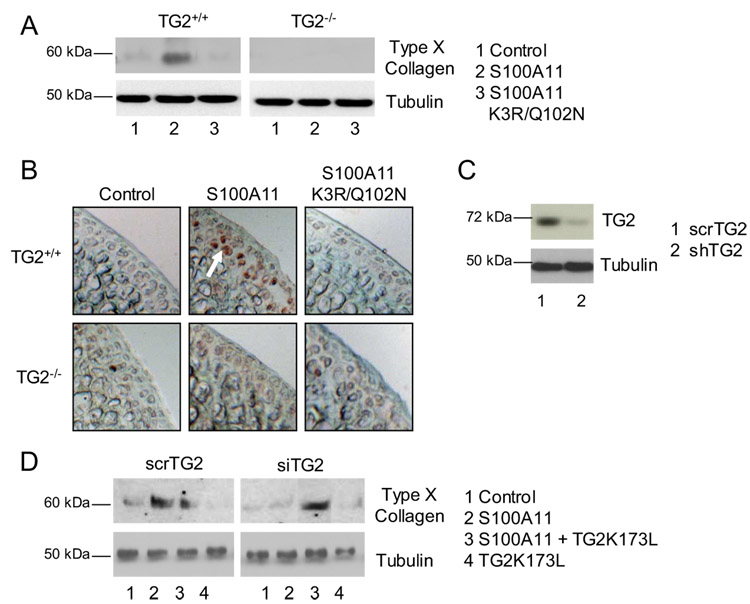
At 4 days in culture, we detected S100A11-induced expression of type X collagen in TG2+/+ chondrocytes and cartilage explants. In contrast, S100A11 K3R/Q102N failed to induce type X collagen in wild-type chondrocytes and cartilage explants (Fig. 6, A and B). As a control, S100A11 bearing a distinct charge-conservative lysine mutation K52R was able to form covalent dimers and to stimulate type X collagen expression (data not shown).
FIGURE 6.
TG2 is required for S100A11 to induce chondrocyte hypertrophy. Mouse chondrocytes (0.1 × 106 cells/12-well dish) (A) or femoral head cartilage explants (B) of the indicated genotypes (TG2−/− and wild-type controls) were stimulated with 100 ng/ml S100A11 or S100A11 K3R/Q102N. SDS-PAGE and Western blotting on cell lysates were performed for type X collagen after 4 days in culture. Frozen sections were examined by immunohistochemistry for type X collagen as described in Materials and Methods. Data are representative of seven different mouse donors. C, For TG2 mRNA knockdown, CH-8 cells (0.25 × 106 cells/6-well dish) were transfected by lipofection with plasmid constructs encoding shTG2 or the control scrambled TG2 (scrTG2), as described in Materials and Methods. TG2 expression in CH-8 cells transfected with scrTG2 vs shTG2, assessed by SDS-PAGE and Western blotting and densitometric analysis of cell lysates, verified >90% knockdown by shTG2. D, After transfection, cells were transferred to 96-well polyHEME-coated plates and 100 ng/ml S100A11 was added with or without 100 ng/ml TG2 K173L. SDS-PAGE and Western blotting on cell lysates were performed for type X collagen after 4 days in culture. Results are representative of three experiments.
Wild-type S100A11 failed to induce type X collagen in TG2−/− chondrocytes and cartilage explants (Fig. 6, A and B). To rule out that adaptive effects of germline TG2 deficiency were responsible for modulating the effects of S100A11 on chondrocytes, we knocked down TG2 in CH-8 cells by RNA interference using shTG2 and scrambled control sequences for TG2. Efficiency of TG2 knockdown by shTG2 was validated at the level of TG2 protein expression showing a >90% decrease in TG2 (Fig. 6C). TG2 knockdown rendered S100A11 unable to induce type X collagen (Fig. 6D). S100A11-induced type X collagen expression was rescued in cells subjected to TG2 knockdown by addition of the human TG2 K173L mutant (Fig. 6D).
TG2 is required to transform S100A11 into an inducer of cartilage matrix catabolism
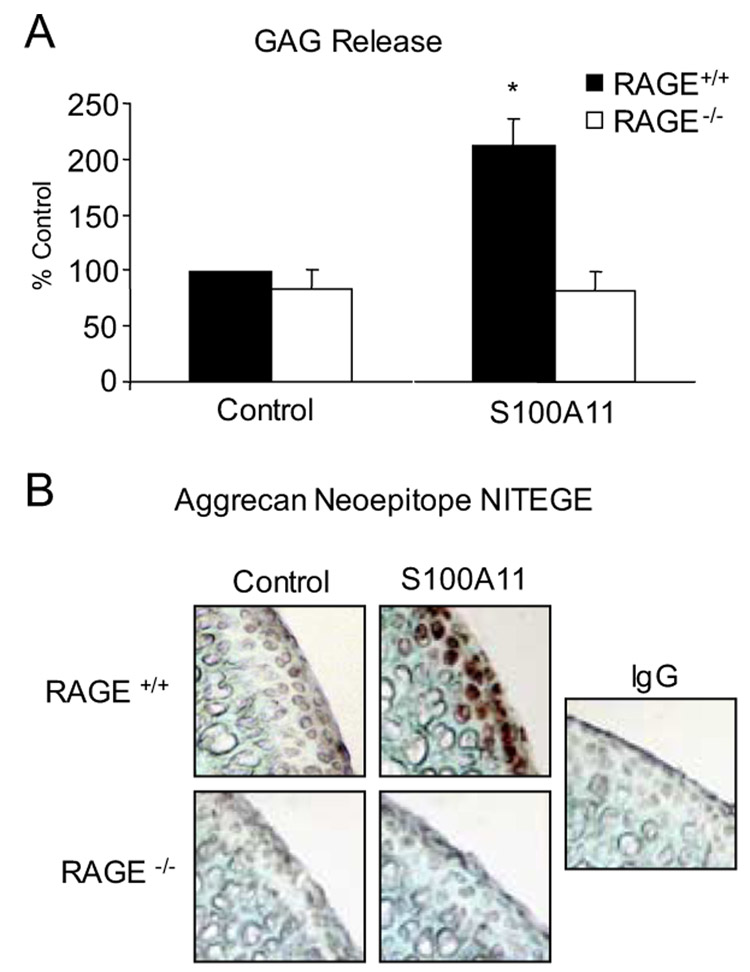
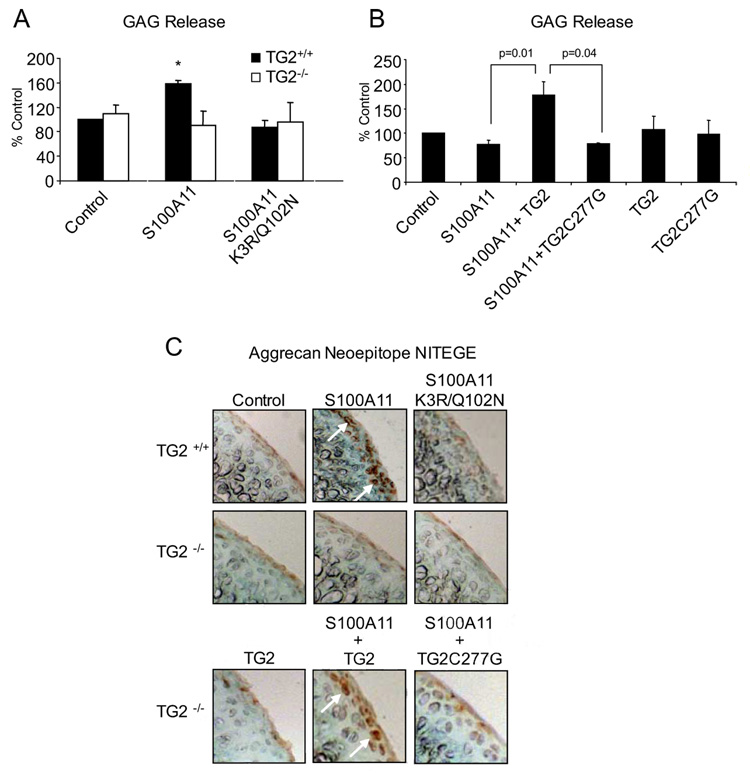
We observed a 2-fold induction of GAG release in response to S100A11 in wild-type (RAGE+/+) cartilage explants (Fig. 7A), an effect comparable to that of IL-1β (Fig. 1A), and which was not observed in RAGE−/− cartilage explants (Fig. 7A). S100A11 induced the aggrecanase neoepitope NITEGE in the RAGE+/+ explants, a response lacking in RAGE−/− cartilage explants (Fig. 7B). S100A11 K3R/Q102N failed to induce GAG release and the aggrecanase neoepitope in wild-type cartilage explants (Fig. 8, A and C), and copper oxidation of K3R/Q102N S100A11 did not render it able to induce GAG release in the TG2−/− cartilage explants (data not shown). Last, failure of S100A11 to induce GAG release and the aggrecanase neoepitope NITEGE in TG2−/− explants demonstrated correction by addition of catalytically active but not catalytically inactive TG2 to the TG2−/− cartilage explants (Fig. 8, B and C).
FIGURE 7.
S100A11 stimulates GAG release in articular cartilage explants. Mouse femoral head cartilage explants were treated with 100 ng/ml S100A11. A, At 48 h, GAG release was measured in the conditioned medium, with results indicated as the percentage of control (with S100A11 inducing 65.5 ± 9.3 mg of GAG release per milligram of tissue in wild-type cartilage explants). Data pooled are from 11 different mouse donors of each genotype in replicates of three. B, Frozen sections were analyzed by immunohistochemistry for the aggrecanase neoepitope NITEGE at 72 h in culture. Data are representative of 5 different mouse donors of each genotype. *, p < 0.05 compared with unstimulated cells.
FIGURE 8.
TG2 mediates the capacity of S100A11 to promote cartilage matrix catabolism. A, TG2+/+ and TG2−/− mouse femoral head cartilage explants were treated with 100 ng/ml S100A11 or S100A11 K3R/ Q102N. At 48 h, GAG release was measured in the conditioned medium. Data pooled are from 15 different mouse donors in triplicates. B, TG2−/− mouse femoral head cartilage was treated with 100 ng/ml S100A11 with or without 100 ng/ml TG2 or 100 ng/ml of the catalytically inactive TG2 C277G mutant. After 2 days, GAG release was measured in the conditioned medium. Data pooled are from eight different mouse donors studied in replicates of three. C, Frozen sections were analyzed by immunohistochemistry for the aggrecanase neoepitope NITEGE at 72 h in culture. Data are representative of at least four different mouse donors of each genotype. *, p < 0.05 compared with unstimulated cells.
Discussion
Increased chondrocyte expression of proinflammatory cytokines such as IL-1βand TNF-α mediate articular cartilage responses to injury in OA through effects on chondrocyte differentiation, as well as shifts from matrix anabolic to catabolic gene expression programs (1). Additional regulatory loops that translate inflammation into cartilage responses to injury in OA include the conversion of inflammatory cytokines to active, secreted forms, as catalyzed by proteases themselves subject to regulation by endoproteolysis (e.g., caspase-1 for IL-1β, and TNF-α converting enzyme for TNF-α).
The capacity of transamidation by TG2 to regulate activation of latent TGF-β (41) has the potential to impact on regulation of inflammatory responses within OA cartilage (8). The primary rationale for the current study of functional linkage between TG2 and S100A11 was based on the observations that multimerization of secreted S100A11 in cultured human chondrocytes occurs in response to CXCL8 (19), and that TG2 plays a major role in mediating CXCL8-induced chondrocyte hypertrophic differentiation (8). In this study, we observed a novel TG2 function in chondrocytes, whereby transamidation by TG2 of S100A11 converted the secreted calgranulin into an inflammatory cytokine-like promoter of chondrocyte hypertrophy and matrix catabolism.
TG2 multifunctionality includes GTPase and fibronectin binding activities (34), but complementary lines of evidence demonstrated that TG2 transamidation catalytic activity was critical for S100A11 to induce chondrocyte hypertrophy and cartilage matrix catabolism. First, the combination of exogenous recombinant S100A11 and the catalytically active but GTP-binding site functional TG2 mutant K173L accelerated chondrocyte hypertrophy. Second, the dual TG2 transamidation substrate site S100A11 mutant K3R/Q102N, which bound to chondrocytes in a RAGE-dependent manner, lost the capacity to induce p38 phosphorylation or hypertrophy, or to suppress proteoglycan synthesis in chondrocytes, and failed to induce the aggrecanase neoepitope NITEGE or GAG release in cartilage explants. Third, S100A11 failed to induce chondrocyte hypertrophy, or to stimulate the aggrecanase neoepitope NITEGE and GAG release in TG2 knockout chondrocytes or cartilage explants.
S100/calgranulins constitutively exist as anti-parallel, noncovalently associated homodimers, heterodimers, and other multimers (16). S100/calgranulin dimer formation is mediated by calcium binding and the interaction of hydrophobic globular domains derived from helix I and helix IV for each monomer (42). TG-dependent modification may inactivate certain S100/calgranulin functions by conformational change (30). However, S100/calgranulin multimerization is thought to enhance RAGE-mediated intracellular signaling cascades at least in part by promoting ligand-induced RAGE oligomerization (26–28). Significantly, there is increasing evidence of receptor oligomerization as a general mechanism for signal propagation by the closely related cytokine family of receptors (43–45). Notably, in this study, the K3R/Q102N S100A11 mutant that lost the capacity to oligomerize also failed to induce chondrocyte hypertrophy, GAG release, and the aggrecanase neoepitope NITEGE.
S100A11 has been defined to be a substrate for TG2-dependent covalent cross-linking via two transamidation substrate domains (at the N-terminal K3 in α helix I and the C-terminal Q102 domain in α helix IV (30)). TGs generally catalyze transamidation in a manner highly selective for glutamine acyl donor substrate motifs (30), with the configuration of the amino acids surrounding the lysine residue being of less importance for formation of N-ε(γ-glutamyl)lysyl isopeptide bonds (46). Though it is not yet clear how TGs differentially recognize and interact with individual glutamines and lysines and their surrounding amino acids, TGs preferentially modify highly accessible glutamine and lysine residues such as in the relatively exposed N termini and C termini of S100A11, and also in S100A10, the calgranulin most closely related to S100A11 (30, 46). It is possible that other chondrocyte-expressed S100/calgranulins could also be TG2 substrates. One example is S100A4, which forms multimers (28, 47), bears highly exposed C-terminal glutamine and lysine residues, stimulates MMP-13 expression in cultured chondrocytes dependent on RAGE (20), and promotes the expression of a variety of MMPs in cells other than chondrocytes (48).
Functional characteristics of multimeric S100 proteins linked by disulfide bonds or noncovalent associations have been studied most extensively in cells other than chondrocytes. In this context, S100B dimers linked together by disulfide bonds induce neurite outgrowth, but dimers without disulfide bonds can induce an in-flammatory response in glial cells (49, 50). Furthermore, noncovalent tetramers of S100B found in human brain extracts appear to induce RAGE multimerization (51). Additionally, the Ca2+-dependent S100A8/A9 tetramer promotes the formation of microtubules (52) and the S100A12 hexamer associates with RAGE (53). Because TG2-induced isopeptide bond formation was critical for S100A11 proinflammatory signaling in chondrocytes, but disulfide-bonded S100A11 multimers generated by copper oxidation did not share this activity, the mode in which S100A11 is multimerized and its consequent conformation appear essential for proinflammatory effects in chondrocytes.
Limitations of this study include lack of kinetic binding studies and assessment of ligand-induced RAGE multimerization in chondrocytes. We did not perform circular dichroism studies for analyses of folding of S100A11 in the presence of TG2 or for possibly altered folding for the S100A11 K3R/Q102N mutant. Glasson et al. (4) have described a critical role for ADAMTS-5-driven aggrecanolysis in a murine instability OA model. In this study, we observed that S100A11 induced both the aggrecanase neoepitope NITEGE and GAG release in cartilage explants, but we did not define the net role in matrix catabolism of ADAMTS-5 activation, relative to potential release and activation of ADAMTS-4 or MMP-3, for example. Moreover, the mechanism of the induction by S100A11 of the aggrecanase neoepitope was beyond the scope of this study. We have observed that S100A11 does not induce ADAMTS-5 transcription in chondrocytes but does increase chondrocyte secretion of ADAMTS-5 protein (D. Cecil, unpublished observation). Regulation of ADAMTS-5 catalytic activity is complex, as it is not simply controlled by furin-induced endoproteolysis (54). S100A11 could theoretically act indirectly by regulating factors that mediate increased ADAMTS-5 catalytic activity. Though TG2 knockout blunted procatabolic responses of chondrocytes and cartilages to S100A11, we did not examine potential effects on S100A11 structure and function exerted by the other major chondrocyte-expressed TG isoenzyme FXIIIA (31). Last, we have detected multimers of S100A11 in extracts of human OA cartilage that retain multimerization under reducing conditions in SDS-PAGE (D. Cecil, unpublished observation). However, we have not quantitatively analyzed differences in S100A11 multimerization in OA relative to normal cartilage. Moreover, we have not yet directly assessed the impact of knockout of S100A11, RAGE, or TG2 on experimental OA in vivo.
TG2 release is critical for determining the phenotype of healing of multiple forms of tissue injury (29). This study reveals that posttranslational modification of S100A11 by TG2 stimulates chondrocyte hypertrophic differentiation and transforms S100A11 into a procatabolic inflammatory cytokine-like molecule for cartilage via RAGE signaling. Therefore, S100A11, subject to transformation by TG2, is able to function as an unconventional inflammatory mediator of altered chondrocyte differentiation and matrix remodeling, analogous to functions of proteolytically derived fibronectin fragments that also are increased within OA cartilages (55). S100A11 release by chondrocytes is regulated partly by conventional cytokines. Nevertheless, the findings of this study, including the retention of responsiveness to IL-1β by RAGE and TG2 knockout mouse cartilage explants, reveal S100A11 to be capable of independently inducing alterations in chondrocyte differentiation and extracellular matrix organization. Hence, S100A11 and TG2 have a unique functional linkage with the potential to translate low-grade cartilage inflammation into the progression of OA.
Acknowledgments
We gratefully acknowledge the assistance of Dr. Kristen Johnson (Veterans Affairs Medical Center, University of California, San Diego, CA) for design and optimization of the TG2 RNA knockdown system.
Footnotes
This work was supported by the Veterans Affairs Research Service and by Research Awards AR54135 and PAG07996 from the National Institutes of Health.
Abbreviations used in this paper: OA, osteoarthritis; GAG, glycosaminoglycan; RAGE, receptor for advanced glycation end product; MMP, matrix metalloproteinase; TG, transglutaminase; shTG, short hairpin TG.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004;(427 Suppl):S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 2.Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 1993;52:870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlaak JF, Pfers I, Meyer Zum Büschenfelde KH, Märker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 1996;14:155–162. [PubMed] [Google Scholar]
- 4.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al. Deletion of active ADAMTS-5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Melchiorri C, Meliconi R, Frizziero L, Silvestri T, Pulsatelli L, Mazzetti I, Borzi RM, Uguccioni M, Facchini A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes. Arthritis Rheum. 1998;41:2165–2174. doi: 10.1002/1529-0131(199812)41:12<2165::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Borzi RM, Mazzetti I, Macor S, Silvestri T, Bassi A, Cattini L, Facchini A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238–242. doi: 10.1016/s0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 7.Nurminskaya M, Linsenmayer TF. Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev. Dyn. 1996;206:260–271. doi: 10.1002/(SICI)1097-0177(199607)206:3<260::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogeneα/CXCL1 induce chondrocyte hypertrophic differentiation. J. Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 9.Terkeltaub RA. What does cartilage calcification tell us about osteoarthritis? J. Rheumatol. 2002;29:411–415. [PubMed] [Google Scholar]
- 10.Chen Q, Johnson DM, Haudenschild DR, Goetinck PF. Progression and recapitulation of the chondrocyte differentiation program: cartilage matrix protein is a marker for cartilage maturation. Dev. Biol. 1995;172:293–306. doi: 10.1006/dbio.1995.0024. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 12.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 13.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Goldring MB. Anticytokine therapy for osteoarthritis. Expert Opin. Biol. Ther. 2001;1:817–829. doi: 10.1517/14712598.1.5.817. [DOI] [PubMed] [Google Scholar]
- 15.Attur MG, Dave MN, Leung MY, Cipolletta C, Meseck M, Woo SL, Amin AR. Functional genomic analysis of type II IL-1β decoy receptor: potential for gene therapy in human arthritis and inflammation. J. Immunol. 2002;168:2001–2010. doi: 10.4049/jimmunol.168.4.2001. [DOI] [PubMed] [Google Scholar]
- 16.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 17.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol. Cell Biol. 2003;23:1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood. 2005;105:2955–2962. doi: 10.1182/blood-2004-07-2520. [DOI] [PubMed] [Google Scholar]
- 19.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J. Immunol. 2005;175:8296–8302. doi: 10.4049/jimmunol.175.12.8296. [DOI] [PubMed] [Google Scholar]
- 20.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 21.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol. Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–353. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell Biochem. 2001;81:102–113. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Barger SW, Wolchok SR, Van Eldik LJ. Disulfide-linked S100 beta dimers and signal transduction. Biochim. Biophys. Acta. 1992;1160:105–112. doi: 10.1016/0167-4838(92)90043-d. [DOI] [PubMed] [Google Scholar]
- 27.Moroz OV, Antson AA, Dodson EJ, Burrell HJ, Grist SJ, Lloyd RM, Maitland NJ, Dodson GG, Wilson KS, Lukanidin E, Bronstein IB. The structure of S100A12 in a hexameric form and its proposed role in receptor signalling. Acta Crystallogr. D. Biol. Crystallogr. 2002;58(Pt 3):407–413. doi: 10.1107/s0907444901021278. [DOI] [PubMed] [Google Scholar]
- 28.Novitskaya V, Grigorian M, Kriajevska M, Tarabykina S, Bronstein I, Berezin V, Bock E, Lukanidin E. Oligomeric forms of the metastasis related Mts1 (S100A4) protein stimulate neuronal differentiation in cultures of rat hippocampal neurons. J. Biol. Chem. 2000;275:41278–41286. doi: 10.1074/jbc.M007058200. [DOI] [PubMed] [Google Scholar]
- 29.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 30.Ruse M, Lambert A, Robinson N, Ryan D, Shon KJ, Eckert RL. S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochemistry. 2001;40:3167–3173. doi: 10.1021/bi0019747. [DOI] [PubMed] [Google Scholar]
- 31.Aeschlimann D, Mosher D, Paulsson M. Tissue transglutaminase and factor XIII in cartilage and bone remodeling. Semin. Thromb. Hemost. 1996;22:437–443. doi: 10.1055/s-2007-999043. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal AK, Gohr CM, Uzuki M, Masuda I. Osteopontin promotes pathologic mineralization in articular cartilage. Matrix Biol. 2006;26:96–105. doi: 10.1016/j.matbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson K, Hashimoto S, Lotz M, Pritzker K, Terkeltaub R. Interleukin- 1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am. J. Pathol. 2001;159:149–163. doi: 10.1016/S0002-9440(10)61682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summey BT, Jr., Graff RD, Lai TS, Greeberg CS, Lee GM. Tissue transglutaminase localization and activity regulation in the extracellular matrix of articular cartilage. J. Orthop. Res. 2002;20:76–82. doi: 10.1016/S0736-0266(01)00064-X. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KA, van Etten D, Nanda N, Graham RM, Terkeltaub RA. Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J. Biol. Chem. 2003;278:18824–18832. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- 36.Johnson KA, Terkeltaub RA. External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification. J. Biol. Chem. 2005;280:15004–15012. doi: 10.1074/jbc.M500962200. [DOI] [PubMed] [Google Scholar]
- 37.Matsui Lee IS, Suzuki M, Hayashi N, Hu J, Van Eldik LJ, Titani K, Nishikimi M. Copper-dependent formation of disulfide-linked dimer of S100B protein. Arch. Biochem. Biophys. 2000;374:137–141. doi: 10.1006/abbi.1999.1595. [DOI] [PubMed] [Google Scholar]
- 38.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 39.Salvat C, Pignet A, Humbert L, Berenbaum F, Thirion S. Immature murine articular chondrocytes in primary culture: a new tool for investigating cartilage. Osteoarthritis Cartilage. 2005;13:243–249. doi: 10.1016/j.joca.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Chambers MG, Kuffner T, Cowan SK, Cheah KS, Mason RM. Expression of collagen and aggrecan genes in normal and osteoarthritic murine knee joints. Osteoarthritis Cartilage. 2002;10:51–61. doi: 10.1053/joca.2001.0481. [DOI] [PubMed] [Google Scholar]
- 41.Falasca L, Iadevaia V, Ciccosanti F, Melino G, Serafino A, Piacentini M. Transglutaminase type II is a key element in the regulation of the anti-inflammatory response elicited by apoptotic cell engulfment. J. Immunol. 2005;174:7330–7340. doi: 10.4049/jimmunol.174.11.7330. [DOI] [PubMed] [Google Scholar]
- 42.Rety S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, Russo-Marie F, Lewit-Bentley A. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat. Struct. Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- 43.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF β complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 44.Naismith JH, Devine TQ, Kohno T, Sprang SR. Structures of the extracellular domain of the type I tumor necrosis factor receptor. Structure. 1996;4:1251–1262. doi: 10.1016/s0969-2126(96)00134-7. [DOI] [PubMed] [Google Scholar]
- 45.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugimura Y, Hosono M, Wada F, Yoshimura T, Maki M, Hitomi K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGASE 2 and Factor XIIIA. J. Biol. Chem. 2006;281:17699–17706. doi: 10.1074/jbc.M513538200. [DOI] [PubMed] [Google Scholar]
- 47.Kiryushko D, Novitskaya V, Soroka V, Klingelhofer J, Lukanindin E, Berezin V, Bock E. Molecular mechanisms of Ca2+ signaling in neurons induced by the S100A4 protein. Mol. Cell Biol. 2006;26:3625–3638. doi: 10.1128/MCB.26.9.3625-3638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc. Natl. Acad. Sci. USA. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winningham-Major F, Staecker JL, Barger SW, Coats S, Van Eldik LJ. Neurite extension and neuronal survival activities of recombinant S100 beta proteins that differ in the content and position of cysteine residues. J. Cell Biol. 1989;109:3063–3071. doi: 10.1083/jcb.109.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koppal T, Lam AG, Guo L, Van Eldik LJ. S100B proteins that lack one or both cysteine residues can induce inflammatory responses in astrocytes and microglia. Neurochem. Int. 2001;39:401–407. doi: 10.1016/s0197-0186(01)00047-x. [DOI] [PubMed] [Google Scholar]
- 51.Ostendorp T, Leclerc E, Galichet A, Koch M, Demling N, Weigle B, Heizmann CW, Kroneck PM, Fritz G. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007;26:3868–3878. doi: 10.1038/sj.emboj.7601805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J. Mol. Biol. 2006;359:961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Xie J, Burz DS, He W, Bronstein IB, Lednev I, Shekhtman A. Hexameric calgranulin C (S100A12) binds to the receptor for advanced glycated end products (RAGE) using symmetric hydrophobic target-binding patches. J. Biol. Chem. 2007;282:4218–4231. doi: 10.1074/jbc.M608888200. [DOI] [PubMed] [Google Scholar]
- 54.Arner EC. Aggrecanase-mediated cartilage degradation. Curr. Opin. Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- 55.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. NF-κB mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]